Abstract
Background
The current gold standard for donor heart preservation is a three-bag-technique and storage in a cooler filled with slush ice. This technique can cause freezing injury with protein denaturation. We report our early experience with a single-use disposable device (SherpaPak™, Paragonix Technologies, MA, USA) specifically designed for sterile permanent temperature-controlled transportation of donor hearts.
Methods
In this case control study with 2:1 matching we identified 21 patients after heart transplantation depending on type of organ transport (standard three-bag-technique vs. SherpaPak™). The outcome after donor heart storage in the SherpaPak™ was compared with donor heart transportation with the standard technique.
Results
Since July 2018 seven patients (5 males; mean age 50.3±13.2years) underwent heart transplantation with the SherpaPak™ system. Cold ischemic time was longer in the SherpaPak™ group (207.7±23.3 vs. 181.6±21.9, P=0.027). SherpaPak™ kept the organ temperature at 5.1±0.8 °C, with an average outside temperature of 21.4±3.6 °C. Among all 21 transplanted patients four developed fatal early graft failure (28.6% vs. 21.4%, P=0.432). Over the first hours we noticed no difference in hemodynamic parameters, CK-MB levels or vasoactive-inotropic score. During first follow-up we noticed slightly better right heart function in the SherpaPak™ group (TAPSE 17.83±2.71 vs. 14.52±2.61 mm, P=0.020). We identified no positive blood cultures in the SherpaPak™ group within the first 30 days after heart transplantation.
Conclusions
The SherpaPak™ provides a constant temperature during transportation with permanent monitoring, never dropping below 4 °C. Organs transported with this novel device showed a normal perioperative function.
Keywords: Heart transplantation; cold storage, organ transportation
Introduction
Despite technological advances and new generations of left ventricular assist devices (LVADs), heart transplantation remains the gold standard for the treatment of end stage heart failure. However, about 40% of deaths in the first 30 days after cardiac transplantation are due to primary graft failure. The registry data of the International Society for Heart and Lung Transplantation Registry identifies two important risk factors, namely graft ischemic time and donor age (1).
The technique of donor heart preservation during transportation has not changed over the last decades. The current standard is a three bag technique with the heart transported in a cooler filled with slush ice. Temperature monitoring is not performed routinely and information on exact temperature of the donor heart is missing. This technique may result in cold injury with protein denaturation. Another important aspect is the lack of sterility of the cooler.
The SherpaPak™ system (Paragonix Technologies, MA, USA) aims to resolve these problematic issues. The SherpaPak™ system preserves the heart in a single-use sterile disposable box, with controlled temperature ranging between 4 to 8 °C. Utilization of this technology has allowed for successful transplantation of hearts with predicted longer cold ischemic times after ex-situ assessment. We are reporting our early clinical experience with the single-use disposable SherpaPak™ device designed for preservation of donor hearts and describe the efficacy and safety of this new system in comparison with conventional cold storage.
The aim of this study was to analyse the donor heart temperature during transportation, donor heart function and systemic infection rate among recipients after transplantation regarding the transportation method used. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1827).
Methods
We retrospectively analysed all adults who underwent cardiac transplantation in our center from January 2008 to August 2019. Between July 2018 and August 2019 we performed 7 heart procurements using the SherpaPak™ device. For a case-control study with 2:1 matching between the two transportation techniques standard cold storage (group C) versus storage in the SherpaPak™ device (group S) a total of 21 patients were identified. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and individual patient consent was waived due to the retrospective chart analysis in a pseudonymized fashion.
All organs were procured from brain dead donors and stored either in the disposable SherpaPak™ system (Figure 1) according to manufacturer’s instructions or with a standard technique consisting of three plastic bags and a cooler. In the control group the innermost bag was filled with Bretschneider cardioplegia (Custodiol HTK®, Dr. Franz Koehler Chemie, Bensheim, Germany), the second bag with ice cold normal saline and the outer bag was empty. The donor heart was procured with antegrade perfusion of 4L cold Custodiol HTK®. In the SherpaPak™ group an extra 500 mL were decanted in the organ canister if the SherpaPak™ was used or for the innermost plastic bag in the control group. The heart connector was attached to the ascending aorta with umbilical tape and the heart was anchored to the organ canister and preserved in the shipper (Figure 1, Video 1). The donor heart temperature was supervised during the transport using the InTemp application (Onset Computer Corporation, Bourne, MA, USA) for mobile phones. Orthotopic heart implantation was performed with the standard bicaval technique. The outcome of the patients receiving a donor heart stored in the SherpaPak™ device was compared with patients whose heart had been procured with the three-bag-technique.
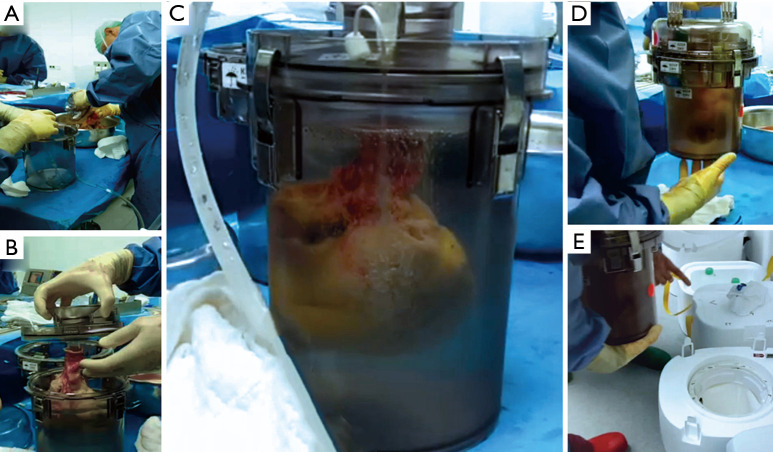
Figure 1.
SherpaPak™ Transport System preparation: after explantation of the donor heart the heart connector with temperature probe is fixed to the aorta and the sterile inner organ canister is filled with cold cardioplegia (A). The donor heart is emerged in the preservation fluid (B). The inner organ canister is deaired with the preservation fluid filled up to the top of the container emerging the donor heart completely allowing homogenous hypothermic protection and reducing the risk of mechanical injury due to organ movement during transport (C) and placed in the outer canister. The closed rigid storage canister set can now safely be moved (D), and is locked in the insulated shipper filled with disposable cold packs with high latent cold storage in the 4–8 °C range (E).
Video 1.
SherpaPak™ Transport System preparation with donor heart during donor heart harvesting.
Patients were matched for recipient and donor age, gender, body mass index (BMI), diagnosis leading to heart transplantation, use of any mechanical circulatory support prior to transplantation, urgency status and pulmonal vascular resistance (Tables 1,2). All parameters were extracted from the hospital database, including indication for heart transplantation, preoperative medical data, comorbidities, length of intensive care unit stay, total length of stay and time until death, laboratory values, intraoperative parameters, inotropic requirements, ventilation times, renal replacement therapy, any mechanical circulatory support. We also compared blood culture findings between groups. The Vasoactive-Inotropic Score (VIS) is an expanded formula for inotropic score previously described by Wernovsky and colleagues (2) now including vasoactive substances as reported by Yamazaki and colleagues (3).
Table 1. Summary of recipient data.
| Variables | SherpaPak™ (n=7) | Standard technique (n=14) | P value |
|---|---|---|---|
| Donor data | |||
| Donor age | 41.71±16.45 | 42.77±16.20 | 0.892 |
| Donor gender: male | 4 (57.1%) | 10 (76.9%) | 0.613 |
| Donor BMI (kg/m2) | 27.86±6.17 | 26.31±3.57 | 0.482 |
| Arterial hypertension | 2 (28.6%) | 2 (15.4%) | 0.587 |
| Smoking | 2 (28.6%) | 7 (50.0%) | 0.642 |
| Diabetes mellitus | 0 (0%) | 1 (11.1%) | 1.000 |
| Recipient data | |||
| Recipient age | 50.29±13.19 | 56.64±8.15 | 0.359 |
| Recipient gender: male | 5 (71.4%) | 11 (78.6%) | 0.717 |
| BMI (kg/m2) | 27.26±3.53 | 26.76±5.89 | 0.840 |
| Arterial hypertension | 5 (71.4%) | 6 (42.9%) | 0.217 |
| Dyslipidemia | 4 (51.7%) | 4 (28.6%) | 0.204 |
| Diabetes mellitus | 4 (57.1%) | 7 (50.0%) | 0.757 |
| Cause of heart failure | |||
| DCM | 5 (71.4%) | 10 (71.4%) | 1.000 |
| ICM | 1 (14.3%) | 2 (14.3%) | |
| Others | 1 (14.3%) | 2 (14.3%) | |
| High urgency status | 5 (71.4%) | 9 (64.3%) | 0.743 |
| LVAD pretransplant | 3 (42.9%) | 5 (35.7%) | 0.751 |
| PVR pretransplant (dynes/sec/cm-5) | 168.00±79.19 | 172.33±68.11 | 0.938 |
| Cold ischemic time (min) | 207.67±23.31 | 181.57±21.89 | 0.027 |
BMI, body mass index; DCM, dilated cardiomyopathy; ICM, ischemic cardiomyopathy; LVAD, left ventricular assist device; PVR, pulmonary vascular resistance.
Table 2. Standardized differences for matching criteria (Cohen’s D) before and after matching with lower and upper 95% confidence intervals.
| Matching criteria | SherpaPak™ (n=7) | Standard technique (n=14) | Cohen’s D before matching (lower and upper 95% CI) | Cohen’s D after matching (lower and upper 95% CI) |
|---|---|---|---|---|
| Donor data | ||||
| Donor age | 41.71±16.45 | 42.77±16.20 | −0.33 (−0.54 to 1.09) | −0.06 (−0.84 to 0.97) |
| Donor gender: male | 0.57±0.50 | 0.77±0.44 | −0.09 (−0.72 to 0.91) | 0.42 (−17.2 to 17.6) |
| Donor BMI (kg/m2) | 27.86±6.17 | 26.31±3.57 | −0.14 (−0.67 to 0.95) | 0.34 (−13.8 to 14.1) |
| Recipient data | ||||
| Recipient age | 50.29±13.19 | 56.64±8.15 | −0.65 (−0.35 to 1.34) | −0.59 (−1.28 to 0.56) |
| Recipient gender: male | 0.71±0.48 | 0.79±0.43 | −0.10 (−0.62 to 0.97) | −0.08 (−0.75 to 1.07) |
| BMI | 27.26±3.53 | 26.76±5.89 | 0.23 (−0.56 to 1.03) | 0.09 (−0.81 to 1.00) |
| aHT | 0.71±0.49 | 0.42±0.53 | −0.67 (−0.13 to 1.47) | −0.57 (−0.36 to 1.48) |
| Dyslipidemia | 0.51±0.51 | 0.28±0.49 | 0.43 (−0.37 to 1.22) | 0.23 (−0.56 to 1.03) |
| DM | 0.57±0.50 | 0.50±0.51 | 0.20 (−0.59 to 0.99) | 0.14 (−0.78 to 1.04) |
| Cause of heart failure | ||||
| DCM | 0.71±0.49 | 0.71±47 | 0.35 (−0.56 to 1.03) | 0.00 (0.00 to 0.00) |
| ICM | 0.14±0.38 | 0.14±0.36 | −0.26 (−0.54 to 1.05) | 0.00 (0.00 to 0.00) |
| Others | 0.14±0.38 | 0.14±0.36 | −0.01 (−0.68 to 0.70) | 0.00 (0.00 to 0.00) |
| High urgency status | 0.71±0.48 | 0.64±0.49 | 0.34 (−0.56 to 1.09) | 0.14 (−0.76 to 1.05) |
| LVAD pretransplant | 0.43±0.53 | 0.36±0.49 | 0.45 (−0.42 to 1.18) | 0.22 (−0.77 to 1.04) |
| Reperfussion time (min) | 102.57±18.67 | 105.5±10.4 | −0.48 (−0.28 to 1.32) | −0.20 (−0.69 to 1.12) |
| PVR pretransplant (dynes/sec/cm5) | 168.00±79.19 | 172.33±68.11 | −0.30 (−1.14 to 1.77) | −0.06 (−0.87 to 0.94) |
Data are presented as mean standard deviation. For categoric data, the mean is the percentage. CI, confidence interval; aHT, arterial hypertension; DM, diabetes mellitus; BMI, body mass index; DCM, dilated cardiomyopathy, ICM, ischemic cardiomyopathy; LVAD, left ventricular assist device; PVR, pulmonary vascular resistance.
Statistical analysis
For matching purposes the standardize difference (Cohen’s D) with 95% upper and lower confidence intervals was calculated. For patients’ clinical characteristics, continuous variables are presented as mean ± standard deviation and categorical variables as frequencies with relative percentage. The normality of the continuous variables was assessed with the Kolmogorov-Smirnov test. Group comparison of continuous variables was performed with the use of Student’s t-test and Mann-Whitney U test. Categorical variables are presented as numbers and percentages and compared using the chi-square or the Fisher’s exact test, as appropriate. A P value of <0.05 was considered significant. All statistics were performed using SPSS software (IBM SPSS Statistics for Windows, Version 23.0, IBM Corp., Armonk, NY, USA).
Results
From January 2008 to August 2019 we had total of 54 heart transplantations in our center. We have used this new system for 7 heart procurements between July 2018 and August 2019. In all other patients standard cold storage using the three bag technique was used. There were 5 men and 2 women with an average age of 50.3±13.2 years. The matched comparison group consisted of 11 men and 3 women (P=0.717) with a mean age of 56.6±8.2 years (P=0.359). Recipient and donor age, gender, BMI, diagnosis leading to heart transplantation, use of any mechanical circulatory support, urgency status, reperfussion organ time and pulmonal vascular resistance were similar between groups (Tables 1,2). Five patients who were listed due to dilated cardiomyopathy (DCM) were matched to 10 patients with DCM, one patient with ischemic cardiomyopathy (ICM) was matched with two ICM patients and one patient with peripartal cardiomyopathy was matched to one myocarditis and another patient with cardiac amyloidosis. One patient with LVAD driveline infection was matched to two patients with driveline infection in the control group.
We have not experienced any system failure, explantation and organ preparation times did not differ between groups. The summary of donor and recipient data are presented in Table 1. Donor and recipient age, sex, BMI and cause of cardiac failure were similar between the two groups. High urgency status according to Eurotrasplant guidelines prior to transplant was equally common in group S and C (71.4% vs. 64.3%). 42.9% of group S and 35.7% of group C patients were bridged with an LVAD before transplantation (P=0.751). The preoperative pulmonary vascular resistance index was similar in both groups: 168.00±79.2 vs. 172.3±68.1 dynes/sec/cm-5 for group S vs. group C, respectively.
Cold ischemic time was under 4 hours in both groups but longer in the SherpaPak™ group with 207.7±23.3 vs. 181.7±21.9 minutes (P=0.027).
The SherpaPak™ system always kept the donor heart temperature between 4 to 8 °C during the entire transport, despite higher ambient temperatures as shown in Figure 2.
Figure 2.
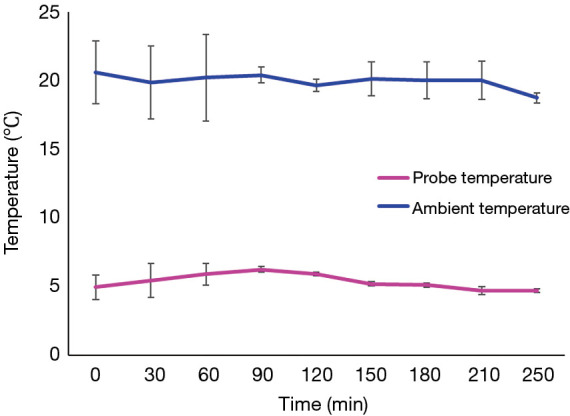
Organ temperature is maintained within a narrow range throughout the transport despite higher ambient temperature with the SherpaPak.
Within the first 30 days after transplant, one patient in the SherpaPak™ group (14.3%) died from severe acute humoral rejection as confirmed by autopsy. One patient (7.1%) in the control group died from sepsis. The early postoperative laboratory values and hemodynamic data are depicted in Table 3.
Table 3. Early postoperative laboratory values and hemodynamic data.
| Variables | SherpaPak™ (n=7) | Standard technique (n=14) | P value |
|---|---|---|---|
| CK-MB baseline | 123.19±40.77 | 134.34±69.52 | 0.703 |
| CK-MB 24 h after transplant | 101.20±67.11 | 119.85±112.5 | 0.702 |
| AST baseline | 165.53±102.92 | 374.84±673.02 | 0.429 |
| AST 6 h after transplant | 248.36±213.12 | 486.59±682.43 | 0.384 |
| ALT baseline | 48.52±40.11 | 108.04±240.97 | 0.529 |
| ALT 6 h after transplant | 83.11±83.27 | 199.66±367.20 | 0.423 |
| VIS Score baseline | 44.01±22.91 | 62.97±47.87 | 0.338 |
| VIS Score 24 h after transplant | 19.48±11.34 | 18.46±14.26 | 0.871 |
| Enoximone (µg/kg/min) | 1.79±0.62 | 2.16±0.69 | 0.254 |
| Norepinephrine (µg/kg/min) | 0.37±0.21 | 0.57±0.047 | 0.318 |
| Lactate arterial | 5.24±3.00 | 6.14±5.62 | 0.702 |
| Lactate venous | 5.43±3.15 | 5.97±5.27 | 0.805 |
| Cardiac Index (L/min/m2) | 2.65±0.26 | 2.53±0.56 | 0.702 |
| Mixed venous saturation (%) | 69.41±7.03 | 67.49±10.61 | 0.674 |
| Central venous pressure (mmHg) | 13±5.03 | 12.54±4.39 | 0.833 |
| Wedge pressure (mmHg) | 11.43±5.593 | 12.42±4.39 | 0.677 |
| Time on respirator (min) | 39.70±27.90 | 42.42±35.39 | 0.881 |
| Defibrillated after de-clamping | 2 (28.6%) | 3 (21.4%) | 0.717 |
| VA-ECMO after transplant | 2 (28.6%) | 2 (14.3%) | 0.432 |
| Positive blood cultures | 0 (0%) | 4 (28.6%) | 0.255 |
CK-MB, creatine kinase muscle-brain type; AST, aspartate transaminase; ALT, alanine transaminase; VIS Score, Vasoactive-inotropic Score; VA-ECMO, veno-arterial Extracorporeal membrane oxygenation.
CK and CK-MB values, aspartate transaminase, alanine transaminase and central venous pressure were similar immediately after and 24 hours after transplant in both groups. The need for defibrillation after releasing the aortic crossclamp did not differ nor did the need for va-ECMO support early after transplant (P=0.432). There was no case of positive blood cultures in SherpaPak™ group, whereas in the control group 4 out of 14 patients were positively tested (P=0.255).
VIS was calculated directly postoperatively and 24 hours after transplantation. Although a trend for higher VIS was observed in the control group postoperatively there was no statistical difference 24 hours after transplant. Analysis of hemodynamic data including cardiac index, central venous pressure, pulmonary capillary wedge pressure and mixed venous saturation did not show any differences.
All patients had induction immunosuppression with high dose corticosteroids and rabbit antithymocyte globulin (rATG) with exception one patient in control group being induced with an IL-2 receptor antagonist. Maintenance immunosuppression consisted of calcineurin inhibitor (CNI), corticosteroids and mycophenolate mofetil (MMF). In case of worsening renal function after transplant we reduced CNI dosage and replaced MMF with the m-TOR inhibitor everolimus. One year after transplant there was no difference in the immunosuppressive regimen between groups (57.1% vs. 64.3%, P=1.000). Further posttransplant medication included trimethoprim/sulfamethoxazole, valganciclovir for 6 months after transplant and lifelong therapy with acetylsalicylic acid combined with statins in all patients.
Cardiac function as assessed by echocardiography before discharge showed no difference in LVEF, systolic pulmonary artery pressure (sPAP) or occurrence of tricuspid valve regurgitation, but better TAPSE values in the SherpaPak™ group indicating better early postoperative right heart function (17.8±2.7 vs. 14.4±2.6 mm, P=0.020). Only one patient in control group had a dilated right ventricle on discharge echocardiography.
Follow-up data 1 year after transplant is provided in Table 4. We noticed slightly higher LVEF in the SherpaPak group (62.40±3.36 vs. 55.27±6.13, P=0.030). TAPSE values improved in both groups after 1 year. There was no difference in sPAP, NT-proBNP or occurrence of tricuspid valve regurgitation between groups.
Table 4. Follow-up data 30 days and 1 year after transplant.
| Variables | SherpaPak™ (n=7) | Standard technique (n=14) | P value |
|---|---|---|---|
| After 30 days | |||
| TAPSE (mm) | 17.83±2.71 | 14.42±2.61 | 0.020 |
| LVEF (%) | 60.60±1.34 | 58.73±3.38 | 0.258 |
| sPAP (mmHg) | 33.5±7.9 | 31.8±5.7 | 0.661 |
| TVR | 0.678 | ||
| Mild | 1 (16.7%) | 1 (7.7%) | |
| Moderate | 0 (0%) | 1 (7.7%) | |
| ISHLT Score 1R on 1. follow-up | 0 (0%) | 4 (33.3%) | 0.516 |
| After 1 year | |||
| TAPSE (mm) | 19.60±2.89 | 16.18±3.89 | 0.103 |
| LVEF (%) | 62.40±3.36 | 55.27±6.13 | 0.030 |
| sPAP (mmHg) | 31.75±3.40 | 31.18±9.34 | 0.908 |
| TVR | 0.380 | ||
| Mild | 1 (20%) | 4 (36.4%) | |
| Moderate | 0 (0%) | 2 (18.2%) | |
| ISHLT Score 1R on 1. year follow-up | 2 (40%) | 4 (36.4%) | 0.889 |
| NT-proBNP (pg/mL) | 811.80±1,121.29 | 1,481.09±1,987.25 | 0.498 |
| Survived after 1 year | 5 (71.4%) | 11 (78.6%) | 0.717 |
TAPSE, tricuspid annular plane systolic excursion; LVEF, left ventricular ejection fraction; ISHLT, International Society of Heart and Lung Transplantation; sPAP, systolic pulmonary artery pressure; TVR, tricuspid valve regurgitation; NT-proBNP, amino-terminal pro-B-type natriuretic peptide.
Discussion
Although normothermic ex vivo heart perfusion showed superiority compared to cold ischemic storage in case of marginal donor allografts, such systems with their associated logistics difficulties and high costs still have limited usage (4,5).
The most important aspect of cold storage are hypothermia at 4 to 8 °C and the chemical constituents of the cardioprotective fluid the donor heart is immersed in. Hypothermia slows metabolic reaction rates and the rate of intracellular enzyme degradation (6). Therefore, hypothermia remains one of the most important tools used to preserve organs for transplantation. Ionic ingredients of cardioprotective fluid facilitate the fast cessation of electric activity due to membrane depolarization by reducing the transmembrane K+ gradient (7).
Despite its protective effect hypothermia can also be harmful to preserved organs through cellular swelling, extracellular edema, cellular acidosis, reperfusion injury, calcium overload and endothelial injury. Temperatures between 4–8 °C are widely accepted as best range for optimal high energy phosphate preservation, low risk of cold injury and good post-transplant function (8). One study involving 186 transplanted organs showed that organ transportation and cold storage using usual packing procedures following the Eurotransplant guidelines led to organ temperatures reaching even below 0 °C, with an average below 2 °C in all packing procedures (9). It is known that temperatures below 2 °C significantly increase the risk of cold injury and frostbite, which can further lead to primary graft failure (10,11).
Current recommendations of the European Committee on Organ transplantation suggest that all packaging materials should be validated for their intended use, with particular attention to the maintenance of temperature within the desired range and for the specified time ensuring highest quality of organs offered for transplant (12). Experiments performed so far showed that the SherpaPak™ can maintain a temperature between 4–8 °C in experimental conditions throughout a longer period of time (13). So far, only one case report reported successful clinical use of SherpaPak™ system for human heart procurement (14).
In our study we showed that donor heart transportation using the SherpaPak™ system is safe. When used in accordance with manufacturer instructions the device keeps the organ temperature in a range between 4–8 °C despite changes in ambient temperature even in summer time. What is even more important is the fact that the temperature never dropped below 4 °C thereby eliminating the risk of cold cell injury. We did not experience any system failure and real-time temperature monitoring using the mobile phone application provided by the manufacturer functioned flawlessly.
Since we do not routinely measure organ temperature during organ transportation with the three bag technique, so we do not have own data to compare but compared our results with data published by Horch et al. (9) previously. These authors showed an average organ temperature below +2 °C in 186 organ procurements raising concerns regarding cold cell injury. The constant temperature range between 4–8 °C as provided by manufacturer and proved in our study remains an advantage of SherpaPak™ system.
Furthermore, we managed to harvest organs from more distant sites as evident from longer ischemic times, narrowing to 4 hours in average. We had similar outcomes compared to our control group. Study on pig hearts showed that this system is able to maintain the temperature within the desired range for more than 12 hours (13). The system’s potential for even longer transportation and heart procurement from more distant sites requires further study.
On echocardiographic control following heart transplantation we measured higher TAPSE (tricuspid annular plane systolic excursion) in the SherpaPak™ group indicating better right heart function. This might be the first positive result of a controlled temperature level during organ harvesting and less cold cell injury.
Another relevant problem with the standard three-bag technique is the questionable sterility of this transportation method. According to some reports postpreservational fluid cultures grew antimicrobial agents in up to 27.5% after cardiac transplantation (15). Other groups show even higher preservation fluid contamination rates in other solid organ transplantation going up to 62.2% of which 17.8% were contaminated with high risk organisms, showing correlation with the development of clinical infection with the same microorganism (16). The second major advantage of the SherpaPak™ system is that it is a sterile shelf-ready to use system, providing a low risk of donor organ contamination. In this study, we analyzed only blood cultures of heart transplanted patients within 30 days after transplantation and found no positive cultures in the SherpaPak™ group. Pathogens detected in our control group requiring antibiotic therapy were Staphylococcus aureus in one, Pseudomonas aeruginosa in two and Enterococcus faecium in one patient. Further investigation with higher procurement numbers are needed to prove SherpaPak™ advantages regarding organ contamination during transport.
Undoubtedly, the SherpaPak™ system is more expensive than the standard technique. However, heart transplantation is a costly procedure anyway and it is our belief that the additional costs are justified because of increased safety for the recipient in terms of sterility and more stable temperature within the recommended range of 4–8 °C.
This investigation was a single-center retrospective study with a limited number of subjects. Patients enrolled in our study underwent heart transplantation in a low volume center during the past 11 years. During that time period, donor and recipient characteristics have changed due to continued donor organ shortage in Europe which might affect our results.
This is the first patient series as a proof of concept and despite small patient numbers we noticed early advantages of using the SherpaPak™ system compared to standard cold organ storage. There is growing interest for pulsatile, normothermic ex vivo heart perfusion and it has been shown that this technique is non-inferior to cold storage (17). Such systems need additional surgical and technical support personnel, equipment, appropriate transport and are inevitably more costly. All this points to the use of such systems for select cases of organ procurement like non-heart beating donors in countries where this is legally permitted. In contrast, SherpaPak™ cold storage might be a good alternative for safe standard donor heart transport at relatively low cost with continuous monitoring of adequate hypothermic state of the donor organ. The SherpaPak™ provides a constant temperature during transportation with permanent monitoring, never dropping below 4 °C. Organs transported with this novel device showed a normal perioperative function, with possiblly better protection of right heart function and no risk of systemic infection due to single use sterile package. Further studies regarding extension of ischemic time and thereby improving donor organ availability using the SherpaPak™ device are needed.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SherpaPack System from Paragonix already has CE mark (is also, to best of our knowledge FDA approved) and as such is approved for use in European Union. We did not need any additional permission to start using this product. We performed retrospective chart analysis of previously acquired patient data as part of regular patient treatment. We do not need extra ethical approval for performing retrospective, descriptive analysis of patient charts and as medical professionals we are entitled to perform retrospective chart analysis as part of quality control, or in the context of medical research for scientific publications according to Article 15§ of Professional code act for medical doctors in Bavaria and Article 25§ of the Bavarian Data Protection Act. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and individual patient consent was waived due to the retrospective chart analysis in a pseudonymized fashion.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1827
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1827
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1827). The authors have no conflicts of interest to declare.
References
- 1.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009-24. 10.1016/j.healun.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 2.Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 1995;92:2226-35. 10.1161/01.CIR.92.8.2226 [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki Y, Oba K, Matsui Y, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth 2018;32:167-73. 10.1007/s00540-018-2447-2 [DOI] [PubMed] [Google Scholar]
- 4.Nutt MP, Fields BL, Belzer FO, et al. Comparison of continuous perfusion and simple cold storage for rabbit heart preservation. Transplant Proc 1991;23:2445-6. [PubMed] [Google Scholar]
- 5.Qayumi AK, Jamieson WR, Rosado LJ, et al. Preservation techniques for heart transplantation: comparison of hypothermic storage and hypothermic perfusion. J Heart Lung Transplant 1991;10:518-26. [PubMed] [Google Scholar]
- 6.Buckberg GD. Myocardial temperature management during aortic clamping for cardiac surgery. Protection, preoccupation, and perspective. J Thorac Cardiovasc Surg 1991;102:895-903. 10.1016/S0022-5223(19)33940-6 [DOI] [PubMed] [Google Scholar]
- 7.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation 1988;45:673-6. 10.1097/00007890-198804000-00001 [DOI] [PubMed] [Google Scholar]
- 8.Jahania MS, Sanchez JA, Narayan P, et al. Heart preservation for transplantation: principles and strategies. Ann Thorac Surg 1999;68:1983-7. 10.1016/S0003-4975(99)01028-0 [DOI] [PubMed] [Google Scholar]
- 9.Horch DF, Mehlitz T, Laurich O, et al. Organ transport temperature box: multicenter study on transport temperature of organs. Transplant Proc 2002;34:2320. 10.1016/S0041-1345(02)03253-0 [DOI] [PubMed] [Google Scholar]
- 10.Tian G, Smith KE, Biro GP, et al. A comparison of UW cold storage solution and St. Thomas' solution II: a 31P NMR and functional study of isolated porcine hearts. J Heart Lung Transplant 1991;10:975-85. [PubMed] [Google Scholar]
- 11.Sukehiro S, Dyszkiewics W, Minten J, et al. Catabolism of high energy phosphates during long-term cold storage of donor hearts: effects of extra- and intracellular fluid-type cardioplegic solutions and calcium channel blockers. J Heart Lung Transplant 1991;10:387-93. [PubMed] [Google Scholar]
- 12.Guide to the Quality and Safety of Organs for Transplantation . Available online: https://www.edqm.eu/en/guide-quality-and-safety-organs-transplantation
- 13.Michel SG, LaMuraglia Ii GM, Madariaga ML, et al. Innovative cold storage of donor organs using the Paragonix Sherpa Pak devices. Heart Lung Vessel 2015;7:246-55. [PMC free article] [PubMed] [Google Scholar]
- 14.Naito N, Funamoto M, Pierson RN, et al. First clinical use of a novel hypothermic storage system for a long-distance donor heart procurement. J Thorac Cardiovasc Surg 2020;159:e121-3. 10.1016/j.jtcvs.2019.05.085 [DOI] [PubMed] [Google Scholar]
- 15.Mossad SB, Avery RK, Goormastic M, et al. Significance of positive cultures from donor left atrium and postpreservation fluid in heart transplantation. Transplantation 1997;64:1209-10. 10.1097/00007890-199710270-00024 [DOI] [PubMed] [Google Scholar]
- 16.Yansouni CP, Dendukuri N, Liu G, et al. Positive cultures of organ preservation fluid predict postoperative infections in solid organ transplantation recipients. Infect Control Hosp Epidemiol 2012;33:672-80. 10.1086/666344 [DOI] [PubMed] [Google Scholar]
- 17.Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015;385:2577-84. 10.1016/S0140-6736(15)60261-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as