Abstract
Background
Excessive activation of immune responses in coronavirus disease 2019 (COVID-19) is considered to be related to disease severity, complications, and mortality rate. The complement system is an important component of innate immunity and can stimulate inflammation, but its role in COVID-19 is unknown.
Methods
A prospective, longitudinal, single center study was performed in hospitalized patients with COVID-19. Plasma concentrations of complement factors C3a, C3c, and terminal complement complex (TCC) were assessed at baseline and during hospital admission. In parallel, routine laboratory and clinical parameters were collected from medical files and analyzed.
Results
Complement factors C3a, C3c, and TCC were significantly increased in plasma of patients with COVID-19 compared with healthy controls (P < .05). These complement factors were especially elevated in intensive care unit patients during the entire disease course (P < .005 for C3a and TCC). More intense complement activation was observed in patients who died and in those with thromboembolic events.
Conclusions
Patients with COVID-19 demonstrate activation of the complement system, which is related to disease severity. This pathway may be involved in the dysregulated proinflammatory response associated with increased mortality rate and thromboembolic complications. Components of the complement system might have potential as prognostic markers for disease severity and as therapeutic targets in COVID-19.
Keywords: COVID-19, SARS-CoV-2, ARDS, Inflammation, Complement, Coagulation
The complement system is persistently activated in hospitalized patients with coronavirus disease 2019 (COVID-19) and associated with disease severity and onset of thromboembolic complications. These findings support the potential use of complement inhibitors to treat severely ill patients with COVID-19.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to the current coronavirus disease 2019 (COVID-19) pandemic, causing high morbidity and mortality rates globally [1]. Clinical presentation and disease course of COVID-19 may vary between individual patients. It can be asymptomatic in up to 80% of infected individuals, causing mild upper respiratory tract illness in many others, but it can also lead to severe viral pneumonia with acute respiratory distress syndrome (ARDS), requiring mechanical ventilatory support in the intensive care unit (ICU) in some individuals [2, 3].
The exact pathogenesis of COVID-19 is still poorly understood. SARS-CoV-2 triggers an excessive and maladaptive systemic inflammatory response, resulting in the sustained release of proinflammatory cytokines [3], development of coagulopathy [4, 5] and endothelitis [6], all leading to an increased risk of thromboembolic complications and unfavorable outcomes in patients with COVID-19. Zhou et al [7] demonstrated that concentrations of biomarkers, such as D-dimer, serum ferritin, and interleukin 6 (IL-6), were significantly elevated in nonsurvivors compared to survivors. Another study in China observed higher plasma concentrations of some cytokines in ICU patients compared with non-ICU patients; these cytokines included interleukin 2, 17, and 10, Granulocyte colony-stimulating factor, interferon-γ–inducible protein 10, monocyte chemoattractant protein 1, and tumor necrosis factor α [3]. These findings could form the basis for the introduction of novel host-directed therapeutic strategies targeting underlying pathophysiological mechanisms, thus possibly improving patient outcomes [8].
The complement system plays a pivotal role in the initial innate immune response to pathogens, including coronaviruses [9]. Complement activation is an important defense mechanism in sepsis and is associated with severity and poor outcome [10, 11]. Beyond its crucial role in eliminating invading pathogens, previous studies have shown that activated complement may induce collateral tissue damage, considered to contribute to the pathogenesis of ARDS [12, 13]. Previous studies have reported that complement blockade could alleviate pulmonary complications in mouse models of Middle Eastern respiratory syndrome coronavirus and severe acute respiratory syndrome–related coronavirus (SARS-CoV), other coronaviruses that caused outbreaks in humans before [9, 14].
A recent preliminary analysis showed enhanced complement component deposition in lung tissue of deceased patients, and increased C5a concentrations in serum samples from patients with severe COVID-19 [15], pointing toward activation of the complement system. In addition, deposition of terminal complement components was detected in the pulmonary microvasculature of patients with severe COVID-19 pneumonitis [16]. Gralinski et al [9] showed that mice deficient for C3 were protected against the effects of SARS-CoV infection, resulting in less respiratory dysfunction, a reduced proinflammatory response, and fewer pathological changes in the lung. These results suggest that targeted inhibition of C3 activation has therapeutic potential. The efficacy of complement inhibition in the treatment of a coronavirus-mediated infection was reported by Jiang et al [14], demonstrating significantly reduced inflammation-mediated tissue destruction in mice when the C5a-C5aR axis was blocked. Moreover, one study, published as a preprint, demonstrated clinical improvement in the first patients treated with anti-C5a monoclonal antibody for SARS-CoV-2 infections [15].
Although these first data suggest a crucial link between activation of the complement system and the dysregulated immune response observed in patients with COVID-19, a comprehensive explorative analysis of the complement system in relation to clinical outcomes is still lacking. In the current study, we assessed the role of the complement system in plasma of patients with COVID-19 and its relation to the host immune response, disease severity, clinical course, and outcomes. We compared plasma complement concentrations in patients with COVID-19 with those in patients with bacterial septic shock and in healthy individuals.
METHODS
Ethics Statement
The study protocol was approved by the local ethics committee (CMO 2020 6344 and CMO 2016 2963) and performed in accordance with the latest version of the declaration of Helsinki and guidelines for good clinical practice.
Patient Inclusion and Sample Collection
This prospective longitudinal study was performed at a tertiary care hospital in the Netherlands. Patients (or their legal representatives) with polymerase chain reaction–proved or presumed SARS-CoV-2 infection admitted to our hospital between March and April were asked for informed consent to participate. Presumed infection was defined based on signs and symptoms, specific computed tomographic findings according the Dutch COVID-19 Reporting and Data System (CO-RADS) classification and final consensus of clinical experts [17].
Plasma samples were collected sequentially (every 48–72 hours) during routine blood withdrawal for laboratory testing. Ethylenediaminetetraacetic acid (EDTA) blood was centrifuged for 10 minutes at 3800 rpm (2954g) at room temperature, plasma samples were collected and stored at −20°C for cytokine analysis and at −80°C for complement factor analysis according to recommended protocols [18, 19]. EDTA plasma and demographic data from healthy controls (the 200 Functional Genomics cohort; http://www.humanfunctionalgenomics.org) and patients with bacterial septic shock according to the Sepsis-3 definition (PROVIDE study, unpublished data; ClinicalTrials.gov NCT 03332225) were used for comparison. The patients with bacterial sepsis were older than those with COVID-19 and the healthy controls (median age [interquartile range], 77 [63–86] years versus 65 [54–72] and 60 [57–66] years, respectively; P < .001) (Table 1) and had a higher mortality rate (62% vs 14%; P < .001). No differences in sex distribution and body mass index (BMI) between the 3 groups were observed.
Table 1.
Patient Characteristics
| Patients With COVID-19a | Healthy Controls (n = 10) | Patients With Sepsis (n = 39) | P Value (All Groups) | ||||
|---|---|---|---|---|---|---|---|
| Total (n = 197) | Non-ICU (n = 115) | ICU (n = 75) | P Value (Non-ICU vs ICU) | ||||
| Age, median (IQR), y | 65 (54–72) | 66 (52–73) | 64 (55–71) | .43 | 60 (57–66) | 77 (63–86) | <.001b |
| Sex, no. (%) | |||||||
| Male | 137 (69) | 76 (66) | 55 (73) | .36 | 4 (40) | 25 (64) | .13 |
| Female | 60 (31) | 39 (34) | 20 (27) | 6 (60%) | 14 (36) | ||
| BMI, median (IQR)c | 26.6 (23.9–29.3) | 26.4 (23.6–29.0) | 27.0 (24.7-2.6) | .45 | 26.9 (23.5–29.4) | 26.2 (21.6–30.9) | .95 |
| Time from first COVID-19 signs to admission, median (IQR), d | 5 (8–10) | 8 (5–10) | 9 (6–10) | .21 | NA | NA | NA |
| Laboratory values, median (IQR)d | |||||||
| C3a, ng/mL | 424 (247–645) | 372 (239–565) | 555 (301–935) | .002 | 67 (20–76) | 5006 (2138–9449) | <.001e |
| C3c, ng/mL | 1359 (1063–1903) | 1365 (1068–1903) | 1383 (1016–2026) | .99 | 852 (713–852) | 3857 (1405–8205) | <.001e |
| TCC, mAU/mL | 4915 (3661–6610) | 4479 (3499–6403) | 6485 (4764–7337) | .003 | 2962 (2677–3434) | 8024 (5886–12 106) | <.001e |
| CRP, mg/L | 97 (58–166) | 90 (51–150) | 150 (85–260) | .008 | NA | NA | NA |
| Ferritin, µg/L | 915 (247–1552) | 866 (424–1400) | 1328 (427–2901) | .10 | NA | NA | NA |
| D-dimer, ng/mL | 1280 (760–2210) | 1240 (750–1850) | 2280 (1260–3640) | .002 | NA | NA | NA |
| IL-6, pg/mL | 65 (30–95) | 53 (26–79) | 157 (78–449) | <.001 | NA | NA | NA |
| Deaths, no. (%) | 27/191 (14) | 10/115 (9) | 16/70 (23) | .002 | NA | 24 (62) | <.001f |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ICU, intensive care unit; IL-6, interleukin 6; NA, not applicable; TCC, terminal complement complex.
aBaseline samples (0–3 days after admission) were available from only 122 of 197 patients (87 of 115 non-ICU and 30 of 75 ICU patients). Data from 7 patients who were initially admitted to the clinical ward and transferred to the ICU during hospital admission (4%) are not shown separately.
bSignificant for COVID-19 versus sepsis and healthy controls versus sepsis.
cBMI was calculated as weightin kilograms divided by height in meters squared.
dCRP, Ferritin, D-dimer, and IL-6 were only available for the patients with COVID-19.
eSignificant difference between all groups.
fSignificant for COVID-19 versus sepsis.
Data Collection
Clinical data and laboratory results were collected from the electronic patient files (EPIC; EPIC System) and recorded in electronic case report forms (Castor EDC). The date of hospital admission (or the date of initial admission for patients transferred from another hospital) was designated as day 0. The first sample was considered a baseline measurement if it was obtained within 3 days after admission. For longitudinal analysis, data were aligned for days after admission and binned into clusters of 3 days.
Complement and Cytokine Assays
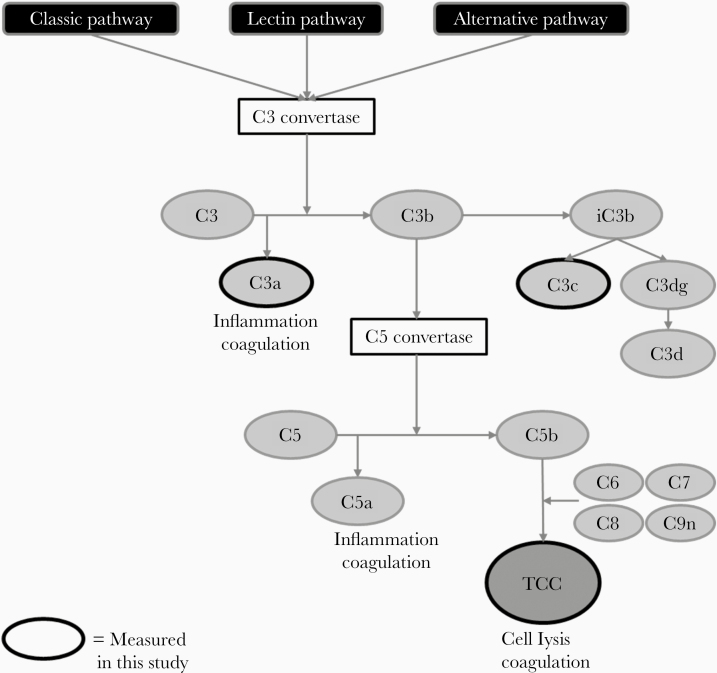
Complement activation was assessed by measuring C3 turnover (C3 vs the C3 activation products C3a and C3c) and downstream C5 turnover (C5 vs the C5 activation product C5a). Moreover, the terminal complement complex (TCC) was assessed as an end-product of the complement cascade. See Figure 1 for a simplified overview of the complement pathway. Concentrations of these complement components were measured in EDTA plasma from patients using commercially available enzyme-linked immunosorbent assays kits (HK 366 [C3], HK354 [C3a], HK368 [C3c], HK390 [C5], HK349 [C5a], and HK328 [TCC]; Hycult Biotech), according to the manufacturer’s protocols. Detailed information is available in the Supplementary Methods. Interassay variation was determined by calculating the coefficient of variation for the quality control samples between assays runs. A coefficient of variation ≤15% was considered low variation. Concentrations of IL-6 were determined batchwise using enzyme-linked immunosorbent assays (Quantikine; R&D Systems), with a lower detection limit of 16 pg/mL.
Figure 1.
Simplified overview of the complement pathway. Activation of the complement system commences via the classical, lectin, and/or alternative pathway, resulting in the formation of C3 convertase, which cleaves C3 into C3a and C3b. C3b further degrades to C3c but also activates C5 convertase, which cleaves C5 into C5a and C5b. C5b combined with other complement factors forms the terminal complement complex (TCC) or membrane attack complex (MAC). C3a and C5a are anaphylatoxins and are inducers of inflammation and coagulation. TCC leads to cell lysis and can also activate the coagulation pathway. The complement factors measured in this study are highlighted with a black outline.
Statistical Analysis
Statistical analysis was performed using SPSS software, version 25.0 (IBM), and GraphPad Prism software, version 8.0 (GraphPad Software). Continuous data are represented as mean with standard deviation following criteria for normal distribution or as medians with interquartile range for nonnormally distributed variables. Nominal data are presented as numbers with percentages. Differences between groups (ICU and non-ICU) were assessed using Mann-Whitney U or Kruskal-Wallis tests for continuous variables and Fisher exact or χ 2 tests for discrete variables. Kinetics of complement factors were assessed using general mixed models on log-transformed data. Correlations between inflammatory markers and complement factors were assessed using Spearman rank correlation tests. Differences were considered statistically significant at P < .05 (2 tailed).
RESULTS
Patient Characteristics
Overall, we collected plasma samples of 197 patients with confirmed COVID-19. Of these, 75 patients were admitted to the ICU, and 115 patients were admitted to the clinical ward at the time of first sampling. Seven patients were initially admitted to the clinical ward but required ICU care during admission. Baseline samples (0–3 days after admission) were available from 122 of the 197 patients (87 of 115 non-ICU and 30 of 75 ICU patients). Table 1 shows that the COVID-19 ICU population had higher concentrations of inflammatory parameters (C-reactive protein [CRP], D-dimer, and IL-6) and a higher mortality rate (23% vs 9%) than the patients who did not require ICU care.
Complement Activation in COVID-19
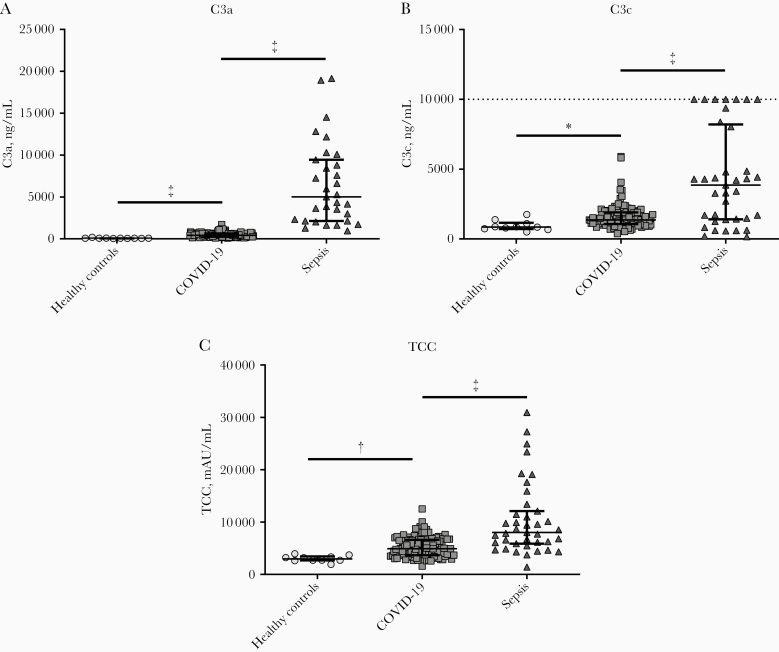
First, we investigated whether complement factor plasma concentrations differ between patients COVID-19, patients with sepsis, and healthy individuals at baseline. Activation of the complement system commences via the classical, lectin, and/or alternative pathway resulting in the formation of C3 convertase, which cleaves C3 into C3a and C3b. C3b further degrades to C3c but also activates C5 convertase, which cleaves C5 into C5a and C5b. C5b combined with other complement factors forms the TCC or membrane attack complex (MAC) (Figure 1). Because C3a, C3c, and TCC are the most stable complement factors, plasma concentrations of these complement factors were measured in healthy controls (n = 10), patients with COVID-19 at baseline (n = 122), and patients with sepsis (n = 39). All markers were significantly elevated in patients COVID-19 compared with healthy controls at baseline (P < .05 for C3a, C3c, and TCC; Figure 2). However, compared to patients with bacterial sepsis, the increase of these complement markers was less profound (P < .001, Figure 2). Moreover, concentrations of C3, C5, and C5a were measured in a subset of 10 healthy controls, 10 patients with COVID-19, and 9 patients with sepsis. C3 and C5 concentrations were significantly lower in patients with sepsis, but no differences were observed between patients with COVID-19 and healthy controls. C5a concentrations were below the detection limit (Supplementary Figure 1).
Figure 2.
Circulating concentrations of complement factors C3a (A), C3c (B), and terminal complement complex (TCC) (C) in healthy controls (n = 10), patients with coronavirus disease 2019 (COVID-19) (n = 122), and patients with sepsis (n = 39). Data are presented as medians with interquartile range. Dotted line in B represents upper detection limit. P values between all groups were <.001 for all complement factors. *P < .05; †P < .01; ‡P < .001.
Complement Activation Correlated With Disease Severity in Patients With COVID-19
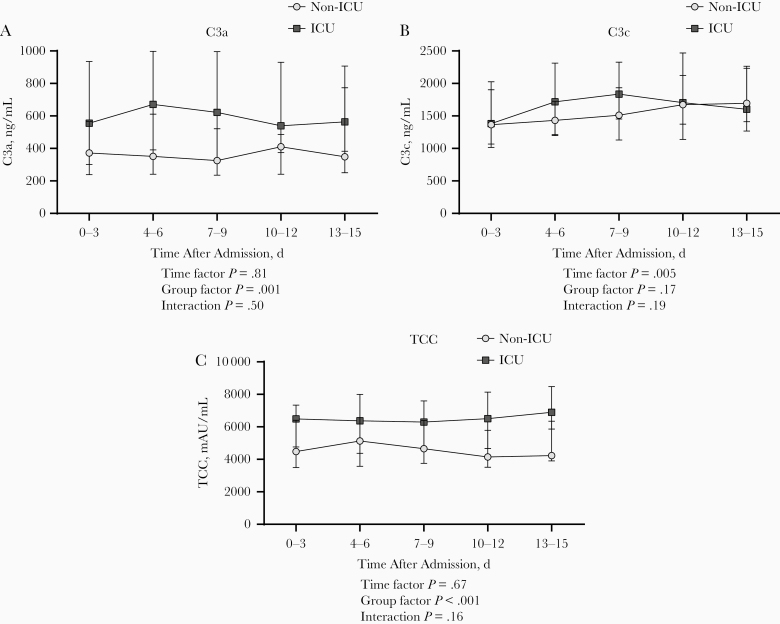
Next, we investigated whether complement factor plasma concentrations differed between ICU and non-ICU patients. The available baseline samples showed significantly higher C3a and TCC concentrations in ICU (n = 30) compared with non-ICU (n = 87) patients (P < .01; Supplementary Figure 2). In contrast, C3c plasma concentrations did not differ significantly between these groups. In addition, longitudinal data from the 115 non-ICU and 75 ICU patients showed significantly higher concentrations of C3a and TCC over time in ICU patients than in patients on the clinical wards (P = .001 and P < .001, respectively; Figure 3A and 3C). Complement activation markers were stable over time for both groups. Furthermore, longitudinal data from 7 patients who were transferred from the clinical ward to the ICU during hospital admission were aligned for day of ICU admission. Increased concentrations of complement markers were observed at the time of ICU admission for 5 patients (Supplementary Figure 3).
Figure 3.
Longitudinal course of C3a (A), C3c (B), and terminal complement complex (TCC) (C) plasma concentrations during hospital admission in non–intensive care unit (non-ICU) patients (n = 115) and ICU patients (n = 75) with coronavirus disease 2019. P values were calculated with general mixed model analyses on log-transformed data. Data are presented as medians with interquartile range.
Complement Activation and Mortality Rates
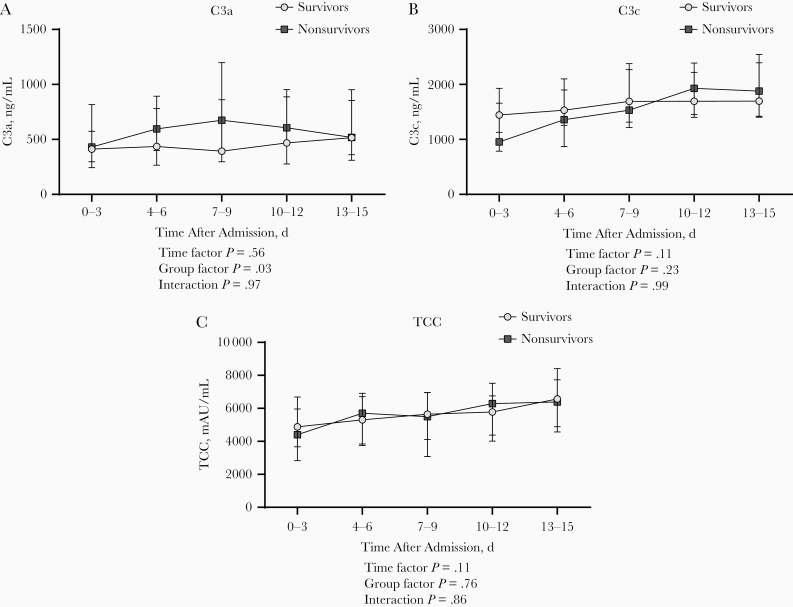
Subsequently, we assessed whether complement activation was correlated with mortality rates. The overall mortality rate was 14% (27 of 191), with rates of 9% in non-ICU (n = 10) and 23% in ICU (n = 16) patients. The available baseline samples in 107 survivors and 11 nonsurvivors showed no differences in concentrations for C3a and TCC, whereas concentrations of C3c were significantly lower in the nonsurvivors (P = .01; Supplementary Figure 4). Of note, we observed that nonsurvivors showed consistently higher concentrations of C3a over time. However, we did not observe the same pattern for the other complement factors (Figure 4A and 4C).
Figure 4.
Longitudinal course of C3a (A), C3c (B), and terminal complement complex (TCC) (C) plasma concentrations during hospital admission in patients with coronavirus disease 19 who survived (n = 164) and in those who died (n = 27). P values were calculated with general mixed model analyses on log-transformed data. Data are presented as medians with interquartile range.
Complement Activation in Patients With Thromboembolic Events
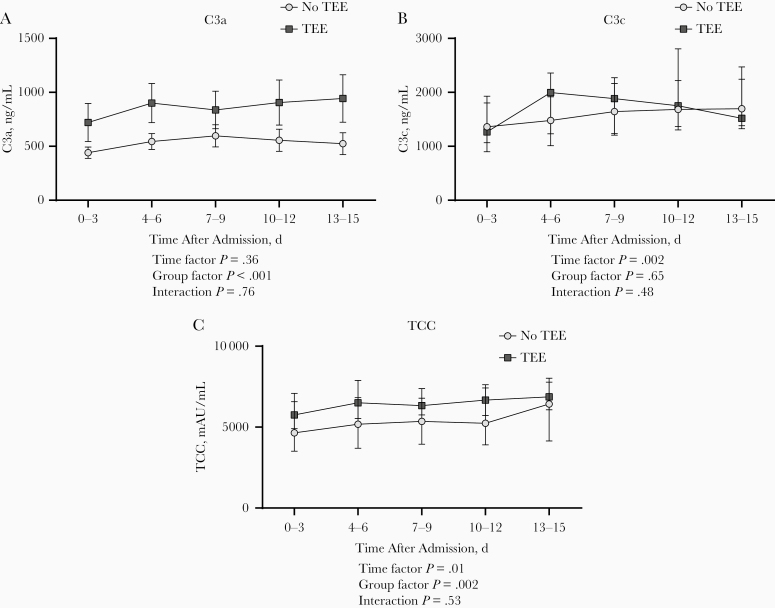
During admission, thromboembolic events were documented in 28 patients (14%); 27 of these patients were admitted to the ICU. Thromboembolic events included pulmonary embolisms (n = 26), cerebrovascular accidents (n = 2) and deep venous thrombosis (n = 1). Baseline samples showed higher concentrations of C3a and TCC in patients who experienced thromboembolic complications (P < .001 and P = .04, respectively; Supplementary Figure 5). Over time, a trend toward higher concentrations of complement markers C3a and TCC in patients with a thromboembolic event was observed (P < .001 and P = .002, respectively; Figure 5A and 5C).
Figure 5.
Longitudinal course of C3a (A), C3c (B), and terminal complement complex (TCC) (C) plasma concentrations during hospital admission in patients with coronavirus disease 2019 with (n = 28) or without (n = 169) thromboembolic events (TEEs). P values were calculated with general mixed model analyses on log-transformed data. Data are presented as medians with interquartile range.
Correlation Between Complement Activation and Other Inflammatory Markers in Patients With COVID-19
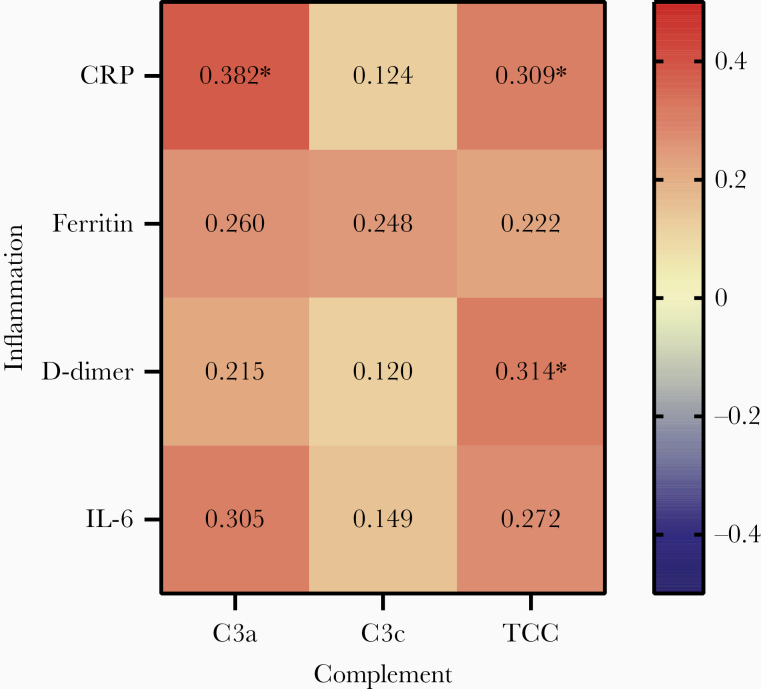
Next, we investigated the relationship between complement factors and inflammatory parameters measured within 3 days after admission. Overall, a trend toward a positive correlation between complement factors and inflammatory markers was observed. However, not all correlations were statistically significant (Figure 6). The strongest correlation was found for CRP (statistically significant for C3a and TCC). The single correlation plots are shown in Supplementary Figures 6, 7, and 8.
Figure 6.
Correlation of complement activation with inflammatory markers in patients with coronavirus disease 2019.. Correlation coefficients (r values, shown in the figure) and P values were calculated using the Spearman rank correlation test. *P < .004 (considered significant after Bonferroni correction for multiple testing). Abbreviations: CRP, C-reactive protein; IL-6, interleukin 6; TCC, terminal complement complex.
DISCUSSION
Complement overactivation plays an important role in the pathogenesis of many different diseases, including infections, renal, autoimmune and hematological diseases, and cancers [20]. The results of the present study demonstrated that the complement system is also activated in COVID-19, particularly in critically ill patients admitted to the ICU. Moreover, longitudinal analysis revealed increased concentrations of complement components over time, suggesting an important role of the complement system in pathophysiology of COVID-19. The continuous complement activation may contribute to tissue damage and the development of long-term complications in hospitalized patients with COVID-19.
The role of the complement system has been widely assessed, and increased activation was previously observed in severe infections, like bacterial sepsis [21]. In line with previous data, we observed profoundly elevated concentrations of C3a, C3c, and TCC in patients with bacterial septic shock in early stages of disease [22]. Compared with the hyperinflammatory state in bacterial sepsis, patients with COVID-19 showed a less profound increase in complement activation markers, which might imply a lower degree of inflammation. It was recently demonstrated that patients with COVID-19 and ARDS had lower levels of circulating cytokines than patients with bacterial sepsis, which suggest that COVID-19 may not be characterized by an excessive “cytokine storm” after all [23]. However, elevated circulating concentrations of complement factors were associated with disease severity (ie, ICU admission and potentially thromboembolic events) and possibly with mortality rates in COVID-19.
In addition, longitudinal data from patients who were transferred to the ICU during their hospital stay owing to disease deterioration demonstrated a possible trend toward elevated complement factors at the time of ICU admission. Unfortunately, in-hospital transfers to ICU occurred in only a very small number of patients in our study. The potential prognostic value of complement markers in this patient group needs further validation in a larger cohort. Collectively, our findings suggest that complement factors may be useful biomarkers of disease severity. In addition, its stable kinetics support the possibility that the complement system could be a useful therapeutic target in hospitalized patients with COVID-19.
In contrast to a previous study [24], we were not able to detect activation of the C5 axis in COVID-19. C5a is, next to C3a, a potent anaphylatoxin and acts as a strong activator of neutrophils, monocytes, and macrophages, leading to proinflammatory cytokine release and induction of inflammation. Given these properties, it is considered to be a promising target to prevent the development of many inflammatory diseases involving the complement system, such as sepsis, rheumatoid arthritis, inflammatory bowel disease, systemic lupus erythematosus, and psoriasis [18, 25–27].
However, the ability to detect these anaphylatoxins, especially C5a, is technically difficult owing to the presence of high-affinity receptors (C5aR) on circulating neutrophils. These receptors will bind C5a, resulting in its very short half-life of approximately 1 minute. C5a can be measured in samples only after C5aR saturation on leukocytes has occurred. Nevertheless, this does not imply that there is no C5a formation during a SARS-CoV-2 infection, as we demonstrated that TCC concentrations are significantly increased in patients COVID-19 compared with healthy controls. Complement activation will lead to cleavage of C5 into the split products C5a and C5b. In turn, TCC is composed of the C5b subunit together with C6, C7, C8, and several C9 molecules. To our knowledge, C5b-9 complex formation without simultaneous release of C5a has thus far never been demonstrated [28, 29].
Because activation of the complement system has a potent inflammatory effect, we assessed the relationship between inflammatory and complement markers. The correlations were clearly present, although not very strong. This may imply interaction of these 2 systems (inflammatory and complement) but also independent stimulatory and regulatory pathways that influence their concentrations.
Coagulation is affected in patients with COVID-19, as reflected by elevated concentrations of D-dimer and a high incidence of thromboembolic events [5, 30]. Our study demonstrated a 14% incidence of thromboembolic events, with increased concentrations of complement markers in these patients. In addition, our data demonstrated a positive correlation between TCC and D-dimer. The relationship between the complement and coagulation pathways has been recognized for many years [31, 32]. Both the end-product of the complement pathway, the C5b-9 complex (or TCC), and the anaphylatoxins C3a and C5a can stimulate the coagulation cascade via several processes. They may activate platelets [33, 34] and promote secretion of von Willebrand factor and P-selectin via activation of endothelial cells [35, 36], and they may increase tissue factor activity [37].
Others have hypothesized about the possible role of complement in the hypercoagulable state in patients with COVID-19, although studies assessing the direct interaction between the complement system and the coagulation pathway in COVID-19 are still lacking [38]. Our results suggest a role of complement in the onset of thromboembolic events in patients with COVID-19. This link might indicate potential effects of complement inhibition in reducing thromboembolic complications in these patients with COVID-19, possibly improving clinical outcomes. Further investigation regarding the pathophysiological mechanism and clinical benefits are still warranted.
The current study has several limitations. First, the study population is relatively small for assessing the role of complement activation on clinical outcomes, such as complications and death. In addition, with only 7 patients transferred to the ICU during their hospital stay, a comprehensive longitudinal analysis regarding parallelism of complement components and disease deterioration was not possible. Therefore, direct conclusions regarding causality cannot be drawn from our observational data. Second, a larger panel of complement factors and inhibitors may be needed to completely decipher the precise pathways through which the final route of complement activation is achieved. Finally, owing to the pragmatic design of this COVID-19 study and a substantial number of transfers from other hospitals, variation in sampling time points has occurred. Therefore, baseline samples were not available for all patients, and the total duration of follow-up varied. Furthermore, disease courses and treatment approaches differed between individual patients. These factors combined have led to heterogeneity in our data set.
Overall, many studies suggest that complement may serve as a potential target for anti-inflammatory treatment in patients with COVID-19 [24, 39–41]. Different components of the complement system could be targeted, because both inhibition of C3 and C5 cleavage have therapeutic potential for COVID-19 by reducing the formation of anaphylatoxins C3a and C5a and the end-product TCC. Moreover, the activation fragments C3a and C5a have robust proinflammatory effects, contributing to tissue damage, reported in COVID-19. Interestingly, Cugno et al [24] suggest the therapeutic use of C5 inhibitors based on the observation of elevated concentrations of C5a and TCC in patients with COVID-19. First results of anti-C5a treatment in patients are promising [15], and several randomized clinical trials targeting the C5 axis are underway (ClinicalTrials.gov NCT04288713, NCT04333420).
Considering that our data support a role for the C3 axis in complement activation in COVID-19 and that C3 acts upstream in the complement system, blocking C3 (preventing the formation not only of C5a but also of the proinflammatory factor C3a) might even be more effective. Indeed, 1 patient with COVID-19 was successfully treated with the anti-C3 agent AMY-101 [39], and a clinical trial will commence shortly (ClinicalTrials.gov NCT04395456). Our study has established the importance of complement activation, including the C3 axis, in COVID-19 and therefore underlines the potential of this trial. Moreover, it would be interesting to investigate the course of complement activation during this treatment, for exploration of potential biomarkers.
In conclusion, our study has shown that complement is activated in patients with COVID-19 and that complement activation correlates with disease severity. Assessment of complement activation markers might be of prognostic value as a monitoring tool for disease severity. Moreover, inhibition of C3 activation, in addition to C5, has potential to serve as a therapeutic strategy for COVID-19.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We would like to thank the entire RCI-COVID-19 study group: Martin Jaeger, Helga Dijkstra, Heidi Lemmers, Liesbeth van Emst, Kiki Schraa, Cor Jacobs, Anneke Hijmans, Trees Jansen, Fieke Weren, Liz Fransman, Jelle Gerretsen, Josephine van de Maat, Gerine Nijman, Simone Moorlag, Esther Taks, Priya Debisarun, Ilse Kouijzer, Heiman Wertheim, Joost Hopman, Janette Rahamat-Langendoen, Chantal Bleeker-Rovers, Jaap ten Oever, Reinout van Crevel, Jacobien Hoogerwerf, Quirijn de Mast, Hans van der Hoeven, Peter Pickkers, Matthijs Kox, Tim Frenzel, Jeroen Schouten, Pleun Hemelaar, Remi Beunders, Sjef van der Velde, Emma Kooistra, Nicole Waalders, Wout Claassen, Hidde Heesakkers, Tirsa van Schaik, Hetty van der Eng, Noortje Rovers, and Margreet Klop-Riehl, all affiliated with the Radboud Center for Infectious Diseases. We also thank Marijke Beenes and Sandra Leijtens for support in measuring complement components.
Financial support. This work was supported by the Netherlands Organization for Scientific Research (Vidi grant to F. L. v. d. V. and Spinoza grant to M. G. N.) and the European Research Council (advanced grant 833247 to M. G. N.).
Potential conflicts of interest. L. W. and E. J. M. T. are employees of Hycult Biotech. E. J. G. B. reports grants and personal fees from Abbott CH, Angelini, InflaRx, MSD Greece, XBiotech, BioMerieux, Thermo Fisher BRAHMS, Horizon 2020 Marie Curie European Sepsis Academy Innovative Training Network, and Horizon 2020 ImmunoSep. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
RCI-COVID-19 study group:
Martin Jaeger, Helga Dijkstra, Heidi Lemmers, Liesbeth van Emst, Kiki Schraa, Cor Jacobs, Anneke Hijmans, Trees Jansen, Fieke Weren, Liz Fransman, Jelle Gerretsen, Josephine van de Maat, Gerine Nijman, Simone Moorlag, Esther Taks, Priya Debisarun, Ilse Kouijzer, Heiman Wertheim, Joost Hopman, Janette Rahamat-Langendoen, Chantal Bleeker-Rovers, Jaap ten Oever, Reinout van Crevel, Jacobien Hoogerwerf, Quirijn de Mast, Hans van der Hoeven, Peter Pickkers, Matthijs Kox, Tim Frenzel, Jeroen Schouten, Pleun Hemelaar, Remi Beunders, Sjef van der Velde, Emma Kooistra, Nicole Waalders, Wout Claassen, Hidde Heesakkers, Tirsa van Schaik, Hetty van der Eng, Noortje Rovers, and Margreet Klop-Riehl
References
- 1. World Health Organization. Coronavirus disease (COVID-19) situation report—171 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200709-covid-19-sitrep-171.pdf?sfvrsn=9aba7ec7_2. Accessed 9 July 2020.
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 2020; 127:104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 2020; 7:e438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395:1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zumla A, Hui DS, Azhar EI, Memish ZA, Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet 2020; 395:e35–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gralinski LE, Sheahan TP, Morrison TE, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio 2018; 9:e01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Charchaflieh J, Rushbrook J, Worah S, Zhang M. Activated complement factors as disease markers for sepsis. Dis Markers 2015; 2015:382463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ren J, Zhao Y, Yuan Y, et al. Complement depletion deteriorates clinical outcomes of severe abdominal sepsis: a conspirator of infection and coagulopathy in crime? PLoS One 2012; 7:e47095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bosmann M, Ward PA. Role of C3, C5 and anaphylatoxin receptors in acute lung injury and in sepsis. Adv Exp Med Biol 2012; 946:147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang R, Xiao H, Guo R, Li Y, Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect 2015; 4:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang Y, Zhao G, Song N, et al. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect 2018; 7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao T, Hu M, Zhang X, et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv [Preprint: not peer reviewed]. 18 June 2020. Available from: 10.1101/2020.03.29.20041962. [DOI] [Google Scholar]
- 16. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020; 220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prokop M, van Everdingen W, van Rees Vellinga T, et al. ; COVID-19 Standardized Reporting Working Group of the Dutch Radiological Society CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology 2020; 296:E97–E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prohászka Z, Nilsson B, Frazer-Abel A, Kirschfink M. Complement analysis 2016: clinical indications, laboratory diagnostics and quality control. Immunobiology 2016; 221:1247–58. [DOI] [PubMed] [Google Scholar]
- 19. Nilsson B, Ekdahl KN. Complement diagnostics: concepts, indications, and practical guidelines. Clin Dev Immunol 2012; 2012:962702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carroll MV, Sim RB. Complement in health and disease. Adv Drug Deliv Rev 2011; 63:965–75. [DOI] [PubMed] [Google Scholar]
- 21. Markiewski MM, DeAngelis RA, Lambris JD. Complexity of complement activation in sepsis. J Cell Mol Med 2008; 12:2245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charchaflieh J, Wei J, Labaze G, et al. The role of complement system in septic shock. Clin Dev Immunol 2012; 2012:407324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. JAMA 2020; 1565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cugno M, Meroni PL, Gualtierotti R, et al. Complement activation in patients with COVID-19: a novel therapeutic target. J Allergy Clin Immunol 2020; 146:215–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ward PA The harmful role of C5a on innate immunity in sepsis. J Innate Immun 2010; 2:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reis ES, Mastellos DC, Hajishengallis G, Lambris JD. New insights into the immune functions of complement. Nat Rev Immunol 2019; 19:503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mastellos DC, Ricklin D, Lambris JD. Clinical promise of next-generation complement therapeutics. Nat Rev Drug Discov 2019; 18:707–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krisinger MJ, Goebeler V, Lu Z, et al. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood 2012; 120:1717–25. [DOI] [PubMed] [Google Scholar]
- 29. Nilsson PH, Thomas AM, Bergseth G, et al. Eculizumab-C5 complexes express a C5a neoepitope in vivo: consequences for interpretation of patient complement analyses. Mol Immunol 2017; 89:111–4. [DOI] [PubMed] [Google Scholar]
- 30. Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020; 191:148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conway EM Complement-coagulation connections. Blood Coagul Fibrinolysis 2018; 29:243–51. [DOI] [PubMed] [Google Scholar]
- 32. Foley JH Examining coagulation-complement crosstalk: complement activation and thrombosis. Thromb Res 2016; 141suppl 2:S50–4. [DOI] [PubMed] [Google Scholar]
- 33. Martel C, Cointe S, Maurice P, et al. Requirements for membrane attack complex formation and anaphylatoxins binding to collagen-activated platelets. PLoS One 2011; 6:e18812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wiedmer T, Esmon CT, Sims PJ. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood 1986; 68:875–80. [PubMed] [Google Scholar]
- 35. Hattori R, Hamilton KK, McEver RP, Sims PJ. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem 1989; 264:9053–60. [PubMed] [Google Scholar]
- 36. Foreman KE, Vaporciyan AA, Bonish BK, et al. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest 1994; 94:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost 1997; 77:394–8. [PubMed] [Google Scholar]
- 38. Fletcher-Sandersjöö A, Bellander BM. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? a literature review. Thromb Res 2020; 194:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mastaglio S, Ruggeri A, Risitano AM, et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol 2020; 215:108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Risitano AM, Mastellos DC, Huber-Lang M, et al. Complement as a target in COVID-19? Nat Rev Immunol 2020; 20:343–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int 2020; 98:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.