Abstract
Objectives
Workplace‐based selective prevention of mental health problems currently relies on subjective evaluation of stress complaints. Hair cortisol captures chronic stress responses and could be a promising biomarker for the early identification of mental health problems. The objective was to provide an overview of the state‐of‐the‐art knowledge on the practical value of hair cortisol in the occupational setting.
Methods
We performed a scoping review of cross‐sectional and longitudinal studies in PubMed, Embase, and PsycINFO up to November 2019 assessing the relations of hair cortisol with work‐related stressors, perceived stress, and mental health outcomes in healthy workers.
Results
We found five longitudinal studies, of which two observed an increase in work‐related stressors to be associated with higher hair cortisol, one found a relation with lower hair cortisol and one did not find a relationship. Findings of cross‐sectional studies were also mixed. The one available longitudinal study regarding mental health showed that hair cortisol was not related to depressive symptoms.
Conclusions
Hair cortisol measurement within occupational health research is still in its early stage and more longitudinal studies are urgently needed to clarify its relationship with work‐related stressors and perceived stress before hair cortisol can be used to identify workers at risk for mental health problems.
Keywords: disease prevention, glucocorticoids, mental stress, work related
1. INTRODUCTION
Chronic stress has adverse effects on many aspects of human development and health, among which the developing human brain, 1 cardiovascular, 2 and mental health. 3 , 4 Chronic stress is also a threat to the mental health and well‐being of the working population. 5 , 6 Various mental and physical stressors can cause chronic stress in workers, these can be either work‐related, related to the private or personal life of the worker, or both. Workplaces are not only a potential source of stress, they also provide a vital context in which to both protect and promote the health of workers. The workplace has been identified as a platform to reach the population with preventive programs. 7 Workers constitute a large proportion of the population and they can be reached through the facilities already in place at the workplace, such as occupational health services and Employee Assistance Programs. Preventive strategies for chronic stress at workplaces can target workers at three levels, all workers (universal prevention), workers at high risk of developing a mental health problems (selective prevention), and workers with a current mental health problem (indicated prevention). Various interventions are available directed at either improving the psychosocial aspects of work in a universal prevention strategy, 8 reducing the negative impact of stress as part of selective prevention, 9 , 10 , 11 or improving work outcomes in workers with mental health problems, constituting an indicated prevention strategy. 12 , 13 , 14
Identification of workers who have a high risk of experiencing chronic stress with the associated (mental) health consequences, is a key part of workplace‐based selective prevention. Current prevention programs rely on the subjective evaluation of work or other stressors and stress complaints by workers with most of the studies using self‐report instruments. These instruments are not equipped to measure unconscious exposure to stressors or the effects of mental stress to the body. Moreover, exposure to stressors is measured at one point in time, 15 , 16 , 17 whereas sustained exposure is likely most harmful to the (mental) health of workers. 4 Epidemiological studies looking at the health effects of cumulative exposure to work stressors are still rare. 15 , 16 , 17
In search for biomarkers of stress, cortisol has been at the focus of attention of researchers, including those in the field of occupational health. In response to a mental or physical stressor, cortisol is involved in energy mobilization by stimulating the production of glucose and the mobilization and brake‐down of fatty acids. Cortisol is also involved in the suppression of the immune‐system via the inhibition of pro‐inflammatory cytokines. 18 , 19 This bodily response of cortisol release in response to stressors is within seconds but not as quick as the response via the autonomous nervous system. The hypothesis is that when the stressor remains or is repeatedly present and the body develops a chronic response to stressors, these responses can become harmful to long‐term health. 20 Studies in occupational health have most often used cortisol in saliva, blood, and urine to examine the link between work stressors and stress reactions. 21 However, these studies either focused on exposure to working conditions that disrupt the biorhythm of workers, such as shift work. 22 Or they were only able to capture short‐term stress responses in reaction to work stressors, as saliva and blood samples represent cortisol concentrations from 20 minutes to several hours before the samples were taken. 23
Advances in the field of stress biomarkers now allow for hair cortisol concentrations (HCC) to provide an easily obtainable index of cortisol levels over a period of several months using the predictable growth rate of hair. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 The test‐retest reliability of HCC within individuals is considered good, 24 , 34 although the more distant parts of the hair seem less reliable. HCC can be determined using various techniques, such as immunoassay ELISA or LC‐MC/MS methods 35 , 36 with both type of analytical methods being well correlated. 37 Although cut off values for HCC have not yet been determined, reference values in healthy people using different analysis techniques have been established. 37 , 38 Promising results have been found, showing a relationship between exposure to stressors and an increase in hair cortisol, 20 and also linking hair cortisol to cardiovascular disease. 39 , 40 , 41 Early identification of increased cortisol could become an important starting point for occupational health selective prevention programs. Nevertheless, several unanswered questions currently preclude the use of hair cortisol in workplace‐based prevention. Evidence for the reliability and feasibility of HCC measurements have now been established albeit not yet particularly within an occupational health setting. Evidence of associations between HCC measurements and work‐related mental stress is now developing.
Scoping reviews are a way of mapping the key concepts that underpin a research area, and they can be particularly useful for bringing together literature in disciplines with emerging evidence. 42 , 43 As the research on cortisol and the relation with biological and psychological stress is still in its early stages and comes from various research fields, the current scoping review aims to deepen the understanding of the potential of measuring HCC to identify high‐risk workers for preventive interventions. In this review, the state‐of‐the‐art knowledge on the value of HCC for occupational healthcare is explored and the types of available evidence are identified for three situations relevant in occupational healthcare:
What evidence is available on the potential use of HCC as:
an indicator of exposure to unfavorable working conditions, that is, what is known on the association between measurement of HCCs and work‐related stressors?
an indicator of current stress reactions in workers, that is, what is known on the association between measurement of HCCs and perceived (work‐related) stress?
a predictor of future common stress‐related health problems, that is, what is known on the predictive value of measuring HCC for depression, anxiety, and cardiovascular disorders?
2. METHODS
For the design of our scoping review, we followed the guidelines as suggested by Peters et al 43 and Arksey et al 44 and we followed the PRISMA‐ScR standard for our reporting. 45 We provide an overview of the existing literature to explore the state‐of the art with regard to HCC and its potential for use in an occupational health setting. We did not register the protocol of our scoping review.
2.1. Search strategy
Two authors (JO, MdJ) independently searched the literature in PubMed, Embase, and PsycINFO up to November 2019. Thereafter, we used a snowball for other potentially relevant studies. As the amount of literature on hair cortisol is still rather limited we decided to use a broad search strategy to answer our questions. We used the following search terms (including synonyms and closely related words) as index terms or free‐text words: (a) cortisol, (b) hair, and (c) stress, depression, anxiety, well‐being, burn‐out, demand, cardiovascular disease, or mortality (see Appendix S1 for details).
2.2. Inclusion criteria
Three authors (FS, GH, and KN) examined the full‐text articles independently to check if the study met the inclusion criteria. Any disagreements that arose were resolved via discussion. Inclusion criteria for all three research questions were as follows: studies reporting on hair cortisol in a working population, written in English or Dutch. For research question one, the association with work‐related stressor was an inclusion criterion. For research question two, an inclusion criterion was the association with stress complaints. For research question three, an inclusion criterion was that the study reported on either depressive, anxiety, or cardiovascular disorders. For the first two research questions, we included both cross‐sectional and longitudinal studies, for the third research question we only included longitudinal studies. Exclusion criteria for all three research questions were a non‐healthy or pregnant population (at baseline), and studies dealing with non‐working populations such as students or informal caregiving.
2.3. Charting and summarizing the results
Information of studies on the potential association between HCC and work‐related stressors or perceived stress were charted according to key study characteristics, that is, study design, population, gender, ethnicity, age, sample size, hair analysis technique, and adjustment procedures. In addition, the results of the studies were synthesized according to a positive, negative or no association.
3. RESULTS
Figure 1 presents the details of the number of studies identified at each stage of the searching process that were used to answer the questions for this scoping review. We found 22 individual studies, reported on in 25 publications, that investigated the association between hair cortisol and work‐related stressors or perceived stress. There were five longitudinal cohort studies and 17 cross‐sectional studies. We found only one study that investigated the association between hair cortisol and future health problems in the working population.
FIGURE 1.
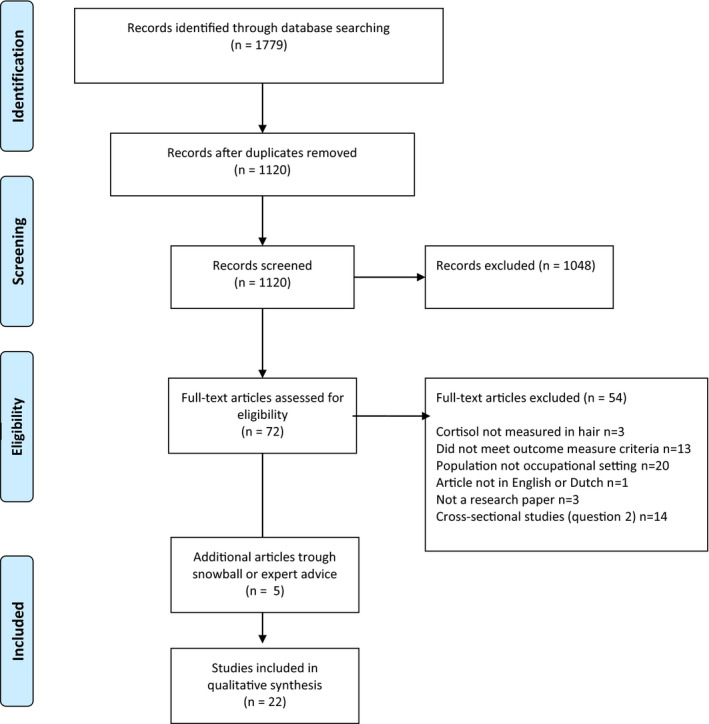
PRISMA flow diagram
3.1. Hair cortisol and work‐related stressors
Hair cortisol concentration in relation to effort‐reward imbalance has been studied in four longitudinal 46 , 47 , 48 , 49 and two cross‐sectional studies 23 , 50 (Tables 1 and 2). Demand‐decision latitude has been studied in five cross‐sectional studies. 50 , 51 , 52 , 53 , 54 One study among 132 UK employees in the public sector observed no association between effort‐reward imbalance and HCC after 3 months follow‐up. 46 Herr et al published two longitudinal studies among men of a metal manufacturing plant and observed in one study among 40 employees with relatively low levels of effort‐reward imbalance that an increase in effort‐reward imbalance was associated with an increase in HCC over a 1‐year follow‐up period. 48 Effort‐reward imbalance was not associated with HCC after 3‐year follow‐up among 66 employees in the Herr et al study. 47 In contrast, another longitudinal study, 49 found an increase in effort‐reward imbalance over 2 years to be associated with a decrease in HCC over time in a sample of 150 workers from various workplaces.
TABLE 1.
Study characteristics of included studies on the associations between hair cortisol and work‐related stressors and perceived stress
| Reference | Design | Duration follow‐up |
Focus Work stressors |
Focus Perceived stress |
Focus Health outcomes |
Population (workplace, country) | Gender (% women) | Age (mean nr yr) and SD | Sample size | Hair analysis technique | Age‐, gender, ethnicity corrected |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boschi et al 2017 67 | CS | NA | The Calgary Symptoms of Stress Inventory |
US England, Norway, Switzerland Private sector organizations |
64% | 41 (9) | 100 | 1‐6 cm strands from posterior vertex. Methanol extracts using a radioimmunoassay | No | ||
| Dettenborn et al 2010 57 | CS | NA | Trier Inventory for the Assessment of Chronic Stress |
Germany Employed versus unemployed |
Employed (57%); unemployed (97%) | Employed: 33 (9); unemployed: 37 (11) | 59 | 3 cm strands from posterior vertex. Immunoassay with chemiluminescence detection | No | ||
| Faresjö et al 2014 62 | CS | NA | Perceived Stress Scale (PSS) |
Sweden Nurses and librarians |
100% | 46 (12) | 112 | 3 cm strands from posterior vertex. Methanol extracts using a radioimmunoassay | Yes (not ethnicity) | ||
| Gidlow et al 2016 46 | LCS | 3 mo | Effort‐Reward Imbalance (ERI) | PSS |
UK Employees of large public sector employers |
81% | 41 (11) | 132 | At least 3 cm strands from posterior vertex. Methanol extracts using ELISA | Yes (not ethnicity) | |
| Hall et al 2018 54 | CS | NA | Job Content Questionnaire (JCQ) | US | 96% | 43 | 106 | 3 cm strands from posterior vertex. Sensitivity enzyme immunoassy kit | No Correlation | ||
| a Herr et al 2018 48 | LCS | 1 yr | ERI |
Germany Metal manufacturing plant |
0% | 48 (6) | 40 | 3 cm strands from posterior vertex. Online solid phase extraction. Liquid chromatography‐tandem mass spectrometry method |
Yes (not ethnicity) |
||
| a Herr et al 2017 47 | LCS | 3 yr | ERI |
Germany Metal manufacturing plant |
0% | 41 (7) | 66 | 3 cm strands from posterior vertex. Online solid phase extraction. Liquid chromatography‐tandem mass spectrometry method | Yes (not ethnicity) | ||
| Janssens et al 2017 53 | CS | NA | JCQ + Copenhagen Psychosocial Questionnaire |
Belgium Production companies |
41% | 43 (10) | 102 | 2‐3 cm strand at vertex posterior. Methanol extracted samples analysed with liquid chromatography tandem mass spectrometry | No | ||
| Kind et al 2018 55 | LCS | 11 mo | Survey about personal boundary violations at the workplace |
Switzerland Caregivers youth residential care |
62% | 23‐61 | 121 | 1.5 cm strands from posterior vertex region. High‐sensitivity cortisol enzyme immunoassay kit | Yes (not ethnicity | ||
| Manenschijn et al 2011 56 | CS | NA | Shift work |
Netherlands Textile company |
0% | Shift workers: 41; day workers: 33 | 122 | 3 cm strand from posterior cortex. ELISA cortisol kit | Yes (not ethnicity) | ||
| Mayer et al 2018 60 , 68 | LCS | 13‐14 mo | PSS | Patient Health Questionnaire (PHQ‐9) |
US Medical Interns |
56% | 25‐33 | 74 | 2 cm strands from posterior vertex. Immunoassay with chemiluminescent detection |
No Within‐person analysis |
|
| Mclennan et al 2016 51 | CS | NA | Maslach Burnout Inventory (MBI) | SF Health Questionnaire |
Germany Nurses geriatric care institutions |
90% | 42 (11) | 246 | 3 mm diameter strands near posterior cortex of 3 cm. Immunoassay with chemiluminescence detection kit |
No Bivariate correlation |
|
| O‐ Brien et al 2013 64 | CS | NA | PSS |
US University staff |
65% | 30 (13) | 135 | 100 strands (3 cm) from the posterior vertex of the head. Enzyme immunoassay | No | ||
| Penz et al 2018 61 | CS | NA | MBI |
Germany/Sweden Workers |
84% | 42 (11) | 314 | 3 cm strands from posterior vertex. Liquid chromatography coupled with tandem mass spectrometry (LC‐MS/MS) | Yes (not ethnicity) | ||
| Penz et al 2019 49 | LCS | 2 yr | ERI |
Germany Workers |
65% | 42 (11) | 150 | 3 cm strands from posterior vertex. LC‐MS/MS | Yes (not ethnicity) | ||
| a Qi et al 2014 23 | CS | NA | ERI |
China Kindergarten teachers |
100% | Median 27, (IQR 24‐30) | 39 | 1 mm diameter hairstrands near posterior cortex of 3 cm. methanol extracted sample analyzed with 3200 QTRAP liquid chromatography tandem mass spectrometer | No | ||
| a Qi et al 2015 52 | CS | NA | JCQ | Need for Recovery (NFR) |
China Kindergarten teachers |
100% | 28 (6) | 43 | 1 mm diameter hairstrands near posterior cortex of 3 cm. Methanol extracted sample analyzed with 3200 QTRAP liquid chromatography tandem mass spectrometer | No | |
| a Qi et al 2017 59 | CS | NA |
Emotional Labor Scale Stressful Life Events Scale |
China Kindergarten teachers |
100% | 28 (6) | 43 | 1 mm diameter hairstrands near posterior cortex of 3 cm. methanol extracted sample analyzed with 3200 QTRAP liquid chromatography tandem mass spectrometer | No | ||
| Rowold et al 2017 58 | CS | NA | Transformational Leadership Inventory |
Germany |
50% | 33 (12) | 131 | 3 cm strand from posterior cortex | No | ||
| Van der Meij et al 2018 50 | CS | NA |
JCQ ERI Questionnaire |
Netherlands |
66% | 39 (12) | 172 | 3 cm strand from to posterior cortex. LC‐MS/MS | Yes (not ethnicity) | ||
| Van Holland et al 2012 63 | CS | NA |
Stress Screener NFR |
Netherlands Meat‐processing industry |
19% | 46 (10) | 29 | 3 cm strand from to posterior cortex | No (correlation) | ||
| Wang et al 2019 66 | CS | NA | MBI |
China Hospitals |
100% |
< 30: n = 22 30‐35: n = 20 >35 n = 26 |
68 | 3 cm strand from occipital position | Yes (not ethnicity) | ||
| Wells et al 2014 65 | CS | NA | PSS |
Canada sample with over‐representation of persons who have mental health, substance use/addiction and violence problems. |
72% | 42 (16) | 324 | 3 cm strand from to posterior cortex. Analyzed on a salivary ELISA kit manufactured by Alpco Diagnostics for quantification | Yes (not ethnicity) |
Abbreviations: CS, cross‐sectional; LCS, longitudinal cohort study; SD, standard deviation.
The Qi publications from 2014, 2015, and 2017 are from the same study, as are the Herr 2017 and 2018 publications.
TABLE 2.
Synthesis of studies investigating the associations between hair cortisol and work‐related stressors and perceived stress
|
+ Higher HCC related to poorer stressor/stress outcome |
0 No significant effect |
‐ Higher HCC related to better stressor/stress outcome |
|
|---|---|---|---|
| Work‐related stressors | |||
| Effort‐reward imbalance | Herr et al 2018 48 ; Qi et al 2014 23 ; Van der Meij et al 2018 50 | Gidlow et al 2016 46 ; Herr et al 2017 47 | Penz et al 2019 49 |
| Demand‐decision latitude | Hall et al 2018 54 | Mclennan et al 2016 51 ; Van der Meij et al 2018 50 ; Qi 2015 52 ; Janssens et al 2017 53 | |
| Shift work | Manenschijn et al 2011 56 (<40 yr) | Manenschijn et al 2011 56 (≥40 yr) | Janssens et al 2017 53 |
| Unemployment | Dettenborn et al 2010 57 | ||
| Verbal and physical aggression | Kind et al 2018 55 | ||
| Surface learning | Qi et al 2017 59 | ||
| Deep learning | Qi et al 2017 59 | ||
| Transformational leadership | Rowold et al 2017 58 | ||
| Instrumental leadership | Rowold et al 2017 58 | ||
| Perceived stress | |||
| Burn‐out | Penz et al 2018 61 (dichotomous outcome); Wang et al 2019 66 (exhaustion & depersonalization) | Mclennan et al 2016 51 (exhaustion & depersonalization); Penz et al 2018 61 (continuous outcome); Wang et al 2019 66 (personal accomplishment) | Mclennan et al 2016 51 (personal accomplishment) |
| Perceived stress | Faresjö et al 2014 62 ; Mayer et al 2018 60 ; O‐Brien et al 2013 64 ; Wells et al 2014 65 | Gidlow et al 2016 46 ; Van Holland et al 2012 63 | |
| Need for recovery | Van Holland et al 2012 63 | Qi et al 2015 52 | |
| Cognitive disorganization | Boschi et al 2017 67 | ||
| Health outcomes | |||
| Depression | Mayer et al 2018 68 | ||
Longitudinal studies are highlighted in bold.
Both cross‐sectional studies observed that higher HCC were significantly associated with higher effort‐reward balance. 23 , 50 HCC were not associated with factors from the demand‐decision latitude model in four available cross‐sectional studies, 50 , 51 , 52 , 53 but HCC were positively associated with iso‐strain (high demand, low control, and low social support) in a cross‐sectional study. 54
Hair cortisol concentration have been studied in relation to various work stressors in one longitudinal study 55 and five cross‐sectional studies 53 , 56 , 57 , 58 , 59 (Tables 1 and 2). A longitudinal study 55 showed that Swiss youth residential caregivers (N = 121) who experienced verbal or verbal and physical aggression had a 1.6 times higher risk of high HCC after 13‐14 months of follow‐up compared to those who experienced no aggression. A Dutch study observed that compared to day workers, shift workers aged <40 years had higher HCC, 56 whereas a Belgium study observed lower HCC levels in shift workers, 53 although the latter study did not correct for the common use of (local) corticosteroids, known to decrease HCC. A small cross‐sectional German study comparing employed and unemployed individuals found that the latter had higher HCC. 57 In addition, in a German cross‐sectional study, instrumental leadership (focused on results, not on relationships) was negatively associated with HCC but transformational leadership (focused on change in tandem with committed members of a group) was not. 58
Finally, high surface acting (ie, modifying affective displays by suppressing their felt emotions) was correlated with high HCC among kindergarten teachers in China. 59
Overall, evidence for an association between HCC and work‐related stressors from longitudinal studies is inconsistent as three studies found a positive association 48 , 55 , 60 and two did not 46 , 47 (Tables 1 and 2). Cross‐sectional studies also show inconsistent results for effort‐reward imbalance and demand‐decision latitude, whereas HCC were positively associated with shift work, aggression, type of leadership, and type of emotional labor in single studies.
3.2. Hair cortisol and perceived work‐related stress
Two longitudinal 46 , 60 and seven cross‐sectional studies 51 , 61 , 62 , 63 , 64 , 65 , 66 investigated the associations between HCC and burnout or perceived stress (Tables 1 and 2). Among 74 medical interns an increase in HCC was significantly correlated with an increase in perceived stress over 1‐year period. 60 In contrast, another longitudinal study observed no significant association between HCC and perceived stress among UK employees in the public sector over a 3‐month follow‐up period. 46 The cross‐sectional studies also showed mixed results, with some studies finding a positive association between HCC and burn‐out 61 , 66 and HCC and perceived stress, 62 , 64 , 65 and other studies finding no association with burn‐out 51 or perceived stress. 63 Two cross‐sectional studies looked at the association between Need for Recovery and HCC, with one study finding no significant association 63 and one finding a significant correlation of HCC with a favorable outcome, a lower Need for Recovery. 52 Finally, one cross‐sectional looked at the stress symptom “cognitive disorganization,” but found no significant relation with HCC. 67
3.3. Predictive value of HCC
There is a lack of longitudinal studies on the relationship between HCC and health outcomes (Tables 1 and 2). The only available longitudinal study 68 showed an increase in HCC and depressive symptoms in medical interns, but the increase in HCC and depressive symptoms were not related to each other.
4. DISCUSSION
4.1. Main results
With this scoping review, we sought for state‐of‐the‐art knowledge on the practical value of HCC within the occupational health context. We found that only a limited number of longitudinal studies were conducted on the relationship between HCC and work‐related stress or stressors. The findings across studies were inconsistent. Most striking was the inconsistency in findings of longitudinal studies of the work stressor Effort‐Reward Imbalance and HCC; The Herr 2018 study found a statistical significant relation of higher HCC related to more imbalance, whereas the Penz 2019 study found a statistical significant relation of higher HCC and less imbalance. And the Herr 2017 and Gidlow 2017 studies both found no statistical significant relation between Effort‐Reward Imbalance and HCC. The inconsistent findings may be partly attributed to the sample sizes and selection bias; both Herr studies had less than 70 participants, primarily men and all from one workplace who had participated in a stress reduction program. While the Gidlow 2017 and the Penz 2019 studies both had larger sample sizes (n = 153 and n = 150, respectively), the Penz 2019 included heterogeneous sample, but the Gidlow 2017 only included workers from two workplaces.
They further used different longitudinal designs. The Gidlow study measured Effort‐Reward Imbalance at baseline and HCC 3 months later, whereas the Penz study measured both Effort‐Reward Imbalance and HCC at baseline, after one and after 2 years of follow‐up. In sum, the status of HCC as an indicator of unfavorable working conditions and of subsequent stress reactions has not been clarified in the currently available literature. We further only found one longitudinal study investigating the relationship between HCC and mental health. This study showed that an increase in HCC was not related to an increase in depressive symptoms. Therefore, the available evidence on HCC as a predictor of future health problems is also limited.
4.2. Comparison with the literature
Occupational healthcare has been focusing more and more on preventing work‐related mental stress. 69 Not only is too much exposure to work stressors related to mental health issues, 70 , 71 there is also a substantial amount of literature linking work stressors, such as job strain and long working hours, to an elevated risk of incident coronary heart disease and stroke. 72 In the majority of studies perceived work stress is measured with questionnaires based on different stress models, such as job demands and control or effort‐rewards imbalance. The prevention of work‐related stress and its consequences could be enhanced by insights on the interplay of work stressors, psychological, and biological processes. However, our findings show that the body of evidence linking HCC to work stressors and stress responses is not sufficient to unravel the combined psychological and biological pathway of work stressors leading to health outcomes. Besides the potential association between mental stress and HCC, the recent interest of occupational healthcare research in linking HCC with other occupational risk factors, such as shift work, highlights the need for more longitudinal studies on HCC within the occupational setting. 28 For now, the practical value of HCC measurements in occupational health is limited. Compared to a meta‐analysis of HCC associations with a wide range of outcomes conducted 3 years earlier than this review, 20 the state of knowledge relevant to occupational health has not substantially increased. In that analysis, only ongoing stress was found to be related to HCC, but no further associations with future stress‐related symptoms or mental disorders were investigated.
In other medical domains, however, the more biological pathway of stressors such a hormonal imbalance due to Cushing Syndrome leading to abdominal obesity and many other health problems, or the relation between stressful life‐events, HCC and psychiatric illness has already been an important topic for researchers. 39 , 73 There indeed seems to be a relationship between hormonal changes or imbalance and disease, but to what extent these hormonal changes can also predict long‐term disease still needs further study. 74 The question remains whether psychological stressors, such as high work load and unperceived stress, 73 can be measured with HCC, and to what extent this measurement is predictive for future disease and related sick leave. We found many small cross‐sectional studies for the assessment of work stressors and perceived work stress. As perceived work stress is not yet standardized with one clear cut‐off level using one particular model integrated into one questionnaire accepted by all, correlating perceived work stress with HCC will remain challenging for future research. Furthermore, the temporal relationship between work stressors and HCC may not be linear, with the possibility of differential effects on cortisol secretion related to short term versus chronic stressors. 75
We suggest a large longitudinal study design using various types of workers and combining both biological and psychological measurements for more understanding of the potential added value of HCC in an occupational context. The first step should be assessing acceptability for workers to have HCC routinely measured as a marker for both perceived as unperceived stress, although large‐scale studies in both population‐based cohorts as well as pediatric or patient cohorts show the feasibility of hair analysis. 30 The next step should be to establish cut‐off scores for those with prolonged high work stress based on accepted standardized measurements for perceived work stress. The last step should then be to assess whether prolonged high HCC is related to an increased risk of disease in the future.
4.3. Methodological considerations
This scoping review used the methodological framework as suggested 43 , 44 to achieve in‐depth and broad results to explore the value of HCC for occupational healthcare. The mapping of the data within this scoping review had the focus of assessing the value of HCC for occupational healthcare. Using this methodology was helpful to identify the available evidence of HCC from the perspective of the use of HCC in occupational health. However, the choice to conduct a scoping review entails that we did not generate a critically appraised and synthesized answer to a strictly defined research question. As we identified 25 papers, one might argue that a systematic review would have been more appropriate. However, these 25 papers covered three separate relationships of HCC, with stressors, stress responses, and health outcomes. And we identified no more than five longitudinal studies. Given the current state of the art, systematic reviews on HCC as an indicator of exposure to stressors and current stress reactions, and as a predictor of future stress‐related health problems should be performed after longitudinal primary studies in large, heterogeneous sample sizes have been conducted with repeated measures of both HCC and the predictor of interest over a longer period of time.
4.4. Conclusion and implications
After having reviewed the literature relevant to the application of hair cortisol measurement in an occupational health setting, we conclude that several prerequisites to such application in practice have not yet been met. Prospective studies in working populations are needed to first show which (duration of) high HCC levels are related to mental and physical health problems. Moreover, to guide preventive efforts in the occupational health setting, the relationship between HCC levels and exposure to perceived work stressors needs to be more clear.
DISCLOSURES
Approval of the research protocol: N/A. Informed consent: N/A. Registry and the registration no. of the study: N/A. Animal studies: N/A. Conflict of interest: None declared.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to this article. FS and KN designed the study, analyzed and interpreted the data, and wrote the manuscript. GH helped with fine‐tuning the design of the study, analyzed and interpreted the data, and contributed to the writing of the manuscript. MJ and JO searched the literature and screened for relevant articles and contributed to writing of the manuscript. EvR advised on the interpretation of the validity of HCC measurements and contributed to the writing of the manuscript. Additionally, all authors approve this version to be published.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We received funding from the Amsterdam Public Health research institute for this research project. EFCvR is supported by a Vidi grant from the Netherlands Organisation for Scientific Research NWO (grant number: 91716453).
Schaafsma FG, Hulsegge G, de Jong MA, Overvliet J, van Rossum EFC, Nieuwenhuijsen K. The potential of using hair cortisol to measure chronic stress in occupational healthcare; a scoping review. J Occup Health. 2021;63:e12189 10.1002/1348-9585.12189
REFERENCES
- 1. Lupien SJ, Juster RP, Raymond C, Marin MF. The effects of chronic stress on the human brain: from neurotoxicity, to vulnerability, to opportunity. Front Neuroendocrinol. 2018;49:91‐105. [DOI] [PubMed] [Google Scholar]
- 2. Steptoe A, Kivimaki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health. 2013;34:337‐354. [DOI] [PubMed] [Google Scholar]
- 3. Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38(8):1220‐1235. [DOI] [PubMed] [Google Scholar]
- 4. Vinkers CH, Joels M, Milaneschi Y, Kahn RS, Penninx BW, Boks MP. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety. 2014;31(9):737‐745. [DOI] [PubMed] [Google Scholar]
- 5. Goetzel RZ, Roemer EC, Holingue C, et al. Mental health in the workplace: a call to action proceedings from the Mental Health in the Workplace—Public Health Summit. J Occup Environ Med. 2018;60(4):322‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hassard J, Teoh KRH, Visockaite G, Dewe P, Cox T. The cost of work‐related stress to society: a systematic review. J Occup Health Psychol. 2018;23(1):1‐17. [DOI] [PubMed] [Google Scholar]
- 7. Burdorf A, Robroek S, Schuring M, et al. Kennissynthese werk(en) is gezond. Rotterdam: Erasmus MC; 2016. [Google Scholar]
- 8. Leka S, Jain A, Cox T, Kortum E. The development of the European framework for psychosocial risk management: PRIMA‐EF. J Occup Health. 2011;53(2):137‐143. [DOI] [PubMed] [Google Scholar]
- 9. Ruotsalainen JH, Verbeek JH, Marine A, Serra C. Preventing occupational stress in healthcare workers. Cochrane Database Syst Rev. 2015;4:CD002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carolan S, Harris PR, Cavanagh K. Improving employee well‐being and effectiveness: systematic review and meta‐analysis of web‐based psychological interventions delivered in the workplace. J Med Internet Res. 2017;19(7):e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhui KS, Dinos S, Stansfeld SA, White PD. A synthesis of the evidence for managing stress at work: a review of the reviews reporting on anxiety, depression, and absenteeism. J Environ Public Health. 2012;2012:515874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nieuwenhuijsen K, Faber B, Verbeek JH, et al. Interventions to improve return to work in depressed people. Cochrane Database Syst Rev. 2014;12:CD006237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Furlan AD, Gnam WH, Carnide N, et al. Systematic review of intervention practices for depression in the workplace. J Occup Rehabil. 2012;22(3):312‐321. [DOI] [PubMed] [Google Scholar]
- 14. Streibelt M, Burger W, Nieuwenhuijsen K, Bethge M. Effectiveness of graded return to work after multimodal rehabilitation in patients with mental disorders: a propensity score analysis. J Occup Rehabil. 2018;28(1):180‐189. [DOI] [PubMed] [Google Scholar]
- 15. de Lange AH, Taris TW, Kompier MA, Houtman IL, Bongers PM. Effects of stable and changing demand‐control histories on worker health. Scand J Work Environ Health. 2002;28(2):94‐108. [DOI] [PubMed] [Google Scholar]
- 16. Godin I, Kittel F, Coppieters Y, Siegrist J. A prospective study of cumulative job stress in relation to mental health. BMC Public Health. 2005;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stansfeld SA, Shipley MJ, Head J, Fuhrer R. Repeated job strain and the risk of depression: longitudinal analyses from the Whitehall II study. Am J Public Health. 2012;102(12):2360‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stalder T, Kirschbaum C, Miller R, Fischer J. Glucocorticoids in hair in relation to cardiometabolic risk markers. J Popul Ther Clin Pharmacol. 2012;19(2):e316. [Google Scholar]
- 19. Stalder T, Steudte S, Alexander N, et al. Cortisol in hair, body mass index and stress‐related measures. Biol Psychol. 2012;90(3):218‐223. [DOI] [PubMed] [Google Scholar]
- 20. Stalder T, Steudte‐Schmiedgen S, Alexander N, et al. Stress‐related and basic determinants of hair cortisol in humans: a meta‐analysis. Psychoneuroendocrinology. 2017;77:261‐274. [DOI] [PubMed] [Google Scholar]
- 21. Siegrist J, Li J. Work stress and altered biomarkers: a synthesis of findings based on the effort‐reward imbalance model. Int J Environ Res Public Health. 2017;14(11):1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen MA, Garde AH, Kristiansen J, Nabe‐Nielsen K, Hansen ÅM. The effect of the number of consecutive night shifts on diurnal rhythms in cortisol, melatonin and heart rate variability (HRV): a systematic review of field studies. Int Arch Occup Environ Health. 2016;89(4):531‐545. [DOI] [PubMed] [Google Scholar]
- 23. Qi X, Zhang J, Liu Y, et al. Relationship between effort‐reward imbalance and hair cortisol concentration in female kindergarten teachers. J Psychosom Res. 2014;76(4):329‐332. [DOI] [PubMed] [Google Scholar]
- 24. Stalder T, Kirschbaum C. Analysis of cortisol in hair—state of the art and future directions. Brain Behav Immun. 2012;26(7):1019‐1029. [DOI] [PubMed] [Google Scholar]
- 25. Gao W, Stalder T, Foley P, Rauh M, Deng H, Kirschbaum C. Quantitative analysis of steroid hormones in human hair using a column‐switching LC‐APCI‐MS/MS assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;928:1‐8. [DOI] [PubMed] [Google Scholar]
- 26. Gao W, Xie Q, Jin J, et al. HPLC‐FLU detection of cortisol distribution in human hair. Clin Biochem. 2010;43(7):677‐682. [DOI] [PubMed] [Google Scholar]
- 27. Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production‐Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34(1):32‐37. [DOI] [PubMed] [Google Scholar]
- 28. Manenschijn L, Koper JW, Lamberts SWJ, Van Rossum EFC. Evaluation of a method to measure long term cortisol levels. Steroids. 2011;76(10):1032‐1036. [DOI] [PubMed] [Google Scholar]
- 29. Skoluda N, Dettenborn L, Stalder T, Kirschbaum C. Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology. 2012;37(5):611‐617. [DOI] [PubMed] [Google Scholar]
- 30. Wester VL, Noppe G, Savas M, van den Akker ELT, de Rijke YB, van Rossum EFC. Hair analysis reveals subtle HPA axis suppression associated with use of local corticosteroids: the lifelines cohort study. Psychoneuroendocrinology. 2017;80:1‐6. [DOI] [PubMed] [Google Scholar]
- 31. Wosu AC, Valdimarsdóttir U, Shields AE, Williams DR, Williams MA. Correlates of cortisol in human hair: Implications for epidemiologic studies on health effects of chronic stress. Ann Epidemiol. 2013;23(12):797‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xiang L, Sunesara I, Rehm K, Marshall GD. Hair cortisol concentrations associate with hair growth rate. Brain Behav Immun. 2016;57:e1. [DOI] [PubMed] [Google Scholar]
- 33. Xie Q, Gao W, Li J, et al. Correlation of cortisol in 1‐cm hair segment with salivary cortisol in human: hair cortisol as an endogenous biomarker. Clin Chem Lab Med. 2011;49(12):2013‐2019. [DOI] [PubMed] [Google Scholar]
- 34. Short SJ, Stalder T, Marceau K, et al. Correspondence between hair cortisol concentrations and 30‐day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology. 2016;71:12‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quinete N, Bertram J, Reska M, Lang J, Kraus T. Highly selective and automated online SPE LC‐MS3 method for determination of cortisol and cortisone in human hair as biomarker for stress related diseases. Talanta. 2015;134:310‐316. [DOI] [PubMed] [Google Scholar]
- 36. Russell E, Kirschbaum C, Laudenslager ML, et al. Toward standardization of hair cortisol measurement: results of the first international interlaboratory round robin. Ther Drug Monit. 2015;37(1):71‐75. [DOI] [PubMed] [Google Scholar]
- 37. Binz TM, Rietschel L, Streit F, et al. Endogenous cortisol in keratinized matrices: Systematic determination of baseline cortisol levels in hair and the influence of sex, age and hair color. Forensic Sci Int. 2018;284:33‐38. [DOI] [PubMed] [Google Scholar]
- 38. Albar WF, Russell EW, Koren G, Rieder MJ, van Umm SH. Human hair cortisol analysis: comparison of the internationally‐reported ELISA methods. Clin Invest Med. 2013;36(6):312–316. [DOI] [PubMed] [Google Scholar]
- 39. Wester VL, van Rossum EF. Clinical applications of cortisol measurements in hair. Eur J Endocrinol. 2015;173(4):M1–M10. [DOI] [PubMed] [Google Scholar]
- 40. Manenschijn L, Schaap L, Van Schoor NM, et al. High long‐term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J Clin Endocrinol Metab. 2013;98(5):2078‐2083. [DOI] [PubMed] [Google Scholar]
- 41. Izawa S, Miki K, Tsuchiya M, Yamada H, Nagayama M. Hair and fingernail cortisol and the onset of acute coronary syndrome in the middle‐aged and elderly men. Psychoneuroendocrinology. 2019;101:240‐245. [DOI] [PubMed] [Google Scholar]
- 42. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141‐146. [DOI] [PubMed] [Google Scholar]
- 44. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19‐32. [Google Scholar]
- 45. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. [DOI] [PubMed] [Google Scholar]
- 46. Gidlow CJ, Randall J, Gillman J, Silk S, Jones MV. Hair cortisol and self‐reported stress in healthy, working adults. Psychoneuroendocrinology. 2016;63:163‐169. [DOI] [PubMed] [Google Scholar]
- 47. Herr RM, Barrech A, Gündel H, et al. Effects of psychosocial work characteristics on hair cortisol—findings from a post‐trial study. Stress. 2017;20(4):363‐370. [DOI] [PubMed] [Google Scholar]
- 48. Herr RM, Almer C, Loerbroks A, et al. Associations of work stress with hair cortisol concentrations—initial findings from a prospective study. Psychoneuroendocrinology. 2018;89:134‐137. [DOI] [PubMed] [Google Scholar]
- 49. Penz M, Siegrist J, Wekenborg MK, Rothe N, Walther A, Kirschbaum C. Effort‐reward imbalance at work is associated with hair cortisol concentrations: prospective evidence from the Dresden Burnout Study. Psychoneuroendocrinology. 2019;109:104399. [DOI] [PubMed] [Google Scholar]
- 50. van der Meij L, Gubbels N, Schaveling J, Almela M, van Vugt M. Hair cortisol and work stress: importance of workload and stress model (JDCS or ERI). Psychoneuroendocrinology. 2018;89:78‐85. [DOI] [PubMed] [Google Scholar]
- 51. McLennan SN, Ihle A, Steudte‐Schmiedgen S, Kirschbaum C, Kliegel M. Hair cortisol and cognitive performance in working age adults. Psychoneuroendocrinology. 2016;67:100‐103. [DOI] [PubMed] [Google Scholar]
- 52. Qi X, Liu Y, Zhang J, et al. Relationship between work strain, need for recovery after work and cumulative cortisol among kindergarten teachers. Int Arch Occup Environ Health. 2015. 10.1007/s00420-015-1033-2. [DOI] [PubMed] [Google Scholar]
- 53. Janssens H, Clays E, Fiers T, Verstraete AG, de Bacquer D, Braeckman L. Hair cortisol in relation to job stress and depressive symptoms. Occup Med. 2017;67(2):114‐120. [DOI] [PubMed] [Google Scholar]
- 54. Hall KL Biobehavioral relationships between job stress and cardiovascular disease risk. US DB—psyh DP—EBSCOhost, ProQuest Information & Learning; 2018. [Google Scholar]
- 55. Kind N, Eckert A, Steinlin C, Fegert JM, Schmid M. Verbal and physical client aggression—a longitudinal analysis of professional caregivers’ psychophysiological stress response and burnout. Psychoneuroendocrinology. 2018;94:11‐16. [DOI] [PubMed] [Google Scholar]
- 56. Manenschijn L, Van Kruysbergen RGPM, De Jong FH, Koper JW, Van Rossum EFC. Shift work at young age is associated with elevated long‐term cortisol levels and body mass index. Journal of Clinical Endocrinology and Metabolism [Internet]. 2011;96(11). 10.1210/jc.2011-1551 [DOI] [PubMed] [Google Scholar]
- 57. Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. Higher cortisol content in hair among long‐term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35(9):1404‐1409. [DOI] [PubMed] [Google Scholar]
- 58. Rowold J, Diebig M, Heinitz K. The effects of transformational and instrumental leadership on followers’ levels of cortisol. Ger J Hum Resour Manag. 2017;31(3):219‐237. [Google Scholar]
- 59. Qi X, Ji S, Zhang J, Lu W, Sluiter JK, Deng H. Correlation of emotional labor and cortisol concentration in hair among female kindergarten teachers. Int Arch Occup Environ Health. 2017;90(1):117‐122. [DOI] [PubMed] [Google Scholar]
- 60. Mayer SE, Lopez‐Duran NL, Sen S, Abelson JL. Chronic stress, hair cortisol and depression: a prospective and longitudinal study of medical internship. Psychoneuroendocrinology. 2018;92:57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Penz M, Stalder T, Miller R, Ludwig VM, Kanthak MK, Kirschbaum C. Hair cortisol as a biological marker for burnout symptomatology. Psychoneuroendocrinology. 2018;87:218‐221. [DOI] [PubMed] [Google Scholar]
- 62. Faresjö Å, Jullander M, Götmalm S, Theodorsson E. Higher perceived stress and poorer health reflected in elevated cortisol concentrations measured in extracts of hair from middle‐aged healthy women. BMC Psychol. 2014;2:1‐9. [Google Scholar]
- 63. van Holland BJ, Frings‐Dresen MH, Sluiter JK. Measuring short‐term and long‐term physiological stress effects by cortisol reactivity in saliva and hair. Int Arch Occup Environ Health. 2012;85(8):849‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O'Brien KM, Tronick EZ, Moore CL. Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress Health. 2013;29(4):337‐344. [DOI] [PubMed] [Google Scholar]
- 65. Wells S, Tremblay PF, Flynn A, et al. Associations of hair cortisol concentration with self‐reported measures of stress and mental health‐related factors in a pooled database of diverse community samples. Stress. 2014;17(4):334‐342. [DOI] [PubMed] [Google Scholar]
- 66. Wang C, Dai J, Li J. Mediating effects of hair cortisol on the mutual association of job burnout and insomnia: A retrospective exploratory study. J Psychiatr Res. 2019;117:62‐67. [DOI] [PubMed] [Google Scholar]
- 67. Boschi H, Trenoweth S, Sheppard ZA. Stress at work: factors associated with cognitive disorganisation among private sector professionals. Health Psychol Open. 2017;4(3):2055102917718376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mayer SE. Examining the relationships between chronic stress, HPA axis activity, and depression in a prospective and longitudinal study of medical internship. US DB—psyh DP—EBSCOhost, ProQuest Information & Learning; 2018. [Google Scholar]
- 69. Macdonald EB, Sanati KA. Occupational health services now and in the future: the need for a paradigm shift. J Occup Environ Med. 2010;52(12):1273‐1277. [DOI] [PubMed] [Google Scholar]
- 70. Nieuwenhuijsen K, Bruinvels D, Frings‐Dresen M. Psychosocial work environment and stress‐related disorders, a systematic review. Occup Med. 2010;60(4):277‐286. [DOI] [PubMed] [Google Scholar]
- 71. Theorell T, Hammarstrom A, Aronsson G, et al. A systematic review including meta‐analysis of work environment and depressive symptoms. BMC Public Health. 2015;15(1):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kivimaki M, Kawachi I. Work stress as a risk factor for cardiovascular disease. Curr Cardiol Rep. 2015;17(9):630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Herane Vives A, De Angel V, Papadopoulos A, et al. The relationship between cortisol, stress and psychiatric illness: new insights using hair analysis. J Psychiatr Res. 2015;70:38‐49. [DOI] [PubMed] [Google Scholar]
- 74. Perez de la Hoz RA, Swieszkowski SP, Cintora FM, et al. Neuroendocrine system regulatory mechanisms: acute coronary syndrome and stress hyperglycaemia. Eur Cardiol. 2018;13(1):29‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rohleder N. Burnout, hair cortisol, and timing: hyper‐ or hypocortisolism? Psychoneuroendocrinology. 2018;87:215‐217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


