TO THE EDITOR—We read with great interest the recent study by Tong et al, who demonstrated the increased expression of endothelial cell adhesion molecules is correlated to coronavirus disease 2019 (COVID-19) severity and may contribute to coagulation dysfunction [1]. The authors examined the expression of 3 endothelial cell adhesion molecules by enzyme-linked immunosorbent assays (ELISA), including vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular adhesion protein-1 (VAP-1).
Here we want to contribute the results focusing on another endothelial cell adhesion molecule, platelet endothelial cell adhesion molecule 1 (PECAM-1 or CD31) [2]. PECAM-1 is a highly glycosylated immunoglobulin-like membrane receptor expressed by leukocytes, platelets, and especially endothelial cells. In addition, PECAM-1 is widely regarded as a marker of endothelium [3]. Just like ICAM-1 and VCAM-1, the extracellular domain of PECAM-1 functions to mediate cell-cell interactions and gives rise to a tight barrier of the endothelium [4, 5]. Elevation of soluble PECAM-1 (sPECAM-1) level has also been shown in the serum of patients with myocardial infarction, acute ischemic stroke, and multiple sclerosis, conditions that involve tissue damage and endothelial cell apoptosis [6, 7]. No study has investigated the associations between sPECAM-1 levels and COVID-19 severity.
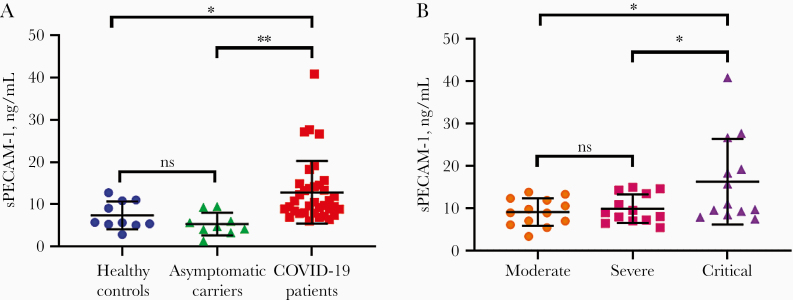
We found that the serum levels of sPECAM-1 (ELISA kit from www.cloud-clone.cn) were not only significantly higher in COVID-19 patients than in healthy controls (12.67 ± 7.40 ng/mL vs 7.40 ± 3.27 ng/mL, P = .035), but also significantly higher than in asymptomatic carriers (5.35 ± 2.66 ng/mL, P = .006) (Figure 1A). In addition, the serum levels of sPECAM-1 were positively correlated with disease severity, and sPECAM-1 levels in patients in critical disease (16.24 ± 10.13 ng/mL) were significantly higher than in patients in moderate (9.07 ± 3.23 ng/mL, P = .023) or severe (9.21 ± 3.86 ng/mL, P = .028) condition (Figure 1B). These results demonstrated that SARS-CoV-2 infection alone is not enough to stimulate endothelial cell activation, which led to sPECAM-1 shedding from endothelium. From our data, together with that of Tong et al [1], it appears that it is the progression of the disease, but not the virus itself, that damages the endothelium, leading to the elevated levels of endothelial-specific adhesion molecules, including sPECAM-1.
Figure 1.
Serum levels of soluble platelet endothelial cell adhesion molecule 1 (sPECAM-1) in different patient groups. A, sPECAM-1 levels in coronavirus disease 2019 (COVID-19) patients and asymptomatic carriers, compared with that in healthy controls. B, Comparison of serum levels of sPECAM-1 among 3 COVID-19 patient groups: moderate, severe, and critical. *P < .05; **P < .01. Abbreviation: ns, nonsignificant.
In addition to the adhesion molecules, we also measured a panel of other indexes in the patient sera, including nitric oxide (NO), superoxide dismutase (SOD) (both kits from http://www.flucky.com.cn), lymphocyte counts, and others, to study the mechanism of the increased levels of circulating adhesion molecules. Of particular interest among these indexes are the consistently higher levels of redox activity. We found that the NO levels were higher in COVID-19 patients than those in healthy controls (42.14 ± 10.27 µmol/L vs 37.28 ± 6.32 µmol/L, P = .161). Importantly, the levels of a reactive oxygen species (ROS) detoxifying enzyme, SOD, were significantly lower in COVID-19 patients than those in healthy controls (150.64 ± 27.10 U/mL vs 182.52 ± 14.61 U/mL, P < .001). Mechanistically, deficiency of PECAM-1 has been associated with a significant decrease in the expression of endothelial nitric oxide synthase (eNOS) and NO in the endothelium [8]. In addition, ROS production has also been implicated in vascular oxidative stress manifested in hypertension, ischemia, hyperoxia, stroke, and other conditions [9].
Taken together with the results of Tong et al [1], these findings consistently demonstrated a damaged endothelium that is correlated with COVID-19 disease severity. Further studies are needed to see if the soluble marker elevations in the COVID-19 patients are due to the enhanced overexpression of the constitutive proteins or due to their increased shedding from the cell surface.
Notes
Acknowledgment. We thank all donors for their participation in this study.
Financial support. This work was supported by the Natural Science Foundation of China (82070142, 31670739, 22077016); National Key R&D Program of China (grant number 2017YFE0103200); Fujian Health and Education Ministry Joint Program (2019-WJ-17); Natural Science Foundation of Fujian Province (grant numbers 2018J01897 and 2018J01729); and Fuzhou Municipal Science and Technology Program (grant number 2020-XG-021).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tong M, Jiang Y, Xia D, et al. Elevated expression of serum endothelial cell adhesion molecules in COVID-19 patients. J Infect Dis 2020; 222:894–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Newman PJ, Berndt MC, Gorski J, et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 1990; 247:1219–22. [DOI] [PubMed] [Google Scholar]
- 3. Caligiuri G. CD31 as a therapeutic target in atherosclerosis. Circ Res 2020; 126:1178–89. [DOI] [PubMed] [Google Scholar]
- 4. Paddock C, Zhou D, Lertkiatmongkol P, Newman PJ, Zhu J. Structural basis for PECAM-1 homophilic binding. Blood 2016; 127:1052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang L, Lin L, Li R, et al. Dimer conformation of soluble PECAM-1, an endothelial marker. Int J Biochem Cell Biol 2016; 77:102–8. [DOI] [PubMed] [Google Scholar]
- 6. Zaremba J, Losy J. sPECAM-1 in serum and CSF of acute ischaemic stroke patients. Acta Neurol Scand 2002; 106:292–8. [DOI] [PubMed] [Google Scholar]
- 7. Losy J, Niezgoda A, Wender M. Increased serum levels of soluble PECAM-1 in multiple sclerosis patients with brain gadolinium-enhancing lesions. J Neuroimmunol 1999; 99:169–72. [DOI] [PubMed] [Google Scholar]
- 8. Park S, DiMaio TA, Scheef EA, Sorenson CM, Sheibani N. PECAM-1 regulates proangiogenic properties of endothelial cells through modulation of cell-cell and cell-matrix interactions. Am J Physiol Cell Physiol 2010; 299:C1468–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 2011; 15:1583–606. [DOI] [PMC free article] [PubMed] [Google Scholar]