Abstract
Background and Aims
As no population-based study has investigated the susceptibility and disease course of COVID-19 among patients with inflammatory bowel diseases [IBD], we aimed to investigate this topic in a population-based setting.
Methods
Two cohorts were investigated. First, a nationwide cohort of all IBD patients diagnosed with COVID-19 was prospectively followed to investigate the disease courses of both diseases. Second, within a population-based cohort of 2.6 million Danish citizens, we identified all individuals tested for SARS-CoV-2 to determine the occurrence of COVID-19 among patients with and without IBD and other immune-mediated inflammatory diseases [IMIDs].
Results
Between January 28, 2020 and June 2, 2020, a total of 76 IBD patients with COVID-19 were identified in the national cohort and prospectively followed for 35 days (interquartile range [IQR]: 25–51). A large proportion [n = 19: 25%] required a COVID-19-related hospitalisation for 7 days [IQR: 2–8.5] which was associated with being 65 years or older (odds ratio [OR] = 23].80, 95% confidence interval [CI] 6.32–89.63, p <0.01) and presence of any non-IMID comorbidity [OR = 8.12, 95% CI 2.55–25.87, p <0.01], but not use of immunomodulators [p = 0.52] or biologic therapies [p = 0.14]. In the population-based study, 8476 of 231 601 [3.7%] residents tested positive for SARS-CoV-2; however, the occurrence was significantly lower among patients with IBD [62 of the 2486 patients = 2.5%, p <0.01] and other IMIDs [531 of 16 492 patients = 3.2%, p <0.01] as compared with patients without IMIDs.
Conclusions
Patients with IMIDs, including IBD, had a significantly lower susceptibility to COVID-19 than patients without IMIDs, and neither immunosuppressive therapies nor IBD activity were associated with the disease course of COVID-19.
Keywords: COVID-19, immune-mediated inflammatory diseases, inflammatory bowel diseases, population-based, disease course
1. Introduction
The coronavirus disease 2019 [COVID-19], caused by the novel coronavirus SARS-CoV-2, is a new form of acute infectious respiratory syndrome, first reported in December 2019, with a wide spectrum of severity.1 COVID-19 has rapidly become a major health emergency, recognised as a pandemic and a Public Health Emergency of International Concern, leading to the lockdown of several states around the world. Patients with immune-mediated inflammatory diseases [IMIDs], such as inflammatory bowel diseases [IBD], are of special interest during this outbreak as a high proportion of these patients receive immunosuppressive therapies which have been shown to increase the risk of infections.2–4 In addition, patients with IBD, including Crohn’s disease [CD] and ulcerative colitis [UC], might express increased concentrations of angiotensin-converting enzyme 2 [ACE2] receptor, which enables the human-to-human transmission of SARS-CoV-2.5,6
The risk and disease course of COVID-19 among patients with IMIDs is still unknown, and only limited epidemiological data are available to guide physicians. A previous retrospective study found no evidence of an increased susceptibility to COVID-19 among patients with IBD,7 and a recent case series found that prednisolone and methotrexate were associated with an increased risk of hospitalisation among patients with COVID-19 and co-occurring IMIDs.8 However, to date available data come from selected cohorts without comparison with the background populations, and therefore these findings have yet to be confirmed in a population-based setting.
In this study, we aimed to determine the prevalence of COVID-19 among Danish patients with IBD as compared with the background population, as well as with Danish patients with other IMIDs. Furthermore, we reported the disease courses of IBD and COVID-19 in patients infected with SARS-CoV-19. Last, we investigated the mental well-being of patients with IBD during the COVID-19 pandemic.
2. Materials and Methods
2.1. Study design and population
We used three study populations to describe the epidemiology and disease course of COVID-19 in patients with IBD. First, we established a prospective nationwide cohort [the Danish COVID-IBD cohort], comprising all adult patients in Denmark with an established diagnosis of IBD [UC or CD] and who tested positive for SARS-CoV-2 genomic material by polymerase chain reaction [PCR] analysis. All internal medicine and gastrointestinal departments in Denmark participated in this study, which was conducted between January 28, 2020 and June 2, 2020. Patients were identified by searching for diagnosis codes for COVID-19 defined by the Danish Health Authority, applied in all Danish health-related electronic systems [DB342A, DB972A and DZ038PA1]9 and for IBD [Supplementary File 1, available as Supplementary data at ECCO-JCC online], as well as by manually screening all hospital patient lists, thereby ensuring identification of all SARS-CoV-2 positive patients with IBD. This cohort was used to describe the clinical characteristics of patients with IBD and the disease course of COVID-19, as well as the course of IBD following the development of COVID-19.
Second, we identified all individuals [both adult and paediatric] who were screened for SARS-CoV-2, by a PCR from either a nasopharyngeal swab or tracheal suctioning, since the first testing took place in Denmark on January 28, 2020 up until June 2, 2020. These individuals were identified though the Epic Health Care System in which all Danish residents living in the Capital Region of Denmark and Region Zealand [i.e. 45.5% of the Danish population] are registered with a unique 10-digit identification number.10 Patients with IMIDs were identified using the International Classification of Diseases [Eighth Revision] and International Classification of Diseases [Tenth Revision] codes, as listed in Supplementary File 1. In this way we could investigate the frequency of COVID-19 among patients with IBD or other IMIDs within a large, well-described population-based sample, and compare it with both the background population and patients with other IMIDs.
Last, a third study population consisted of patients with IBD [with and without COVID-19] who were willing to participate in a survey distributed by the Danish IBD Patient Association [CCF] and a major patient-oriented information website.11 The survey was centred on information sources for COVID-19, adherence to IBD-related therapy, and mental well-being as assessed by the Perceived Stress Scale during the first 2 months of the pandemic.12,13
2.2. Outcomes, definitions, and data collection
In the national cohort, all patients with IBD who tested positive for COVID-19 in any internal medicine or gastrointestinal department in Denmark were followed for up to 3 months after infection. The data collected included patient demographics, COVID-19 symptoms, and disease course [treatments, hospitalisations, admission to intensive care units, requirement of respiratory support in terms of noninvasive or invasive ventilators, and mortality] and IBD characteristics (IBD sub-type, Montreal Classification,14 disease activity at diagnosis of COVID-19 using either the Simple Clinical Colitis Activity Index [SCCAI] for UC or IBD unclassified,15 or the Harvey‐Bradshaw Index [HBI] for CD,16 and Mayo Endoscopic Score for both CD and UC,17 IBD-related treatments and changes in treatments, and disease activity). The data were registered in the Research Electronic Data Capture system [REDCap].18
Data collected for the population-based study concerned the prevalence of positive SARS-CoV-2 PCR test results of nasopharyngeal swabs or tracheal suctioning among all residents living in the Capital Region of Denmark and Region Zealand between January 28, 2020 and June 02, 2020. The residents were grouped according to the presence or absence of diagnosis codes for IMIDs, as specified in Supplementary File 1.
Finally, the data collected from the survey, which was constructed by three authors of this paper [MA, JBS, and JB], included information sources for COVID-19, patients’ adherence to their current IBD treatment, and their mental well-being as assessed by the Perceived Stress Scale which encompasses the perceived predictability, controllability, and manageability of COVID-19, its personal impact and meaning, and the individual’s perceived capacity to cope with the pandemic.12,13 In the Perceived Stress Scale, scores 0–13, 14–26, and 27–40 defined low, moderate, and high levels of perceived stress, respectively.
2.3. Statistical analysis
Statistical analyses were performed using R version 3.6.1 developed by R Foundation for Statistical Computing.19 Descriptive statistics were used to describe prevalences. Differences between UC and CD were investigated by Fisher’s exact test and chi square test. In addition, binary linear and logistic regression models were used to investigate possible continuous and categorical variables as predictive factors for the disease course of COVID-19 and IBD; p-values smaller than 0.05 were considered to be significant.
2.4. Ethical considerations
This study was approved by the Danish Patient Safety Authority and Knowledge Centre on Data Protection Compliance. According to Danish law, no permission from the National Committee on Health Research Ethics was required for this non-interventional study.
2.5. Data availability statement
All data are incorporated into the article and its online Supplementary Material, available as Supplementary data at ECCO-JCC online.
3. Results
3.1. Baseline characteristics of patients with IBD infected with COVID-19 in Denmark
During the observation period, a total of 76 patients with IBD [45 with UC and 31 with CD] were diagnosed with COVID-19 in Denmark according to the presence of PCR-confirmed SARS-CoV-2 genomic material. As summarised in Table 1, UC was most often a left-sided colitis [n = 14: 31.1%] and CD was mostly confined to the colon [n = 11: 35.4%] and had a non-stricturing and non-penetrating behaviour [n = 19: 61.3%]. A total of 45 patients [59%] were males and the median age at diagnosis was 51 years (interquartile range [IQR]: 39–66). In addition, 35 patients [46%] were active or former smokers at the time of their COVID-19 diagnosis and the median body mass index was 27 [IQR 23–29]. Regarding IBD disease activity at the time of the COVID-19 diagnosis, 51 [67%], 52 [68%], and 13 [17%] patients were in clinical, biochemical, and/or endoscopic remission, respectively.
Table 1.
Baseline characteristics of patients with IBD and COVID-19.
| N | Ulcerative colitis [UC] | Crohn’s disease [CD] | p-value | |
|---|---|---|---|---|
| Patients | 45 | 31 | ||
| Age at diagnosis of COVID-19 [years] | Median [IQR] | 51 [39–70] | 54 [38–62] | |
| Female gender | [%] | 15 [33.3] | 16 [51.6] | 0.11 |
| IBD localisation and behaviour | ||||
| E1: ulcerative proctitis | [%] | 13 [28.9] | ||
| E2: left-sided UC | [%] | 14 [31.1] | ||
| E3: Extensive colitis | [%] | 9 [20.0] | ||
| L1: ileal CD | [%] | 5 [16.1] | ||
| L2: colonic CD | [%] | 11 [35.4] | ||
| L3: ileocolonic CD | [%] | 7 [22.6] | ||
| L4: upper gastrointestinal CD | [%] | 0 | ||
| B1: non-stricturing, non-penetrating CD | [%] | 19 [61.3] | ||
| B2: stricturing CD | [%] | 4 [12.9] | ||
| B3: penetrating CD | [%] | 2 [6.5] | ||
| Perianal disease | [%] | 0 | ||
| Smoking history | [%] | 19 [42.2] | 16 [51.6] | 0.42 |
| Body mass index [kg/m2] | Median [IQR] | 26.6 [22.1–28.1] | 27.7 [25.6–29.8] | |
| Co-occurring IMIDs | [%] | 13 [28.9] | 9 [29.0] | 1.00 |
| Comorbidities other than IMIDs | [%] | 14 [28.9] | 12 [38.7] | 0.49 |
| IBD disease activity at diagnosis of COVID-19 | ||||
| Clinical remission | [%] | 29 [64.4] | 22 [71.0] | 0.55 |
| Biochemical remission | [%] | 30 [66.7] | 22 [71.0] | 0.69 |
| Endoscopic remission | [%] | 8 [17.8] | 5 [16.1] | 0.85 |
| IBD-related treatment at time of COVID-19 | ||||
| None | [%] | 9 [20.0] | 10 [32.2] | 0.22 |
| Topical 5-ASA | [%] | 17 [37.8] | 1 [3.2] | <0.01 |
| Systemic 5-ASA | [%] | 23 [51.1] | 2 [6.4] | <0.01 |
| Topical steroids | [%] | 2 [4.4] | 1 [3.2] | 0.79 |
| Systemic steroids | [%] | 1 [2.2] | 2 [6.4] | 0.35 |
| Immunomodulators | [%] | 4 [8.9] | 12 [38.7] | <0.01 |
| Biologic therapies | [%] | 8 [17.8] | 10 [32.2] | 0.14 |
Localisation and behaviour of IBD is categorized according to Montreal Classification.14 The p-value is based on a chi square test, with a p <0.05 [bold] considered to be significant.
IBD, inflammatory bowel disease, IMID, immune-mediated inflammatory disease, 5-ASA, 5-aminosalicylates; UC, ulcerative colitis; CD, Crohn’s disease; IQR, interquartile range.
At least one comorbidity was present in 43 [57%] patients with IBD, and ccomorbity was equally distributed among IMIDs [n = 22: 29%] and non-IMIDs [n = 25: 33%]. The most frequent IMIDs were asthma [n = 6: 7.9%], spondyloarthropathies [n = 6: 7.9%], and psoriasis [n = 2: 2.6%].
One in four patients with IBD [n = 19: 25%] received no IBD-related treatment at the time of their COVID-19 diagnosis; the most frequent treatment for IBD was with systemic 5-aminosalicylates [5-ASA] [n = 25: 22.9%], topical 5-ASA [n = 18: 23.7%], biologic therapies [n = 18: 23.7%], and immunomodulators, including azathioprine and 6-mercaptopurine [n = 16: 21.1%].
3.2. COVID-19 symptomatology and disease course of COVID-19 in patients with IBD
Among the 76 patients with IBD included in the national cohort, the most frequent symptoms of COVID-19 were coughing [n = 33: 43.4%], fever [n = 31: 40.8%], tiredness [n = 16: 21.1%], dyspnoea [n = 16: 21.1%], and muscle pain or arthralgia [n = 14: 18.4%]. Only three [3.9%] of the patients experienced diarrhoea, and only two [2.6%] experienced abdominal pain, as debut symptoms of COVID-19.
A large proportion of patients with IBD [n = 19: 25%] required hospitalisation due to COVID-19, with a median admission duration of days [IQR: 2–8.5], for the purpose of medical therapy, including antibiotics [n = 15: 19.7%] and oxygenation [n = 14: 18.4%]. Of these, one in four [6.6%] patients required admission to an intensive care unit and three patients [3.9%] required respiratory support by ventilator.
A total of four patients [three UC and one CD], all elderly patients with a median age of 86 years [IQR: 80–89], died of COVID-19. Half of these were men with cardiovascular comorbidities, and one patient had asthma. In terms of IBD, three of these four patients had active IBD at the time of their COVID-19 diagnosis; two patients received systemic 5-ASA as monotherapy, one patient received topical steroids as monotherapy, and the last patient [who was in clinical IBD remission] did not receive any IBD-related therapy. None of the patients received immunosuppressive therapies.
A significant association was found between COVID-19-related hospitalisation and being older than 65 years (odds ratio [OR] = 23].8, 95% CI 6.3–89.6, p <0.01] and the presence of a non-IMID comorbidity [OR = 8.1, 95%CI 2.6–25.9, p <0.01]. In addition, the presenting symptoms of COVID-19 of fever, tiredness, cough, and dyspnoea were associated with hospitalisation, as shown in Table 2. However, no association was found between gender, smoking, disease activity, extent of IBD, or IBD-related therapy and the risk of COVID-19 related hospitalisation. The only risk factor for admission into an intensive care unit was dyspnoea as a presenting symptom of COVID-19 [OR = 19.7, 95% CI 2.0–191.8, p = 0.01] which was also the only significant risk factor for COVID-19-related death [p = 0.03].
Table 2.
Association between clinical risk factors and COVID-19-related outcomes.
| Hospitalisation | Admission to intensive care unit | Requirement of ventilators | Death | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Living in Capital Region | 0.22 | 0.07–0.66 | 0.01 | 1.82 | 0.28–11.78 | 0.53 | 0.18 | 0.02–2.05 | 0.17 | NA | 0.99 | |
| Age > 65 years | 23.80 | 6.32–89.63 | <0.0001 | 4.76 | 0.73–30.93 | 0.10 | 1.42 | 0.12–16.58 | 0.78 | NA | 1.00 | |
| IBD sub-type UC | 1.47 | 0.49–4.42 | 0.50 | 0.92 | 0.14–5.87 | 0.93 | 0.29 | 0.03–3.39 | 0.33 | 1.91 | 0.19–19.28 | 0.58 |
| Body mass index >25 | 2.08 | 0.49–8.82 | 0.32 | 0.52 | 0.03–8.76 | 0.65 | 0.52 | 0.03–8.76 | 0.65 | 0.52 | 0.03–8.76 | 0.65 |
| Smoking | 1.49 | 0.49–4.51 | 0.48 | 1.17 | 0.18–7.56 | 0.87 | 1.58 | 0.14–18.35 | 0.72 | 0.76 | 0.10–5.75 | 0.79 |
| Disease activity of IBD at diagnosis of COVID-19 Clinical remission |
1.89 | 0.55–6.52 | 0.31 | 1.96 | 0.21–18.5 | 0.56 | NA | - | 1.00 | 0.14 | 0.01–1.42 | 0.10 |
| Biochemical remission | 1.02 | 0.31–3.34 | 0.97 | 0.61 | 0.10–3.95 | 0.61 | NA | - | 1.00 | 0.40 | 0.05–3.04 | 0.38 |
| Localisation of IBD L1: ileal CD |
2.12 | 0.33–13.75 | 0.43 | 4.19 | 0.38–46.73 | 0.25 | 8.63 | 0.64–116.53 | 0.11 | NA | - | 1.00 |
| L2: colonic CD | 1.15 | 0.27–4.86 | 0.85 | 1.53 | 0.15–15.08 | 0.72 | 3.15 | 0.26–38.05 | 0.37 | NA | - | 1.00 |
| L3: ileocolonic CD | 0.47 | 0.05–4.20 | 0.50 | NA | - | 1.00 | NA | - | 1.00 | NA | - | 1.00 |
| L4: upper gastrointestinal CD | NA | - | NA | - | NA | - | NA | - | ||||
| E1: ulcerative proctitis | 2.19 | 0.62–7.75 | 0.23 | 3.64 | 0.54–24.34 | 0.18 | 2.54 | 0.21–30.32 | 0.46 | 5.55 | 0.71–43.62 | 0.10 |
| E2: left-sided UC | 0.19 | 0.02–1.55 | 0.12 | NA | - | 1.00 | NA | - | 1.00 | NA | - | 1.00 |
| E3: extensive colitis | 0.34 | 0.04–2.92 | 0.33 | 1.39 | 0.22–8.91 | 0.73 | NA | - | 1.00 | 2.67 | 0.25–28.80 | 0.42 |
| Comorbidities Comorbidities other than IMIDs |
8.12 | 2.55–25.87 | <0.0001 | 1.39 | 0.22–8.91 | 0.73 | 1.02 | 0.09–11.83 | 0.99 | 2.13 | 0.28–16.08 | 0.46 |
| IMIDs | 0.84 | 0.26–2.70 | 0.77 | 0.60 | 0.06–5.65 | 0.65 | 1.24 | 0.11–14.40 | 0.87 | 0.81 | 0.08–8.23 | 0.86 |
| IBD-related treatment Topical 5-ASA |
0.82 | 0.23–2.88 | 0.76 | NA | - | 1.00 | NA | - | 1.00 | NA | - | 1.00 |
| Systemic 5-ASA | 0.92 | 0.30–2.81 | 0.89 | 1.39 | 0.22–8.91 | 0.73 | NA | - | 1.00 | 2.13 | 0.28–16.08 | 0.46 |
| Topical steroid | NA | - | 0.99 | NA | - | 1.00 | NA | - | 1.00 | 11.67 | 0.81–167.49 | 0.07 |
| Systemic steroid | 1.53 | 0.13–17.86 | 0.74 | NA | - | 1.00 | NA | - | 1.00 | NA | - | 1.00 |
| Immunomodulators | 1.49 | 0.44–5.03 | 0.52 | 2.71 | 0.41–17.83 | 0.30 | 8.43 | 0.71–99.64 | 0.09 | NA | - | 1.00 |
| Biologic therapy | 0.30 | 0.06–1.46 | 0.14 | 0.79 | 0.08–7.60 | 0.84 | 1.65 | 0.14–19.30 | 0.69 | NA | - | 1.00 |
| No treatment | 1.10 | 0.34–3.59 | 0.88 | 0.74 | 0.08–7.02 | 0.79 | 1.53 | 0.13–17.86 | 0.74 | NA | - | 1.00 |
Localisation of IBD is categorised according to Montreal Classification.14 The p-values originate from regression analyses and p <0.05 [bold] was considered to be significant.
OR, odds ratio; CI, confidence interval; IBD, inflammatory bowel disease. IMID, immune-mediated inflammatory disease. 5-ASA, 5-aminosalicylates. NA, not applicable
3.3. IBD course during COVID-19
Of 76 patients, 15 [19.7%] underwent a clinical assessment of IBD 35 days [IQR: 25–51] after their diagnosis of COVID-19; eight of these patients [53.3%] experienced no change in IBD activity, and three patients experienced a relapse based on a clinical assessment. One patient required initiation of systemic steroids. As presented in Supplementary File 2, available as Supplementary data at ECCO-JCC online, no risk factors—including gastrointestinal symptoms of COVID-19 or changes in IBD-related therapy—were found to be associated with any IBD-related relapse.
3.4. Occurrence and prognosis of COVID-19 in patients with IBD, other immune-mediated diseases, and in the normal population
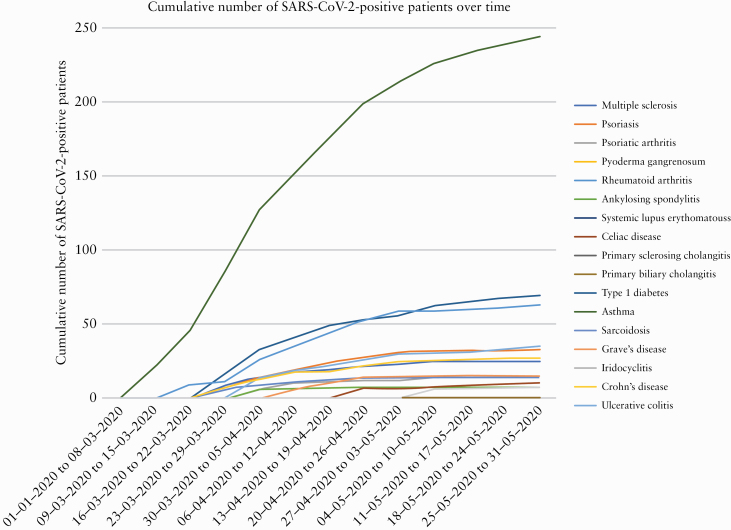
In order to investigate the prevalence of COVID-19, we analysed all individuals tested for SARS-CoV-2 in the geographical area of Capital Region of Denmark and Region Zealand. Between January 28, 2020 and June 2, 2020, a total of 2 649 091 individuals [45.5% of the Danish population] were living in the uptake area, of whom 18 336 [0.7%] had IBD. During the observation period, a total of 231 601 individuals, including 2486 [1.1%] patients with IBD, were screened for SARS-CoV-2. The first case of COVID-19 was detected on February 27, 2020. The proportions of SARS-CoV-2 positive patients with various IMIDs are presented in Table 3 and illustrated in Figure 1; Figures 2 and 3 illustrate the testing over time. Overall, 8476 individuals [3.7%] were found positive for COVID-19 during the study period. The corresponding prevalence among patients with IBD was 62 out of 2486 patients [2.5%], which was significantly lower than among non-IBD patients [3.7%; p <0.01]. As shown in Table 3, this difference can be attributed especially to patients with UC. In contrast, patients with CD and co-occurring IMIDs had an increased frequency of COVID-19 [9.4% vs 3.7%, p = 0.04]. Of note, all 62 patients were also identified in the national cohort.
Table 3.
Prevalence of COVID-19 among patients with various IMIDs and in the general population.
| Overall N of patients | N tested | Proportion of patients tested [%] | N | Frequency of COVID-19 [%] | p-value | |
|---|---|---|---|---|---|---|
| COVID-19 | ||||||
| Overall in the general population | 2649091 | 231601 | 8.74 | 8476 | 3.66 | |
| No IMIDs | 2531740 | 215109 | 8.50 | 7945 | 3.69 | Ref |
| Any IMID | 117351 | 16492 | 14.05 | 531 | 3.22 | <0.0001 |
| Asthma | 42571 | 7176 | 16.86 | 244 | 3.4 | 0.21 |
| Rheumatoid arthritis | 12501 | 1583 | 12.66 | 63 | 3.98 | 0.56 |
| Type 1 diabetes | 14331 | 1578 | 11.01 | 69 | 4.37 | 0.17 |
| Ulcerative colitis [UC] | 11458 | 1451 | 12.66 | 35 | 2.41 | 0.01 |
| Psoriasis | 8024 | 1086 | 13.53 | 33 | 3.04 | 0.27 |
| Crohn’s disease [CD] | 6878 | 1035 | 15.05 | 27 | 2.61 | 0.07 |
| Psoriatic arthritis | 4398 | 589 | 13.39 | 15 | 2.55 | 0.15 |
| Multiple sclerosis | 6628 | 766 | 11.56 | 25 | 3.26 | 0.54 |
| Sarcoidosis | 2743 | 461 | 16.81 | 12 | 2.6 | 0.23 |
| Grave’s disease | 3453 | 433 | 12.54 | 14 | 3.23 | 0.62 |
| Coeliac disease | 3023 | 382 | 12.64 | 10 | 2.62 | 0.28 |
| Iridocyclitis | 2538 | 284 | 11.19 | 6 | 2.11 | 0.17 |
| Primary sclerosing cholangitis | 1289 | 247 | 19.16 | 4 | 1.62 | 0.09 |
| Ankylosing spondylitis | 1926 | 226 | 11.73 | 7 | 3.1 | 0.65 |
| Systemic lupus erythematosus | 1107 | 191 | 17.25 | 5 | 2.62 | 0.45 |
| UC and any other IMID | 1044 | 177 | 16.95 | 8 | 4.52 | 0.58 |
| CD and any other IMID | 638 | 53 | 8.31 | 5 | 9.43 | 0.04 |
| Primary biliary cholangitis | 616 | 79 | 12.82 | 1 | 1.27 | 0.26 |
| Pyoderma gangrenosum | 207 | 41 | 19.81 | 2 | 4.88 | 0.70 |
The p-value is based on a chi square test, with p <0.05 [bold] considered to be significant.
IMID, immune-mediated inflammatory disease; NA, not applicable.
Figure 1.
Proportion of SARS-CoV-2-positive patients with various immune-mediated inflammatory diseases [IMIDs].
Figure 2.
Number of patients with immune-mediated inflammatory diseases testing positive with SARS-CoV-2 over time.
Figure 3.
Number of patients with immune-mediated inflammatory diseases tested for SARS-CoV-2 over time.
Patients with IMIDs generally had a lower occurrence of COVID-19 than did patients without IMIDs (531 of 16 492 [3.2%] vs 7945 of 215 109 [3.7%], p <0.01]. Comparing IBD with other IMIDs, we also found that COVID-19 occurred less frequently among patients with IBD than among patients with other IMIDs [2.5% vs 3.3%, p = 0.03].
Nonetheless, the mortality among patients with COVID-19 did not seem to differ according to presence or absence of IMIDs [7.5% vs 5.8%, p = 0.13]. However, as presented in Table 4, mortality was significantly increased among patients with IMIDs and COVID-19 compared with patients with IMIDs but without COVID-19, and this was particularly attributed to patients with rheumatoid arthritis, IBD, type 1 diabetes, and asthma.
Table 4.
Mortality among patients with or without IMIDs in relation to COVID-19.
| Mortality, N [%] | ||||
|---|---|---|---|---|
| Overall N of patients | Patients with COVID-19 | Patients without COVID-19 | p-value | |
| Overall | 2649091 | 500 [5.90] | 11186 [0.42] | <0.0001 |
| Absence of any IMIDs | 2531740 | 460 [5.79] | 10437 [0.41] | <0.0001 |
| Presence of any IMID | 117351 | 40 [7.50] | 749 [0.64] | <0.0001 |
| Asthma | 42571 | 16 [6.56] | 199 [0.47] | <0.0001 |
| IBD | 18336 | 4 [6.45] | 75 [0.41] | <0.0001 |
| Type 1 diabetes | 14331 | 6 [8.70] | 103 [0.72] | <0.0001 |
| Rheumatoid arthritis | 12501 | 12 [19.05] | 149 [1.19] | <0.0001 |
| Psoriasis | 8024 | NA | 59 [0.74] | NA |
| Multiple sclerosis | 6628 | NA | 47 [0.71] | NA |
| Psoriatic arthritis | 4398 | NA | 20 [0.45] | NA |
| Grave’s disease | 3453 | NA | NA | NA |
| Coeliac disease | 3023 | NA | 9 [0.30] | NA |
| Sarcoidosis | 2743 | NA | 17 [0.62] | NA |
| Iridocyclitis | 2538 | NA | 15 [0.58] | NA |
| Ankylosing spondylitis | 1926 | NA | NA | NA |
| Primary sclerosing cholangitis | 1289 | NA | 69 [5.26] | NA |
| Systemic lupus erythomatouss | 1107 | NA | NA | NA |
| Primary biliary cholangitis | 616 | NA | 11 [1.77] | NA |
| Pyoderma gangrenosum | 207 | NA | NA | NA |
The p-value is based on a chi square test comparing the proportion of mortality among patients with and without COVID-19, with p <0.05 [bold] considered to be significant.
IMID, immune-mediated inflammatory disease; NA, not applicable.
3.5.IBD patients’ perspective of the COVID-19 pandemic
A month after the outbreak of COVID-19 in Denmark, a total of 2000 patients with IBD participated in an online survey exploring their stress levels, information sources, and adherence to IBD-related therapies, regardless of whether they had been diagnosed with COVID-19. Overall, 382 [19.1%] patients were not taking any IBD-related medication, 672 [33.6%] patients were receiving biologic therapies, 592 [29.6%] were receiving immunomodulators [azathioprine, mercaptopurine, or methotrexate], and 56 [2.8%] were receiving systemic steroids. In general, most patients with IBD were adherent to their IBD-related therapies, as only 30 [1.5%] patients discontinued the treatments on their own, and 67 [3.4%] and six [0.3%] patients discontinued their therapy in agreement with a gastroenterologist or general practitioner, respectively.
Patients with IBD sought out COVID-19 related information using the Danish health authorities [80.4%], patient associations [53.8%], gastroenterologists at hospital departments [23.4%], and social media [22.3%]. Only 5.6% sought out information from their nurse, and only 4.0% did so from the Danish Society of Gastroenterology and Hepatology.
The outbreak of COVID-19 appears to have affected the mental health of patients with IBD, as 52.8% reported a moderate level of stress and 7.2% a high level of stress, according to the Perceived Stress Scale, and 40% have experienced low-grade stress since the outbreak of COVID-19. Patients receiving biologic therapies were at greater risk of having a high level of stress [10% vs 6%, chi square test p <0.01] or moderate-to-high levels of stress [65% vs 56%, p <0.01]. However, patients with high scores on the Perceived Stress Scale were not at increased risk of discontinuing their IBD-related treatment [p = 0.85].
4. Discussion
Data about the risk of COVID-19 and its disease course among patients with IBD remain scarce but are urgently needed to ensure informed decision making by physicians caring for patients with IBD, as well as to address patients’ concerns. This is the first population-based study of COVID-19 among patients with IBD, and we have found that the prevalence of COVID-19 among Danish patients with IBD and other IMIDs overall was low and significantly lower than in the background population.
Concerns have been raised about a potentially increased risk of COVID-19 among patients with IBD, due to immunosuppressive therapy and, possibly, increased levels of ACE-2 mediating the entry of SARS-CoV-2 into human cells. However, a recent meta-analysis and our own population-based study, suggest a low prevalence of COVID-19 among patients with IBD compared with the general population.20 For example, in a North American selected cohort, the prevalence of COVID-19 was 3.0% among patients with IBD compared with 8.2% in the reference group.21 Denmark was among the first European countries to initiate a lockdown in order to contain the spread of the virus. On January 15, 2020, the Danish Health Authority drew up its first guidelines for a national containment strategy in which only symptomatic patients were tested for COVID-19. Subsequently, the containment strategy was changed into a mitigation strategy on March 12, 2020, with widening of the screening criteria. Since May 18, 2020, all individuals living in Denmark were provided free testing for COVID-19 regardless of presence of symptoms and without the need for a physician prescribing the test. Patients receiving immunosuppressive therapy such as that used for IBD regardless of the indication, are categorised as high-risk patients and are advised by the Danish government to exhibit extreme caution to limit the risk of infections. Therefore, it is possible that patients with IBD may have acted more carefully and hence limited the risk of infections by social distancing and hygiene. However, the exact reasons for the lower prevalence remain unknown as such a behaviour would also have affected other IMIDs, but this pattern was not observed, suggesting other factors might be at play for IBD.
As with IBD, few data have been gathered about the prevalence of COVID-19 among patients with other IMIDs. A recent retrospective and exploratory study of patients with rheumatological diseases in Spain found an increased prevalence of COVID-19 of 0.78% compared with the general population [0.58%].22 The prevalence rates were considerably lower than those found in our population, which might be explained by the fact that the Spanish study included only patients attending hospital emergency departments and thereby excluded patients with subclinical disease courses.
In this study, we found a relatively large proportion of patients with IBD and COVID-19 requiring hospitalisation [20%] and admission to intensive care units [5%] for COVID-19 related reasons. These data are comparable to a recent Italian study of 79 patients, in which 28% required hospitalisation and 9% admission to an intensive care unit.23 Interestingly, the Italian study found a significant association between IBD disease activity and the risk of COVID-19 hospitalisation, something which we could not confirm. In fact, we did not identify any association between IBD-related treatment, including immunosuppressive therapies, and the risk of hospitalisation or respiratory therapies among IBD patients with COVID-19, which is in accordance with previous studies.21,23 In contrast, the largest publication to date provided by SECURE IBD, predominantly including patients from USA. found 5-ASA and systemic corticosteroids to be associated with a severe disease course of COVID-19 requiring treatment at intensive care units or respiratory support with mechanical ventilation, or resulting in death.24 This discrepancy could represent selection bias due reliance on physician reporting in the registry study, whereas this population-based study did not rely on manual reporting.
Furthermore, we described the disease course of IBD following a COVID-19 diagnosis and found no apparent risk of relapse among our cohort. However, this finding needs to be interpreted with care as only 15 patients, with a median of just 35 days of follow-up, were available for analysis. Data concerning the course of IBD following viral infections are very limited. One intriguing study found that a large proportion of patients [45% and 7% for UC and CD, respectively] experienced a mild relapse during the first week after contracting the H1N1v virus; however, that cohort consisted of only 25 patients.25 Viral infections may in general be associated with an alteration of IBD disease activity, and this remains to be confirmed using larger cohorts.
In our cohort, patients with IBD as well as patients with other IMIDs had a higher test rate [149–177%] than the population in general, indicating a lower threshold of being tested. This could, however, reinforce the difference in occurrence of COVID-19 among patients with and without IBD or other IMIDs as a higher rather than a lower occurrence of COVID-19 would be expected. A key question arising from this finding is whether immunological factors could play a role in reducing the risk of COVID-19 among patients with IMIDs. Emerging studies are indicating a beneficial association between immunosuppressive therapies and COVID-19 outcomes, among both patients with IBD and patients with other IMIDs.26,27 Although such an association remains to be demonstrated in larger cohorts, we hypothesise that the inflammatory pathways of COVID-19 and those of IBD and other IMIDs are not overlapping.
Last, scarce data exist regarding the mental well-being of patients with IBD during the COVID-19 pandemic. Survey studies have reported good overall adherence to IBD therapy, but also fears of COVID-19 infection and lifestyle changes including less travelling and increased usage of disinfectants.28,29 In this study, we report a close adherence to IBD-related therapy, but undergoing immunosuppressive therapy was associated with an increased risk of severe stress, according to the validated Perceived Stress Scale. The patients in our cohort sought guidance regarding COVID-19 from reliable sources but found it stressful to actively seek for information. We therefore suggest actively reaching out to patients with IBD, even those without symptoms of COVID-19, to provide reliable information, advice, and guidelines.
This study has several strengths. First, this is the first prospective population-based study of patients with IBD and COVID-19, all with PCR-validated diagnoses of the latter. The variety of approaches for including patients and the active involvement of all departments caring for patients with IBD in Denmark, as well as the independence of patient-reported diagnoses of COVID-19, ensured the elimination of geographical and selection bias. Second, there are currently no large-scale published reports of the prevalence of COVID-19 among patients with and without specific IMIDs; as such, this study serves to address that blind spot.
Nonetheless, some limitations must be borne in mind. As with almost any other COVID-19 related study, indications for testing for it have varied considerably since its outbreak. This could potentially bias our results as to its true prevalence among patients with and without IMIDs. However, this narrow testing strategy has been updated and includes screening more broadly, and the prolonged inclusion period of this study helps to offset the potential bias. In addition, we were unable to determine predictive factors for several outcomes of interest due to the low event rates and/or small number of patients. A larger study population is needed to further clarify the predictive factors for these outcomes and to include asymptomatic patients who might be less motivated to get tested. A recent systematic review estimated that 12.9% of all patients are asymptomatic carriers of SARS-CoV-2.30 Finally, data were only available for the number of individuals tested and their test results in two of the five administrative regions of Denmark; however, Capital Region of Denmark and Region Zealand include almost half of the Danish population and are where most cases of COVID-19 have been identified. Nonetheless, our findings might overestimate the prevalence of COVID-19 somewhat.
In conclusion, the epidemiological data presented here confirm similar or lower COVID-19 rates among patients with IBD and other IMIDs than in the general population and no increased mortality among patients with IMIDs and COVID-19 compared with patients without IMIDs. In addition, we found no correlation between the use of immunosuppressive therapies and COVID-19-related outcomes. The short-term IBD disease course appears to be unaffected by COVID-19. However, immunosuppressive therapies are associated with high levels of perceived stress, emphasising the importance of providing advice and guidelines to patients.
Supplementary Material
Acknowledgements
The authors are grateful to all members of the Danish COVID-IBD Study Group who have participated in screening the outpatient lists for COVID-19. The group consists of Inge Nordgaard Lassen [Hvidovre University Hospital], Flemming Bendtsen [Hvidovre University Hospital], Ida Vind [Hvidovre University Hospital], Marianne Kiszka-Kanowitz [Hvidovre University Hospital], Gitte Pedersen [Hvidovre University Hospital], Trine Boysen [Hvidovre University Hospital], Anette Mertz Nielsen [Hvidovre University Hospital], Pia Munkholm [Nordsjaelland University Hospital], Lars Kristian Munck [Zealand University Hospital], Jens Frederik Dahlerup [Aarhus University Hospital], Christian Lodberg Hvas [Aarhus University Hospital], Asser Mathiassen Oppfeldt [Horsens Region Hospital], Henning Glerup [Silkeborg Region Hospital], Søren Lyhne [Randers Region Hospital], Torben Knudsen [Esbjerg University Hospital], Jens Kjeldsen [Odense University Hospital], Torben Nathan [Sygehus Lillebælt], Søren Jensen [Sygehus Lillebælt], Wojciech Cebula [Nykoebing Falster Sygehus], Kim Therkelsen [Hjoerring Sygehus], and Gunnar Lauge Nielsen [Farsoe Sygehus].
Funding
This research did not receive any grants.
Conflict of Interest
JBS: research grants from Takeda and the Capital Region Denmark; and as national coordinator of studies from AbbVie, Arena Pharmaceuticals, Ely Lilly, and Boehringer Ingelheim. None of these pertain to the research submitted here. JB: personal fees from AbbVie, Janssen-Cilag, Celgene, Samsung Bioepis, and Pfizer; grants and personal fees from Takeda, MSD, and Tillots Pharma; grants from Novo Nordisk Foundation and Bristol Meyers Squibb. None of these pertain to the research submitted here.
Author Contributions
MA: study concept design, patient inclusion, data extraction, analysis and interpretation of data, and drafting of manuscript. AP, NP, KT, LL, TJ, MKVA, MRH, KH, ABL, AM, AN, AW: patient inclusion, data extraction, and critical revision of manuscript. JBS, JB: study concept design, patient inclusion, data extraction, critical revision of manuscript, and supervision. All authors approved the final version of the manuscript, including the authorship list.
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology 2018;155:337–46.e10. [DOI] [PubMed] [Google Scholar]
- 3. Zabana Y, Rodríguez L, Lobatón T, et al. Relevant infections in inflammatory bowel disease, and their relationship with immunosuppressive therapy and their effects on disease mortality. J Crohns Colitis 2019;13:828–37. [DOI] [PubMed] [Google Scholar]
- 4. Grijalva CG, Chen L, Delzell E, et al. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalisation for infection in patients with autoimmune diseases. JAMA 2011;306:2331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garg M, Burrell LM, Velkoska E, et al. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: A pilot study. J Renin Angiotensin Aldosterone Syst 2015;16:559–69. [DOI] [PubMed] [Google Scholar]
- 7. Monteleone G, Ardizzone S. Are patients with inflammatory bowel disease at increased risk for covid-19 infection? J Crohns Colitis. 2020.. PMID: 32215548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haberman R, Axelrad J, Chen A, et al. Covid-19 in immune-mediated inflammatory diseases—case series from New York. N Engl J Med. 2020, April. NEJMc2009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sundhedsdatastyrelsen; Indberetni ng til Landspatientregisteret i forbindelse med COVID-19 samt om koder kun til lokal brug [National Health Data Authority. Reporting to The National Patient Register during COVID-19 and regarding codes only for local use.]. 2020.https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjW0obxk-TqAhUIyaQKHRZUB9oQFjAAegQIAhAB&url=https%3A%2F%2Fsundhedsdatastyrelsen.dk%2F-%2Fmedia%2Fsds%2Ffiler%2Frammer-og-retningslinjer%2Fpatientregistrering%2Flpr_indberetningsvejle Accessed April 1, 2020. [Google Scholar]
- 10. Larsen D. Population in Denmark. Statistics Denmark; 2020. https://www.dst.dk/en/Statistik/emner/befolkning-og-valg/befolkning-og-befolkningsfremskrivning/folketal Accessed June 2, 2020. [Google Scholar]
- 11. Lev Med IBD (Live with IBD) 2020. levmedibd.dk Accessed July 5, 2020.
- 12. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 13. Eskildsen A, Dalgaard VL, Nielsen KJ, et al. Cross-cultural adaptation and validation of the Danish consensus version of the 10-item Perceived Stress Scale. Scand J Work Environ Health 2015;41:486–90. [DOI] [PubMed] [Google Scholar]
- 14. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19[Suppl A]:5A–36A. [DOI] [PubMed] [Google Scholar]
- 15. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 17. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. N Engl J Med 1987. PMID: 3317507. [DOI] [PubMed] [Google Scholar]
- 18. Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture [REDCap] ‐ a metadatadriven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. R Core Team. R: A Language and Environment for Statistical Computing. . 2017. https://www.r-project.org/ Accessed July 1, 2020. [Google Scholar]
- 20. Burgueño J, Abreu M. Reply to the letter to the editor: The incidence and outcomes of COVID-19 in patients with IBD: a rapid review and meta-analysis. Inflamm Bowel Dis 2020;26:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gubatan J, Levitte S, Balabanis T, Patel A, Sharma A, Habtezion A. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in Northern California. Gastroenterology 2020;159:1141–4.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pablos JL, Abasolo L, Alvaro-Gracia JM, et al. ; RIER investigators group. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis 2020;79:1170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bezzio C, Saibeni S, Variola A, et al. ; Italian Group for the Study of Inflammatory Bowel Disease [IG-IBD]. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut 2020;69:1213–7. [DOI] [PubMed] [Google Scholar]
- 24. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rahier JF, Papay P, Salleron J, et al. Influenza A [H1N1]v infection in patients with inflammatory bowel disease: a case series. Aliment Pharmacol Ther 2011;33:499–500. [DOI] [PubMed] [Google Scholar]
- 26. Papa A, Gasbarrini A, Tursi A. Epidemiology and the impact of therapies on the outcome of COVID-19 in patients with inflammatory bowel disease. Gastroenterology 2020;159:481‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Michot JM, Albiges L, Chaput N, et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol 2020;31:961–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grunert P, Reuken P, Stallhofer J, Teich N, Stallmach A. Inflammatory bowel disease in the COVID-19 pandemic—the patients’ perspective. J Crohns Colitis 2020, Jun 20. doi: 10.1093/ecco-jcc/jjaa126. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D’Amico F, Rahier JF, Leone S, Peyrin-Biroulet L, Danese S. Views of patients with inflammatory bowel disease on the COVID-19 pandemic: a global survey. Lancet Gastroenterol Hepatol 2020;5: 631–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al-Sadeq DW, Nasrallah G. The incidence of the novel coronavirus SARS-CoV-2 among asymptomatic patients: a systematic review. Int J Infect Dis 2020;S1201:30533–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.