Abstract
Background
Advanced age is a well-known risk factor for poor prognosis in COVID-19. However, few studies have specifically focused on very old inpatients with COVID-19. This study aims to describe the clinical characteristics of very old inpatients with COVID-19 and identify risk factors for in-hospital mortality at admission.
Methods
We conducted a nationwide, multicenter, retrospective, observational study in patients ≥ 80 years hospitalized with COVID-19 in 150 Spanish hospitals (SEMI-COVID-19) Registry (March 1–May 29, 2020). The primary outcome was in-hospital mortality. A uni- and multivariate logistic regression was performed to assess predictors of mortality at admission.
Results
A total of 2772 consecutive patients (49.4% men, median age 86.3 years) were analyzed. Rates of atherosclerotic cardiovascular disease, diabetes mellitus, dementia, and Barthel Index < 60 were 30.8%, 25.6%, 30.5%, and 21.0%, respectively. The overall case-fatality rate was 46.9% (n: 1301) and increased with age (80–84 years: 41.6%; 85–90 years: 47.3%; 90–94 years: 52.7%; ≥95 years: 54.2%). After analysis, male sex and moderate-to-severe dependence were independently associated with in-hospital mortality; comorbidities were not predictive. At admission, independent risk factors for death were: oxygen saturation < 90%; temperature ≥ 37.8°C; quick sequential organ failure assessment (qSOFA) score ≥ 2; and unilateral–bilateral infiltrates on chest x-rays. Some analytical findings were independent risk factors for death, including estimated glomerular filtration rate < 45 mL/min/1.73 m2; lactate dehydrogenase ≥ 500 U/L; C-reactive protein ≥ 80 mg/L; neutrophils ≥ 7.5 × 103/μL; lymphocytes < 0.8 × 103/μL; and monocytes < 0.5 × 103/μL.
Conclusions
This first large, multicenter cohort of very old inpatients with COVID-19 shows that age, male sex, and poor preadmission functional status—not comorbidities—are independently associated with in-hospital mortality. Severe COVID-19 at admission is related to poor prognosis.
Keywords: Age ≥ 80, COVID-19, Mortality, Prognostic factors, SARS-CoV-2
Since the beginning of the COVID-19 pandemic, advanced age has been identified as one of the strongest risk factors for poor outcomes, complications, and mortality (1–10). Paradoxically, few studies have focused on the clinical characteristics of COVID-19 in older patients (≥65 or ≥60 years of age) (11–17). Furthermore, up until now, there has been very limited information on COVID-19 specifically in very older patients (≥80 years old) available (12,14) despite the fact that this population has the highest mortality rate (18).
Spain is one of the countries with the highest number of patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) relative to the size of the population in the world (19). The first COVID-19 infection in the country was confirmed on January 31, 2020 and since then, 245 575 confirmed cases have been recorded, with 124 806 hospitalizations, 11 633 admissions to intensive care units, and 28 315 deaths at July 20 (20). Furthermore, Spain has one of the oldest populations in the world, with 6.2% of the population ≥ 80 years old of age at present (21).
To our knowledge, this is the first large, multicenter cohort study of very old patients hospitalized with COVID-19. The objectives of this study were to describe the clinical presentation of patients ≥ 80 years old of age hospitalized with COVID-19 and to identify risk factors for in-hospital mortality at admission. We hypothesized that prognostic factors for mortality due to COVID-19 could be different in patients ≥ 80 years old of age compared to the general population.
Materials and Methods
Study Design and Population
We carried out an observational, multicenter, nationwide study of patients ≥ 80 years old of age hospitalized with COVID-19 in Spain from March 1 to May 29, 2020. All patient data were obtained from the Spanish Society of Internal Medicine’s SEMI-COVID-19 Registry, in which 150 hospitals in Spain participate. The SEMI-COVID-19 Registry includes all consecutive patients ≥ 18 years old admitted to hospitals for COVID-19; this study analyzes the subpopulation of patients ≥ 80 years old.
Variables
The SEMI-COVID-19 Registry retrospectively compiles data from the first admission of patients ≥ 18 years old of age with COVID-19 confirmed microbiologically by a reverse transcription polymerase chain reaction (RT-PCR) test of a nasopharyngeal swab sample, sputum specimen, or bronchoalveolar lavage. In brief, this registry collects sociodemographic data, previous medical history, routine treatment, clinical presentation, clinical condition, laboratory test results, radiological findings, clinical management, in-hospital complications, length of hospital stay, early readmissions, referral to long-term care or skilled-nursing facilities, and in-hospital deaths. More in-depth information on the justification, objectives, methodology, and preliminary results of the SEMI-COVID-19 Registry have recently been published (22). Data were collected retrospectively by clinicians using an online electronic data capture system.
To assess preadmission functional status, we used the Barthel Index (23) (independent or mild dependence: 100–91; moderate dependence: 90–61; and severe dependence: ≤60). The burden of comorbidities was assessed by the age-adjusted Charlson Comorbidity Index (24). DSM5 criteria were used for the diagnosis of dementia (25). Atherosclerotic cardiovascular disease was defined as a previous history of ischemic cardiopathy (myocardial infarction, acute coronary syndrome, angina, or coronary revascularization), cerebrovascular disease (stroke, transient ischemic attack), or peripheral arterial disease (intermittent claudication, revascularization, lower limb amputation, or abdominal aortic aneurysm). Nonatherosclerotic cardiovascular disease included atrial fibrillation and heart failure. Obesity was defined as a body mass index ≥ 30 kg/m2. Patients were considered to have hypertension, diabetes mellitus, or dyslipidemia if they had a prior clinical diagnosis or have been on pharmacological treatment for these conditions. Chronic pulmonary disease was defined as a diagnosis of chronic obstructive pulmonary disease and/or asthma. Malignancy included solid tumors and/or hematologic neoplasia (excluding nonmelanoma skin cancer). Moderate-to-severe renal disease was defined as an estimated glomerular filtration rate < 45 mL/min/1.73 m2 according to the CKD-EPI equation (26). The preadmission comorbidity was collected from the patient’s electronic medical record, obtained from each hospital. The laboratory data (blood gases, metabolic panel, complete blood count, coagulation) and diagnostic imaging tests were collected at admission.
The treatments used at admission were classified as antimicrobial therapy (beta-lactam, hydroxychloroquine, azithromycin, or lopinavir/ritonavir), immunomodulatory therapy (systemic corticosteroids, interferon beta-1b, or tocilizumab), or anticoagulant therapy (low-molecular-weight heparin in low doses as prophylaxis [40 mg of enoxaparin or equivalent], in intermediate doses as prophylaxis [enoxaparin 1 mg/kg/day or equivalent], or in high doses as an anticoagulant [1 mg/kg/12 hours or equivalent of another heparin]).
In-hospital complications included the presence of secondary bacterial pneumonia, acute respiratory distress syndrome, acute heart failure, arrhythmia, acute coronary syndrome, myocarditis, epileptic seizures, stroke, shock, sepsis, acute kidney failure, disseminated intravascular coagulation, venous thromboembolism, multiple organ dysfunction syndrome, and acute limb ischemia. The definition of complications during the hospitalization was defined pre hoc and was available from the online electronic data capture system. Ventilation support included invasive and noninvasive mechanical ventilation and high-flow oxygen therapy (27). Readmission within 30 days of hospital discharge was considered early rehospitalization.
The end point of the study was all-cause in-hospital mortality expressed as the case-fatality rate, or the proportion of in-hospital deaths in relation to the total number of patients hospitalized with COVID-19.
Statistical Analysis
Patients’ epidemiological and clinical characteristics were analyzed using descriptive statistics. Continuous and categorical variables are expressed as medians (interquartile ranges) or mean (standard deviation [SD]) and as absolute values (%), respectively. The continuous variables were classified into two categories. Patients were included in either the survivor or nonsurvivor group. The differences between the groups were determined using the two-sample Student’s t test or the Mann–Whitney U test for continuous variables and Pearson’s chi-square test for categorical variables. Values were considered to be statistically significant when p < .05. The measure of association was presented as odds ratio and 95% confidence interval.
A multivariate analysis was performed to compensate for confounding variables. The regression analysis values were expressed as adjusted odds ratios (AORs) with a 95% confidence interval. Multiple logistic regression analysis was used to identify independent predictors of in-hospital mortality and complications associated with in-hospital mortality. First, a univariate analysis of each variable was carried out to identify variables which had a significant result. All variables with no significant contribution or a confounding role were removed from the model. Statistically significant variables identified in the univariate analysis (p < .01) were entered into a multivariate logistic regression. Due to the fact that there were some missing values, variables which were not recorded for >25% of patients were excluded from the analysis (eg, serum ferritin, D-dimer, interleukin-6, procalcitonin, venous lactate, and aspartate aminotransferase). The variables included as part of combined variables, such as the quick sequential organ failure assessment (qSOFA) model, were also excluded (eg, tachypnea [≥20 breaths per minute], hypotension [systolic blood pressure < 100 mmHg], and confusion). Statistical data analysis was performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY).
Ethical Aspects
The STROBE statement guidelines were followed in the conduct and reporting of the study (Supplementary Material). All the patients gave their informed consent. When there were biosafety concerns and/or when the patient had already been discharged, verbal informed consent was requested and noted on the medical record. Data confidentiality and patient anonymity were maintained at all times, in accordance with Spanish regulations on observational studies. Patient identifiable information was deleted before the database was analyzed; thus, it is not possible to identify patients on an individual level either in this article or in the database. This study was carried out in accordance with the Declaration of Helsinki and was approved by the Institutional Research Ethics Committees of each participating hospital.
Results
Patients and Outcome
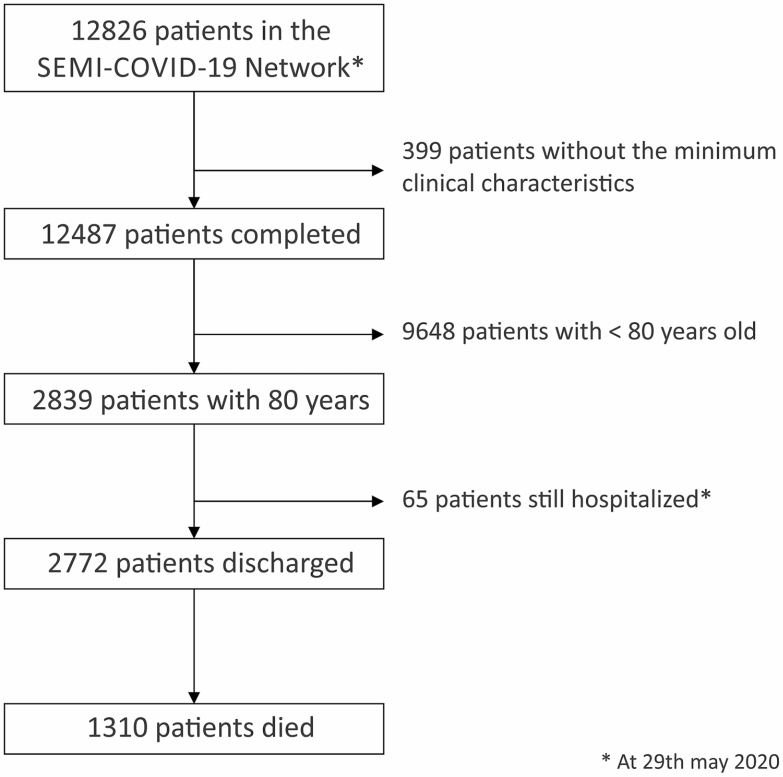
As of May 29, 2020, of a total 12 487 patients diagnosed with COVID-19 included in the SEMI-COVID-19 Registry, 2839 (22.7%) were ≥80 years of age. Sixty-seven (2.4%) remained hospitalized as of the cutoff date and were not included in this study. Of the total of 2772 patients included, 1471 (53.1%) were alive after hospital discharge and 1301 (46.9%) died during their hospital stay (in-hospital mortality) (Figure 1). The overall case-fatality rate was 46.9% and showed a significant increase that correlated with age, from a fatality rate of 41.6% (368/885 patients) in patients aged 80–84 years to the highest mortality rate of 54.2% (77/142 patients) in patients ≥95 years of age (Supplementary Table 1).
Figure 1.
Patient inclusion flowchart
Demographics and Clinical Characteristics
The percentages of males in the nonsurvivor and survivor groups were 53% and 46.2%, respectively (p < .001) (Table 1). The proportion of patients living in nursing homes prior to admission was 21.6%, a figure which was similar in the nonsurvivor and survivor groups. The comorbidities, symptoms, and physical examination findings of the very old patients with COVID-19 are shown in Table 2. The percentages of moderate and severe dependence in the nonsurvivor group were higher than in the survivors group (28.4% and 24.7% vs 24.3% and 17.8%, respectively, p < .001).
Table 1.
Clinical Characteristics, in Hospitalized Patients ≥ 80 Years Old With COVID-19
| Total N (%) (n = 2772) | Nonsurvivors N (%) (n = 1301) | Survivors N (%) (n = 1471) | p Value | |
|---|---|---|---|---|
| Age, median (IQR), years | 86.3 (83.2–89.6) | 86.6 (83.6–90.2) | 85.9 (82.5–89.3) | <.001 |
| Sex, Male | 1367 (49.4) | 688 (53) | 679 (46.2) | <.001 |
| Acquisition | .044 | |||
| Community | 1954 (70.5) | 915 (70.3) | 1039 (70.6) | |
| Nosocomial | 222 (8.0) | 120 (9.2) | 101 (6.8) | |
| Nursing home | 599 (21.6) | 267 (20.5) | 331 (22.5) | |
| Degree of dependence | <.001 | |||
| Independent or mild | 1463 (52.8) | 611 (47.0) | 852 (57.9) | |
| Moderate | 727 (26.2) | 369 (28.4) | 357 (24.3) | |
| Severe | 583 (21.0) | 320 (24.7) | 262 (17.8) | |
| Comorbidities | ||||
| CCI, mean (SD) | 6.3 (1.9) | 6.5 (2.1) | 6.0 (1.9) | <.001 |
| Hypertension | 2080 (75) | 971 (74.6) | 1109 (75.4) | .621 |
| Dyslipidemia | 1399 (50.5) | 644 (49.5) | 755 (51.3) | .365 |
| Nonatherosclerotic cardiovascular diseases* | 952 (34.4) | 483 (37.1) | 469 (31.9) | .004 |
| Atherosclerotic cardiovascular diseases** | 855 (30.8) | 449 (34.5) | 406 (27.6) | <.001 |
| Dementia | 844 (30.5) | 428 (32.9) | 416 (28.3) | .009 |
| Diabetes mellitus | 710 (25.6) | 346 (26.6) | 366 (24.9) | .302 |
| Chronic pulmonary disease*** | 547 (19.7) | 262 (20.1) | 285 (19.4) | .680 |
| ObesityΦ | 444 (16.0) | 229 (17.6) | 215 (14.6) | .04 |
| MalignancyΦΦ | 364 (13.1) | 186 (14.3) | 178 (12.1) | .089 |
| Moderate-to-severe renal disease | 324 (11.7) | 182 (14.0) | 142 (9.6) | <.001 |
| Symptoms | ||||
| Shortness of breath | 1705 (61.5) | 925 (71.1) | 780 (53.0) | <.001 |
| Cough | 1694 (61.1) | 804 (61.9) | 890 (60.5) | .447 |
| Fatigue | 1026 (37.0) | 492 (37.9) | 534 (36.3) | .403 |
| Anorexia | 608 (21.9) | 293 (22.5) | 315 (21.4) | .005 |
| Diarrhea | 396 (14.3) | 159 (12.3) | 237 (16.1) | .005 |
| Vomiting | 150 (5.4) | 77 (6.0) | 73 (5.0) | .25 |
| Physical exam | ||||
| Oxygen saturation < 90% (pulse oximetry) | 729 (26.3) | 524 (40.3) | 204 (13.9) | <.001 |
| Temperature ≥ 37.8°C | 590 (21.3) | 332 (25.5) | 258 (17.5) | <.001 |
| Hypotension (systolic blood pressure < 100 mmHg) | 225 (8.1) | 143 (10.9) | 82 (5.5) | <.001 |
| Tachycardia (>100 beats per minute) | 541 (19.5) | 320 (24.5) | 224 (15.2) | <.001 |
| Tachypnoea (≥22 breaths per minute) | 1189 (42.9) | 752 (57.8) | 437 (29.7) | <.001 |
| Confusion | 815 (29.4) | 491 (37.7) | 324 (22.0) | <.001 |
| Pulmonary rales | 1551 (55.9) | 804 (61.8) | 747 (50.8) | <.001 |
| qSOFA score | ||||
| Low risk (≤1) | 2253 (81.3) | 935 (71.9) | 1317 (89.5) | <.001 |
| High risk (≥2) | 519 (18.7) | 365 (28.1) | 154 (10.5) |
Notes: CCI = Charlson Comorbidity Index; IQR = interquartile range; N (%) = number of cases (percentage); qSOFA = quick sequential organ failure assessment; SD = standard deviation.
ΦObesity: body mass index ≥ 30 kg/m2.
ΦΦMalignancy includes solid tumors or hematological neoplasia.
*Nonatherosclerotic cardiovascular diseases include atrial fibrillation or/and heart failure.
**Atherosclerotic cardiovascular diseases include coronary, cerebrovascular, and/or peripheral vascular disease.
***Chronic pulmonary disease includes chronic obstructive pulmonary diseases and/or asthma.
Table 2.
Radiology and Laboratory Findings, in Hospitalized Patients ≥ 80 Years Old With COVID-19
| Total N (%) (n = 2772) | Nonsurvivors N (%) (n = 1301) | Survivors N (%) (n = 1471) | P Value | |
|---|---|---|---|---|
| Chest x-ray findings | <.001 | |||
| Normal | 500 (19.0) | 157 (12.1) | 343 (23.3) | — |
| Unilateral infiltrates | 582 (21) | 243 (18.7) | 338 (23.0) | — |
| Bilateral infiltrates | 1690 (61.1) | 900 (69.2) | 790 (53.7) | — |
| Laboratory findings | ||||
| Complete blood count | ||||
| Leukocytes ≥ 10.0 × 103/μL | 610 (22.0) | 377 28.9) | 233 (15.8) | <.001 |
| Neutrophils ≥ 7.5 × 103/μL | 740 (26.7) | 452 (34.7) | 288 (19.5) | <.001 |
| Lymphocytes < 0.800 × 103/μL | 1170 (42.3) | 660 (50.7) | 510 (34.7) | <.001 |
| Eosinophils < 0.030 × 103/μL | 2150 (77.6) | 1059 (81.4) | 1091 (74.2) | .001 |
| Monocytes < 0.500 × 103/μL | 1447 (52.2) | 727 (55.9) | 719 (48.9) | <.001 |
| Hemoglobin < 11 g/dL | 434 (15.6) | 206 (15.8) | 228 (15.5) | .684 |
| Platelet count < 150 × 103/μL | 860 (31.0) | 422 (32.4) | 438 (29.8) | .141 |
| Arterial blood gases | ||||
| pH < 7.40 (n = 1515) | 191 (21.1) | 191 (24.8) | 128 (17.2) | <.001 |
| PCO2 < 35 mmHg (n = 1515) | 763 (50.4) | 382 (49.7) | 380 (51.1) | .585 |
| PO2 ≤ 60 mmHg (n = 1515) | 643 (42.4) | 411 (53.5) | 231 (31.0) | <.001 |
| PO2/FiO2 ratio ≤ 200 (n = 1395) | 313 (22.4) | 228 (32) | 85 (12.4) | <.001 |
| Serum biochemistry | ||||
| Glucose > 126 mg/dL (n = 2627) | 1173 (44.7) | 650 (55.4) | 523 (44.6) | <.001 |
| eGFR < 45 mL/min/1.73 m2 | 889 (36.5) | 597 (46.6) | 392 (39.6) | <.001 |
| Lactate dehydrogenase ≥ 500 U/L (n = 2200) | 406 (18.5) | 272 (27.7) | 134 (11.0) | <.001 |
| AST ≥ 70 U/L (n = 2422) | 129 (5.3) | 73 (6.5) | 56 (4.3) | .013 |
| ALT ≥ 70 U/L (n = 1971) | 221 (11.2) | 138 (15.4) | 83 (3.3) | <.001 |
| C-reactive protein ≥ 80 mg/L (n = 2612) | 124 (47.7) | 731 (60.6) | 515 (36.7) | <.001 |
| Venous lactate ≥ 2.20 mmol/L (n = 1296) | 368 (28.4) | 237 (36.1) | 131 (20.5) | <.001 |
| Procalcitonin ≥ 0.150 ng/mL (n = 1306) | 66 (51.1) | 386 (66.0) | 281 (39.0) | <.001 |
| Interleukin-6 ≥ 40 pg/mL (n = 255) | 113 (44.3) | 57 (66.3) | 56 (33.1) | <.001 |
| D-dimer ≥ 1000 ng/mL (n = 1930) | 994 (57.5) | 476 (57.5) | 518 (47.0) | <.001 |
| Serum ferritin ≥ 450 /μg/L (n = 956) | 488 (51.0) | 202 (64.7) | 286 (44.4) | <.001 |
Notes: ALT = alanine aminotransferase; AST = aspartate aminotransferase; eGFR = estimated glomerular filtration rate; FiO2 = fraction of inspired oxygen; N (%) = number cases (percentage).
The mean Charlson Comorbidity Index was elevated in the whole population, but higher in the nonsurvivor group (p < .001). The most prevalent comorbidities were hypertension (75%), dyslipidemia (50.5%), nonatherosclerotic cardiovascular diseases (34.4%), atherosclerotic cardiovascular diseases (30.8%), dementia (30.5%), and diabetes (25.6%). The proportion of cardiovascular diseases, dementia, moderate-to-severe renal disease, and obesity was significantly higher in the nonsurvivor group (p < .05), but no differences were observed between survivor and nonsurvivor groups in regard to other comorbidities such as hypertension, diabetes, chronic pulmonary diseases, and malignancy. The most common symptoms were shortness of breath (61.5%) and cough (61.1%). Presence of dyspnea and anorexia were higher in the nonsurvivor group, whereas diarrhea was more common in the survivor group (p < .001). Signs of severe clinical disease at admission (oxygen saturation < 90%, temperature ≥ 37.8°C, hypotension, tachypnea, confusion, or qSOFA score ≥ 2) as well the presence of rales in the chest examination were more common in nonsurvivor group (p < .001).
Laboratory and Radiological Findings
A higher proportion of patients in the nonsurvivor group had leukocytosis, neutrophilia, monocytopenia, and lymphocytopenia (p < .001) as well as higher levels of plasma glucose, creatinine, lactate dehydrogenase, aminotransferases, C-reactive protein, lactate, procalcitonin, ferritin, D-dimer, and interleukin-6 (p ≤ .001) (Table 2). Hypoxemia and a PaO2/FiO2 ratio ≤ 200 were more common in the nonsurvivor group (p < .001). The presence of unilateral and, especially, bilateral infiltrates were significantly more common in the nonsurvivor group (p < .001).
In-Hospital Treatment
Hydroxychloroquine and lopinavir/ritonavir were used in 75.7% and 40.9% of patients, respectively (Table 3). Antimicrobial therapy, including beta-lactam and azithromycin, was indicated in 75.9% and 54.5% of patients, respectively. The main immunomodulatory therapy was systemic glucocorticoids, which was used in 39.0% of patients. Moreover, interferon beta-1b, tocilizumab, and colchicine were used in 7.4%, 2.8%, and 1.5%, respectively. The percentages of patients treated with hydroxychloroquine and azithromycin in the nonsurvivor and survivor groups were 68.9% versus 81.7% and 50.5% versus 58.1%. respectively (p < .001). The proportion of patients treated with beta-lactam, interferon beta-1b, and systemic corticosteroids in the nonsurvivor and survivor groups were 79.0% versus 73.2%, 10.2% versus 4.95%, and 43.4% versus 35.2%, respectively (p < .001). Finally, the proportion of use of low-molecular-weight heparin in the nonsurvivor and survivor groups was 76.9% versus 85.5% (p < .001). The most common ventilation therapies used were high-flow nasal cannula oxygen (7.3%) and noninvasive therapy (3.9%).
Table 3.
In-Hospital Treatment Used in Hospitalized Patients ≥ 80 Years Old With COVID-19
| Total N (%) (n = 2772) | Nonsurvivors N (%) (n = 1301) | Survivors N (%) (n = 1471) | p Value | |
|---|---|---|---|---|
| Antimicrobial therapy | ||||
| Beta-lactam antibiotics | 2104 (75.9) | 1028 (79.0) | 1077 (73.2) | <.001 |
| Hydroxychloroquine | 2098 (75.7) | 910 (68.9) | 1188 (81.7) | <.001 |
| Azithromycin | 1511 (54.5) | 667 (50.5) | 844 (58.1) | <.001 |
| Lopinavir/ritonavir | 1134 (40.9) | 555 (42.0) | 579 (39.9) | .250 |
| Immunomodulatory therapy | ||||
| Systemic corticosteroids | 1081 (39.0) | 573 (43.4) | 508 (35.2) | <.001 |
| Interferon beta-1b | 205 (7.4) | 135 (10.2) | 70 (4.9) | <.001 |
| Tocilizumab | 78 (2.8) | 44 (3.3) | 34 (2.3) | .140 |
| Colchicine | 42 (1.5) | 17 (1.3) | 25 (1.7) | .341 |
| Anakinra | 11 (0.4) | 5 (0.4) | 6 (0.4) | .932 |
| Baricitinib | 10 (0.4) | 1 (0.1) | 9 (0.8) | .024 |
| Immunoglobulin | 6 (0.2) | 3 (0.2) | 3 (0.2) | .891 |
| Ventilation therapy | ||||
| High-flow nasal cannula oxygen | 203 (7.3) | 153 (11.6) | 50 (3.7) | <.001 |
| Noninvasive mechanical ventilation | 107 (3.9) | 18 (1.4) | 89 (0.3) | <.001 |
| Invasive mechanical ventilation | 22 (0.8) | 4 (0.3) | 18 (1.4) | .001 |
| Anticoagulant therapy | ||||
| Oral anticoagulants* | 183 (6.6) | 70 (5.3) | 113 (7.9) | .004 |
| Low-molecular-weight heparin: | 2251 (81.5) | 1016 (76.9) | 1235 (45.6) | <.001 |
| No | 513 (18.5) | 305 (23.1) | 208 (14.5) | — |
| Low (prophylaxis) dose | 1624 (58.6) | 729 (55.2) | 895 (61.5) | <.001 |
| Intermediate dose | 208 (7.8) | 86 (6,5) | 122 (9.0) | .051 |
| High (anticoagulant) dose | 419 (15.1) | 198 (15.2) | 221 (15) | .149 |
Notes: N (%) = number of cases (percentage).
*Vitamin K and non-vitamin K antagonist oral anticoagulants.
Complications
Acute respiratory distress syndrome was the most common complication (43% [severe 26.5%, moderate 9.9%, and mild 7.0%]), followed by acute kidney failure (24.6%) (Table 4). Pneumonia, acute heart failure, multiple organ dysfunction syndrome, and sepsis occurred in 14.2%, 13.9%, 10.8%, and 9.3% of inpatients, respectively. Overall, most of the complications were more common in the nonsurvivor group. Only 33 patients (1.2%) were admitted to the intensive care unit; they had a case-fatality rate of 75.8%. The median length of hospital stay was 14 days, with shorter stays observed in the nonsurvivor group (p < .001).
Table 4.
Complications and Outcomes in Hospitalized Patients ≥ 80 Years Old With COVID-19
| Total N (%) (n = 2772) | Nonsurvivors N (%) (n = 1301) | Survivors N (%) (n = 1471) | p Value | |
|---|---|---|---|---|
| Complications | ||||
| ARDS | ||||
| No | 1567 (56.6) | 355 (27.3) | 1212 (82.4) | |
| Mild | 195 (7.0) | 53 (4.1) | 141 (9.6) | .157 |
| Moderate | 275 (9.9) | 193 (14.8) | 82 (5.6) | <.001 |
| Severe | 738 (26.5) | 701 (53.9) | 37 (2.5) | <.001 |
| Acute kidney failure | 684 (24.6) | 441 (33.9) | 246 (16.5) | <.001 |
| Pneumonia | 394 (14.2) | 234 (18.0) | 160 (10.9) | <.001 |
| Acute heart failure | 386 (13.9) | 246 (18.9) | 1340 (9.5) | <.001 |
| Multiple organ dysfunction syndrome | 299 (10.8) | 289 (22.2) | 10 (0.7) | <.001 |
| Sepsis | 259 (9.3) | 216 (16.6) | 43 (2.9) | <.001 |
| Arrhythmia | 177 (5.3) | 99 (7.6) | 78 (5.3) | .014 |
| Shock | 115 (4.1) | 111 (8.5) | 4 (0.3) | <.001 |
| Acute coronary syndrome | 38 (1.4) | 35 (2.7) | 3 (0.2) | <.001 |
| Venous thromboembolism | 40 (1.5) | 12 (0.9) | 28 (1.9) | .032 |
| Myocarditis | 27 (1.0) | 20 (1.6) | 7 (0.5) | .004 |
| Intravascular coagulation | 36 (1.3) | 31 (2.4) | 5 (0.3) | <.001 |
| Epileptic seizures | 25 (0.9) | 16 (1.2) | 9 (0.6) | .084 |
| Stroke | 25 (0.9) | 13 (1.0) | 12 (0.8) | .603 |
| Outcome | ||||
| Intensive care admission | 33 (1.2) | 25 (1.9) | 8 (0.5) | .001 |
| Days of hospitalization, median (IQR) | 14 (9–21) | 11 (7–16) | 17 (12–23) | <.001 |
Notes: ARDS = acute respiratory distress syndrome; IQR = interquartile range; N (%) = number of cases (percentage).
Multivariate Analysis of Risk Factors at Admission Associated With In-Hospital Mortality
The only demographic characteristics independently associated with all-cause in-hospital mortality were age (AOR: 1.03), male sex (AOR: 1.28), and severe degree of dependence (AOR: 1.76) (Table 5). In contrast, comorbidities were not related with mortality. Oxygen saturation < 90% (AOR: 2.70), temperature ≥ 37.8°C (AOR: 1.37), and qSOFA score ≥ 2 (AOR: 1.68) at admission were independently associated with mortality. Finally, the laboratory parameters at admission that were predictors of mortality were estimated glomerular filtration rate < 45 mL/min/1.73 m2 (AOR: 2.14); lactate dehydrogenase ≥ 500 U/L (AOR: 1.63); C-reactive protein ≥ 80 mg/L (AOR: 1.65); neutrophil count ≥ 7.5 × 103/μL (AOR: 1.44); lymphocyte count < 0.800 × 103/μL (AOR: 1.27); and monocyte count < 0.500 × 103/μL. Also, both unilateral (AOR: 1.05) and bilateral infiltrates (AOR: 2.10) in chest x-rays at admission were independent predictors of in-hospital mortality. In this model, the p value for the Hosmer–Lemeshow test of goodness of fit was .662. The results of logistic regression model of risk factors at admission associated with in-hospital mortality did not change after the inclusion of different medical therapies (hydroxychloroquine, beta lactams, azithromycin, interferon-beta, steroids, and low-molecular-weight heparin).
Table 5.
Risk of Mortality According to Comorbidities, Symptoms, Physical Examination, Radiology, and Laboratory Findings in Hospitalized Patients ≥ 80 Years Old With COVID-19 (bivariate and multivariate analysis)
| OR (CI 95%) | AOR (CI 95%) | p Value | |
|---|---|---|---|
| Age | 1.04 (1.03–1.06) | 1.03 (1.00–1.06) | .024 |
| Sex, male | 1.31 (1.12–1.54) | 1.27 (1.01–1.59) | .036 |
| Degree of dependence | |||
| Independent or mild | 1 | 1 | |
| Moderate | 1.44 (1.20–1.72) | 1.17 (0.89–1.58) | .293 |
| Severe | 1.70 (1.40–2.08) | 1.76 (1.20–2.57) | .003 |
| Comorbidities | |||
| CCI | 1.12 (1.07–1.16) | NI | — |
| Nonatherosclerotic cardiovascular disease* | 1.25 (1.07–1.47) | 1.07 (0.85–1.35) | .552 |
| Atherosclerotic cardiovascular diseases** | 1.38 (1.17–1.64) | 1.22 (0.96–1.54) | .102 |
| Dementia | 1.24 (1.05–1.46) | 1.05 (0.78–1.38) | .747 |
| ObesityΦ | 1.24 (1.00–1.54) | NI | |
| Moderate–severe renal disease | 1.52 (1.20–1.92) | 0.84 (0.59–1.86) | .322 |
| Symptoms | |||
| Shortness of breath | 2.17 (1.85–2.54) | 1.21 (0.96–1.55) | .102 |
| Anorexia | 1.06 (0.88–1.28) | 0.93 (0.72–1.22) | .687 |
| Diarrhea | 0.73 (0.59–0.91) | 0.83 (0.62–1.34) | .832 |
| Physical exam | |||
| Oxygen saturation < 90% (pulsi oximetry) | 4.19 (3.47–5.05) | 2.70 (2.10–3.50) | <.001 |
| Temperature ≥ 37.8°C | 1.60 (1.32–1.93) | 1.37 (1.06–1.78) | .016 |
| Hypotension (systolic blood pressure < 100 mmHg) | 2.12 (1.58–2.84) | NI | |
| Tachycardia (>100 beats per minute) | 1.80 (1.48–2.19) | NI | — |
| Tachypnoea (≥20 breaths per minute) | 3.23 (2.76–3.79) | NI | — |
| Confusion | 2.14 (1.81–2.53) | NI | — |
| Pulmonary rales | 1.56 (1.36–1.89) | 1.13 (0.90–1.41) | .282 |
| qSOFA score ≥ 2 (high risk) | 3.33 (2.71–4.09) | 1.68 (1.25–2.26) | .001 |
| Chest x-ray | |||
| Normal | 1 | 1 | |
| Unilateral infiltrates | 1.56 (1.20–2.00) | 1.50 (1.03–2.18) | .033 |
| Bilateral infiltrates | 2.49 (2.00–3.07) | 2.10 (1.51–2.93) | <.001 |
| Laboratory findings | |||
| Leukocytes ≥ 10.0 × 103/μL | 2.15 (1.79–2.60) | NI | — |
| Neutrophils ≥ 7.5 × 103/μL | 2.19 (1.84–2.61) | 1.44 (1.11–1.86) | .004 |
| Lymphocytes < 0.800 × 103/μL | 1.90 (1.66–2.26) | 1.27 (1.01–1.60) | .038 |
| Eosinophils < 0.030 × 103/μL | 1.51 (1.29–1.82) | 1.23 (0.42–1.61) | .101 |
| Monocytes < 0.500 × 103/μL | 1.32 (1.13–1.549 | 1.26 (1.00–1.59) | .043 |
| pH < 7.40 | 1.59 (1.24–2.05) | NI | — |
| PO2 ≤ 60 mmHg | 2.55 (2.06–3. 17) | NI | — |
| PO2/FiO2 ratio ≤ 200 | 3.31 (2.54–4.39) | NI | — |
| Glucose > 126 mg/dL | 1.84 (1.58–2.15) | 1.12 (0.90–1.48) | .283 |
| eGFR < 45 mL/min/1.73 m2 | 2.30 (1.96–2.70) | 2.14 (1.68–2.78) | <.001 |
| Lactate dehydrogenase ≥ 500 U/L | 3.09 (2.46–3.89) | 1.63 (1.35–2.05) | <.001 |
| AST ≥ 70 U/L | 1.56 (1.09–2.23) | NI | |
| ALT ≥ 70 U/L | 2.16 (1.68–2.88) | NI | — |
| C-reactive protein ≥ 80 mg/L | 2.65 (2.26–3.11) | 1,65 (1.32–2.07) | <.001 |
| Venous lactate ≥ 2.20 mmol/L | 2.19 (1.71–2.82) | NI | — |
| Procalcitonin ≥ 0.150 ng/mL | 3.03 (2.42–3.81) | NI | — |
| Interleukin-6 ≥ 40 pg/mL | 3.96 (2.28–6.89) | NI | — |
| D-dimer ≥ 1000 ng/mL | 1.52 (1.27–1.82) | NI | — |
| Serum ferritin ≥ 450 /μg/L | 2.29 (1.73–3.09) | NI | — |
Notes: ALT = alanine aminotransferase; AOR = adjusted odds ratio; AST = aspartate aminotransferase; CCI = Charlson Comorbidity Index; CI 95% = coefficient interval; eGFR = estimated glomerular filtration rate; NI = not included in the multivariate analysis; OR = odds ratio; qSOFA = quick sequential organ failure assessment.
ΦObesity = body mass index ≥ 30 kg/m2.
*Nonatherosclerotic cardiovascular disease includes atrial fibrillation or/and heart failure.
**Atherosclerotic cardiovascular diseases include coronary, cerebrovascular, and/or peripheral vascular disease.
Discussion
This study confirms the high mortality rate from severe COVID-19 among very old patients. We found a case-fatality rate in hospitalized patients ≥ 80 years old of age (46.9%) that was similar to other hospital cohorts reported in Madrid and New York, but lower than what was reported in Italy or China (4,5,8,28).
The predictors of a poor prognosis in COVID-19 have been described in many cohorts of hospitalized patients. They include older age, male sex, comorbidities (hypertension, obesity, diabetes, cardiovascular diseases, chronic pulmonary diseases, chronic kidney disease, and malignancy), signs of severe clinical presentation at admission (dyspnea, low oxygen saturation, high respiratory rate, hypotension, and a low level of consciousness), evidence of a hyperinflammatory response (leukocytosis, neutrophilia, and elevated C-reactive protein and ferritin levels), and organ and coagulation dysfunction (increased plasma glucose, serum creatinine, lactate dehydrogenase, and D-dimer levels) (1–10).
Moreover, complications such as acute respiratory distress syndrome and multiple organ dysfunction syndrome are very common in patients hospitalized with COVID-19 and result in poor prognosis (1–8) These complications are also found in geriatric series (17). Worse outcomes in older patients with COVID-19 have been related to uncontrolled local and systemic hyperinflammation (23,29).
So far, only one small, single-center study specifically focused on very old patients hospitalized with COVID-19 has been published. Covino et al (14) described the clinical characteristics and prognostic factors in 69 patients ≥ 80 years old of age (median age 84 [82–89] years old) admitted to an Italian referral center for COVID-19. They found a 30-day mortality rate of 33.3%. They suggested that the risk of death was not age-dependent in patients ≥ 80 years old of age and that severe dementia was a relevant risk factor for mortality in this population. Furthermore, indications of severe COVID-19, such as elevated lactate dehydrogenase and low oxygen saturation figures at admission, were also associated with a poor prognosis (14).
Our study suggests that the importance of some of these risk factors could be different in very old patients. First, in contrast to the work by Covino et al (14), our study suggests that age remains an independent risk factor for mortality, even in very old patients hospitalized for COVID-19. Also, male sex was found to imply a poor prognosis for COVID-19 in very old patients (30), as well as in younger adults (1–8).
Second, although dyspnea has been associated with mortality in many reports (3,31,32), in our study, it was not associated with death. This fact could be explained by the difficulty in registering this symptom in ill and very old patients. In fact, qSOFA––a simple scale easily performed at admission that evaluates respiratory rate, systolic blood pressure, and mental status—is a predictor of mortality by sepsis (33) and also a good predictor of a poor prognosis in patients with COVID-19 (34); it remains useful in patients ≥ 80 years old of age. In view of our results, we strongly recommend the use of qSOFA at admission in older patients with COVID-19.
Third, although comorbidities were very common in our study, as seen in the literature regarding patients ≥ 65 years of age with COVID-19 (15,35,36), the absence of a correlation between comorbidities—including dementia—and mortality in our very old population is striking. It is plausible that the high prevalence of comorbidities (hypertension, dyslipidemia, heart diseases, dementia, and other such chronic conditions) among the older population contributes to the weak association between comorbidities and mortality. While empirical evidence shows that mild obesity may improve the survival rate (“obesity paradox”) in old patients with chronic diseases (37) or community-acquired pneumonia (38), obesity has been identified as a risk factor for mortality in COVID-19, a finding that was confirmed in our very old population.
Fourth, an elevated plasma glucose level was not a predictor of mortality in our cohort, which is in contrast with previous reports (19,39). Acute kidney injury, a common complication in hospitalized adults, is associated with poor prognosis. It has recently been related to higher mortality in patients ≥ 65 years old with COVID-19 (40), a finding that was also observed in our study.
Finally, our study highlights the prognostic importance of preadmission clinical status in older adults more than 80-year patients with COVID-19. Remarkably, we found that a severe degree of dependence (defined by a Barthel Index ≤ 60) was an independent predictor of death. Surprisingly, functional status had not been previously evaluated in relation to mortality in geriatric patients with COVID-19 (11–17).
Several prognostic scores for mortality in hospitalized older patients have been proposed, such as the Multidimensional Prognostic Index (MPI) (36), the Hospital-patient One-year Mortality Risk (HOMR) score (41,42), and the Identification of Seniors At Risk-Hospitalized Patients (ISAR-HP) score (43). However, a simpler way to predict in-hospital mortality in the older adults is to assess functional status using the Barthel Index, which evaluates the ability to perform activities of daily living and has been demonstrated to be a good predictor of mortality in geriatric patients hospitalized for any reason (44). Our study is the first report that has found that preadmission functional status is related to mortality in older adults aged more than 80 years old patients with COVID-19.
An unexpected finding in our study was the similar survival rates among older people living in nursing homes compared to those living at home, considering that we observed a close relationship between dependence and mortality and the fact that it is well-known that older people living in nursing homes have higher rates of severe dependence. Selection bias is the most plausible explanation for this finding, given that older patients sent to hospitals from nursing homes could be less dependent.
This study has some limitations. First, this is a retrospective series focused on hospitalized patients. Given that these patients presented with more severe disease and a higher mortality rate, our data may overestimate overall mortality in the entirety of the older adults aged more than 80 years old with COVID-19. Second, as a retrospective cohort study, data were collected by a large number of researchers, which could have led to heterogeneity in data input and validation. Third, more than one-third of patients did not have laboratory data for some biochemical values, such as procalcitonin, venous lactate, D-dimer, interleukin-6 serum levels, and serum ferritin levels, which may have led to an underestimation of their potential predictive value. Finally, the data were retrospectively collected during the peak of the COVID-19 pandemic in Spain, which was a period of overwhelming demand on the health care system. Thus, despite the large number of clinical and complementary variables included in the registry, other important variables, such as delirium, undernutrition, and other hospital-acquired geriatric syndromes, were not included. For the same reason, we could not collect any frailty scale data in this study. Recently, it has been reported that the level of frailty is a useful predictor of short-term COVID-19 outcomes in geriatric patients (45). However, a fair relationship between frailty and the Barthel Index in hospitalized older patients has been demonstrated (46).
Conclusion
In very old patients hospitalized with COVID-19, clinical data of severe COVID-19 at admission were associated with high all-cause mortality. Age, male sex, poor preadmission functional status, and severe symptoms—but not comorbidities—were independently associated with in-hospital mortality. Therefore, functional assessment is of critical importance for establishing a prognosis for older patients with COVID-19.
Funding
None declared.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
List of the SEMI-COVID-19 Network members (Supplementary Appendix).
Author Contributions
J.-M.R.-R., R.G.-H., and J.-M.C.-R. designed the study, had full access to all data in the study, and takes responsibility for the integrity and accuracy of the data analysis. V.B., M.R., J.M.-C., D.P.-R., M.C.-M., M.R.-R., J.-L.B.-P., F.A.-F., D.M.-M., J.-A.V.-N., G.A.-R., M.M.-B., I.P.-F., G.-M.G.-G., P.G.-V., A.A.-K., M.-R.P.-R., C.R.-F.-V., A.M.-M., A.L.-R., M.-J.G.-J., and J.-M.C.-A. contributed to data acquisition and data interpretation. J.-M.R.-R. and R.G.-H. were responsible for literature search, manuscript writing, and data analysis. J.-M.R.-R., R.G.-H., and J.-M.C.-R. were responsible for supervision of data collection and data management. All authors reviewed and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. J.-M.R.-R. and R.G.-H. are the guarantor.
References
- 1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang WH, Guan WJ, Li CC, et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicenter) and outside Hubei (non-epicenter): a nationwide analysis of China. Eur Respir. 2020;55:2000562 doi: 10.1183/13993003.00562-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bialek S, Boundy E, Bowen V, et al. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Docherty AB, Harrison EM, Green CA, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO clinical characterisation protocol. BMJ. 2020;239:2020.04.23.20076042. doi: 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borobia A, Carcas A, Arnalich F, et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med. 2020;9:1733. doi: 10.3390/jcm9061733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garnier-Crussard A, Forestier E, Gilbert T, Krolak-Salmon P. Novel coronavirus (COVID-19) epidemic: what are the risks for older patients? J Am Geriatr Soc. 2020;68:939–940. doi: 10.1111/jgs.16407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim T. Improving preparedness for and response to coronavirus disease 19 (COVID-19) in long-term care hospitals in Korea. Infect Chemother. 2020;52:133–141. doi: 10.3947/ic.2020.52.2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen TL, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020;75:1788–1795. doi: 10.1093/gerona/glaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niu S, Tian S, Lou J, et al. Clinical characteristics of older patients infected with COVID-19: a descriptive study. Arch Gerontol Geriatr. 2020;89:104058. doi: 10.1016/j.archger.2020.104058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun H, Ning R, Tao Y, et al. Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: a retrospective study. J Am Geriatr Soc. 2020;68:E19–E23. doi: 10.1111/jgs.16533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Covino M, De Matteis G, Santoro M, et al. Clinical characteristics and prognostic factors in COVID-19 patients aged ≥80 years. Geriatr Gerontol Int. 2020;20:704–708. doi: 10.1111/ggi.13960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palmieri L, Vanacore N, Donfrancesco C, et al. Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. J Gerontol A Biol Sci Med Sci. 2020;75:1796–1800. doi: 10.1093/gerona/glaa146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee JY, Kim HA, Huh K, et al. Risk factors for mortality and respiratory support in elderly patients hospitalized with COVID-19 in Korea. J Korean Med Sci. 2020;35:e223. doi: 10.3346/jkms.2020.35.e223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fumagalli S, Salani B, Gabbani L, Mossello E, Ungar A. Covid-19 cases in a no-Covid-19 geriatric acute care setting. A sporadic occurrence? Eur J Intern Med. 2020;77:141–142. doi: 10.1016/j.ejim.2020.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang S, Ma P, Zhang S, et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. 2020;63:102–2111. doi: 10.1007/s00125-020-05209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ministerio de Sanidad. Situación de COVID-19 en España. Centro de 382 Coordinación de Alertas y Emergencias Sanitarias. Enfermedad por el coronavirus (COVID-19) 2020. https://cnecovid.isciii.es/covid19/. Accessed June 30, 2020.
- 21. Eurostat-Data Explorer. Population: Structure Indicators 2020. https://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do. Accessed June 30, 2020.
- 22. Casas Rojo JM, Antón Santos JM, Millán J, et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain. SEMI-COVID-19 Registry. Rev Clin 2020. S0014-2565(20)30206-X. doi: 10.1016/j.rce.2020.07.003 [DOI] [Google Scholar]
- 23. Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging (Albany NY). 2020;12:9959–9981. doi: 10.18632/aging.103344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rius C, Pérez G, Martínez JM, et al. An adaptation of Charlson comorbidity index predicted subsequent mortality in a health survey. J Clin Epidemiol. 2004;57:403–408. doi: 10.1016/j.jclinepi.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 25. American Psychiatric Association. Neurocognitive Disorders. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association; 2013 [Google Scholar]
- 26. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 28. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 29. Boccardi V, Ruggiero C, Mecocci P. COVID-19: a geriatric emergency. Geriatrics (Basel). 2020;5:24. doi: 10.3390/GERIATRICS5020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Promislow DEL. A geroscience perspective on COVID-19 mortality. J Gerontol A Biol Sci Med Sci. 2020;75:e30–e33. doi: 10.1093/gerona/glaa094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kashani KB. Hypoxia in COVID-19: sign of severity or cause for poor outcomes. Mayo Clin Proc. 2020;95:1094–1096. doi: 10.1016/j.mayocp.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95:1138–1147. doi: 10.1016/j.mayocp.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramos-Rincón JM, Fernández-Gil A, Merino E, et al. The quick sepsis-related organ failure assessment (qSOFA) is a good predictor of in-hospital mortality in very elderly patients with bloodstream infections: a retrospective observational study. Sci Rep. 2019;9:15075. doi: 10.1038/s41598-019-51439-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Su Y, Tu GW, Ju MJ, et al. Comparison of CRB-65 and quick sepsis-related organ failure assessment for predicting the need for intensive respiratory or vasopressor support in patients with COVID-19. J Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atkins JL, Masoli JAH, Delgado J, et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020;75:2224–2230. doi: 10.1093/gerona/glaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Landi F, Onder G, Gambassi G, Pedone C, Carbonin P, Bernabei R. Body mass index and mortality among hospitalized patients. Arch Intern Med. 2000;160:2641–2644. doi: 10.1001/archinte.160.17.2641 [DOI] [PubMed] [Google Scholar]
- 38. Nie W, Zhang Y, Jee SH, Jung KJ, Li B, Xiu Q. Obesity survival paradox in pneumonia: a meta-analysis. BMC Med. 2014;10:12. doi: 10.1186/1741-7015-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu J, Huang J, Zhu G, et al. Elevation of blood glucose level predicts worse outcomes in hospitalized patients with COVID-19: a retrospective cohort study. BMJ Open Diabetes Res Care. 2020;8:e001476. doi: 10.1136/bmjdrc-2020-001476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yan Q, Zuo P, Cheng L, et al. Acute kidney injury is associated with in-hospital mortality in older patients with COVID-19. J Gerontol A Biol Sci Med Sci. 2020:glaa181. doi: 10.1093/gerona/glaa181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Walraven C. The hospital-patient one-year mortality risk score accurately predicted long-term death risk in hospitalized patients. J Clin Epidemiol. 2014;67:1025–1034. doi: 10.1016/j.jclinepi.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 42. van Walraven C, Forster AJ. The HOMR-Now! model accurately predicts 1-year death risk for hospitalized patients on admission. Am J Med. 2017;130:991.e9–991.e16. doi: 10.1016/j.amjmed.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 43. William G, Adrian W. New horizons: urinary incontinence in older people. Age Ageing. 2017;43:157–163. doi: 10.1093/AGEING [DOI] [PubMed] [Google Scholar]
- 44. Ryg J, Engberg H, Mariadas P, et al. Barthel Index at hospital admission is associated with mortality in geriatric patients: a Danish nationwide population-based cohort study. Clin Epidemiol. 2018;10:1789–1800. doi: 10.2147/CLEP.S176035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hägg S, Jylhävä J, Wang Y, et al. Age, frailty and comorbidity as prognostic factors for short-term outcomes in patients with COVID-19 in geriatric care. J Am Med Dir Assoc. 2020. S1525-8610(20)30704-0. doi: 10.1016/j.jamda.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marcucci M, Franchi C, Nobili A, Mannucci PM, Ardoino I; REPOSI Investigators Defining aging phenotypes and related outcomes: clues to recognize frailty in hospitalized older patients. J Gerontol A Biol Sci Med Sci. 2017;72:395–402. doi: 10.1093/gerona/glw188 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.