Significance
The noise pollution accompanying industrialization is a risk factor to human health. Here, we show in a rodent model that even moderate-level noise at ∼65 dB SPL that has little effect on stress status can substantially impair hippocampus-related learning and memory by altering the plasticity of synaptic transmission. It is possible that because moderately loud noise does not affect peripheral hearing per se, the negative impacts of chronic exposure to such noise are currently not well characterized. Our results indicate the importance of more thoroughly defining these possibly hitherto unappreciated hazards of noise pollution in modern human environments.
Keywords: behavior, hippocampus, noise exposure, plasticity, rat
Abstract
The neural mechanisms underlying the impacts of noise on nonauditory function, particularly learning and memory, remain largely unknown. Here, we demonstrate that rats exposed postnatally (between postnatal days 9 and 56) to structured noise delivered at a sound pressure level of ∼65 dB displayed significantly degraded hippocampus-related learning and memory abilities. Noise exposure also suppressed the induction of hippocampal long-term potentiation (LTP). In parallel, the total or phosphorylated levels of certain LTP-related key signaling molecules in the synapses of the hippocampus were down-regulated. However, no significant changes in stress-related processes were found for the noise-exposed rats. These results in a rodent model indicate that even moderate-level noise with little effect on stress status can substantially impair hippocampus-related learning and memory by altering the plasticity of synaptic transmission. They support the importance of more thoroughly defining the unappreciated hazards of moderately loud noise in modern human environments.
The noise pollution accompanying industrialization is a risk factor to human health. Earlier studies have extensively examined the deleterious impacts of noise in the auditory systems of both humans and animal models (1–6), showing that noise exposure either early or late in life can induce progressive hearing loss, change neural coding along the auditory pathway, and alter auditory-related perception and behavior.
The auditory system, however, contains direct and indirect pathways to other systems and structures of the brain that are necessary for functional integration. For example, earlier studies found that the hippocampus, the core area of the brain associated with learning and memory processes, receives neuronal inputs from the auditory system through the lemniscal and nonlemniscal pathways (7–11). It is thus conceivable that noise-evoked activities might be transmitted via these connections to the hippocampus, thereby affecting learning and memory. Indeed, animal studies have shown that exposure to loud noise (e.g., above a sound pressure level [SPL] of 95 dB) that induces temporary or permanent shifts in the auditory threshold disrupts hippocampal histology, decreases neurogenesis in the hippocampus, and impairs hippocampus-related learning and memory abilities (12–16). In addition, epidemiological studies have demonstrated that environment noise has substantially negative effects on children’s learning outcomes and cognitive abilities (17–19). While the usual explanations for the origins of these noise-induced effects on nonauditory functions have relied on stress-related processes (15, 16, 20–23), the underlying neural mechanisms remain largely unknown.
In this study, we exposed rat pups to structured noise delivered at ∼65 dB SPL for a 7-wk period. Exposure to a moderate level of modulated broad-spectrum noise more realistically models the noise environments people encountered in industrial workplaces and other modern acoustic settings (2, 4, 24–26). We then evaluated the behavioral consequences of noise exposure on hippocampus-related learning and memory for these noise-exposed rats. In addition, we explored the mechanisms underlying possible postexposure changes in learning and memory via physiological and molecular assessments of the hippocampus.
Results
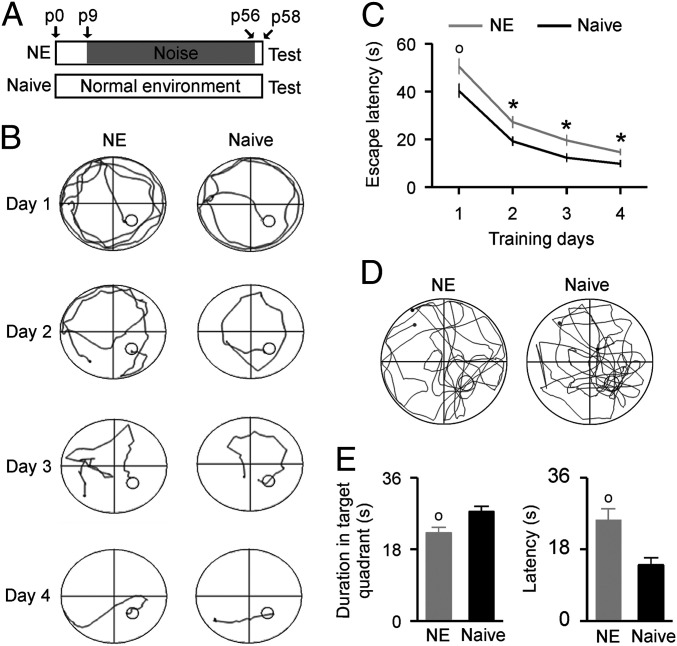
Rat pups were exposed to a moderate level of noise (∼65 dB SPL) in a sound-shielded chamber from postnatal day 9 (p9) to p56. These rats were thereafter referred to as the noise-exposed (NE) rats (Fig. 1A). As shown in SI Appendix, Fig. S1, the average thresholds of the auditory brainstem response (ABR) for the NE rats were comparable to that of the naïve rats, indicating intact peripheral hearing of the rats after exposure to moderate-level noise. In addition, no measurable changes were observed for the NE rats in both the open field test (SI Appendix, Fig. S2) and the elevated-zero maze test (SI Appendix, Fig. S3), manifesting normal locomotor activities and anxiety-related behaviors of the NE rats. Furthermore, the plasmatic corticosterone concentrations that assess the intensity of stress were similar in the NE and naïve rats (SI Appendix, Fig. S4). Thus, the moderate-level noise exposure applied in this study had little effect on rats’ stress-related processes.
Fig. 1.
Behavioral performance in the Morris water maze test. (A) Experimental timelines for the NE and naïve rats. Note that all tests started 2 d after the end of noise exposure (i.e., on p58). (B) Sample swimming traces of a NE rat (Left) and a naïve rat (Right) during the training phase. Small circles show the escape platforms. (C) Average latencies to find the escape platform during the training phase for the NE (n = 27) and naïve (n = 27) rats. Error bars represent SEM. *P < 0.05; o, P < 0.01. (D) Sample swimming traces of a NE rat (Left) and a naïve rat (Right) during the test phase. Small circles show the position of former escape platforms that were removed during the test phase. (E) Average time spent in the target quadrant (Left) and average latencies of first entering the former platform position (Right) for the NE and naïve rats during the test phase.
Morris Water Maze Test.
We used the Morris water maze test to evaluate hippocampus-related spatial learning and memory abilities (27–29). During the training phase, latencies for both the NE and naïve rats to find the platform submerged in the water maze progressively decreased with training time (Fig. 1 B and C; two-way ANOVA, P < 0.001). However, the NE rats required a significantly longer time period to find the platform compared to the naïve rats on each training day (Fig. 1C; two-way ANOVA with Student-Newman-Keuls post hoc test, P < 0.05 to 0.01). During the test phase, the NE rats demonstrated worse learning bias when navigating toward the target quadrant that previously contained the platform; they spent less time in this quadrant than did the naïve controls (Fig. 1 D and E, Left; unpaired Student’s t test, P = 0.0041). The latencies for their first entrance into the former platform position were also significantly longer than those of the naïve rats (Fig. 1 E, Right; unpaired Student’s t test, P = 0.0011). No significant difference in the total swim distance, however, was found between the NE and naïve rats (22.9 ± 0.9 m for the NE rats vs. 22.5 ± 0.5 m for the naïve rats; unpaired Student’s t test, P = 0.74). These results indicate that the NE rats had impaired spatial memory.
Y-Maze Test.
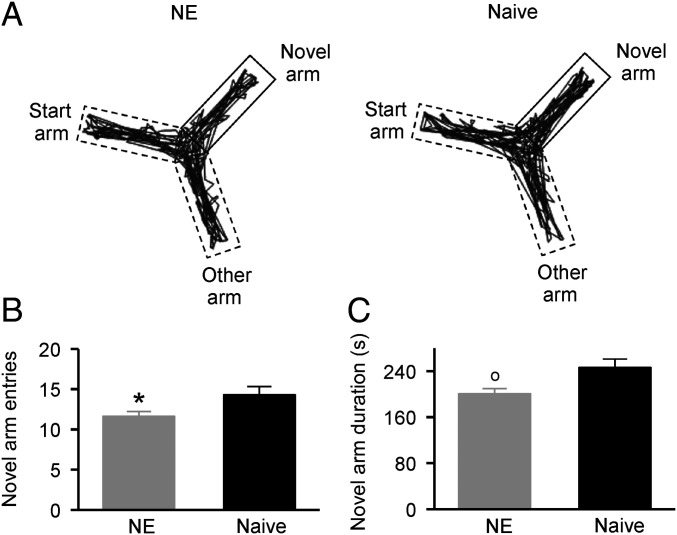
We used the Y-maze test to assess the rats’ short-term spatial memory and exploratory activity in a novel environment, both of which are highly dependent on hippocampal function (30). As shown in Fig. 2A, there was less path density for the NE rat in the novel arm compared to the naïve rat during the test period. A statistical analysis revealed that the NE rats exhibited a significantly smaller number of entries to the novel arm compared to the naïve rats (Fig. 2B; unpaired Student’s t test, P = 0.023). They also spent less time in the novel arm than did the naïve controls (Fig. 2C; unpaired Student’s t test, P = 0.0096). These results indicate that early noise exposure disrupts the short-term spatial memory and exploratory activity of rats.
Fig. 2.
Behavioral performance in the Y-maze test. (A) Sample movement traces of an NE rat (Left) and a naïve rat (Right) during the test phase. (B) Average numbers entering the novel arm for the NE (n = 12) and naïve (n = 11) rats during the test phase. Error bars represent SEM. *P < 0.05. (C) Average time spent in the novel arm for the NE and naïve rats during the test phase. o, P < 0.01.
Novel Object Recognition Test.
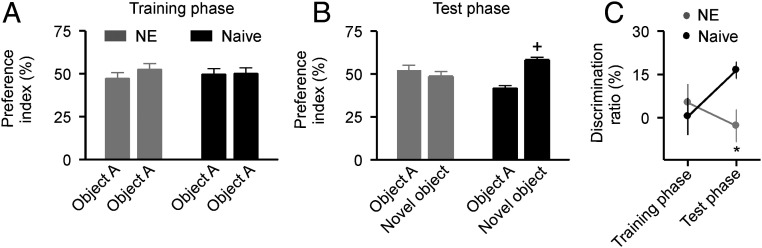
The novel object recognition test, which exploits rodents’ natural tendency to explore novel stimuli, is a widely used test for hippocampus-related memory formation (31). As described in the Materials and Methods section, we evaluated the recognition memory of rats by calculating the preference index. We did not find any significant differences in the preference indices for both the NE and naïve rats during the training phase (Fig. 3A; two-way ANOVA, P = 0.39), indicating that they spent a comparable amount of time exploring the two identical objects. While the naïve rats showed significantly higher preference indices for the novel object than for the familiar one during the test phase (Fig. 3B; two-way ANOVA with Student-Newman-Keuls post hoc test, P < 0.001), the NE rats had comparable indices for both the novel and familiar objects (two-way ANOVA with Student-Newman-Keuls post hoc test, P = 0.38). The same was true for the discrimination ratio; the NE rats had no preference for either object during the training and test phases (Fig. 3C; two-way ANOVA with Student-Newman-Keuls post hoc test, P = 0.342). This was in significant contrast to the naïve rats, which displayed increased exploration of the novel object during the test phase (two-way ANOVA with Student-Newman-Keuls post hoc test, P = 0.028). Taken together, these results indicate that early noise exposure impacts the recognition memory of rats.
Fig. 3.
Behavior performance in the novel object recognition test. (A) Average preference indices of the NE (n = 13) and naïve (n = 18) rats during the training phase. Error bars represent SEM. (B) Average preference indices for both groups of rats during the test phase. +, P < 0.001. (C) Average discrimination ratios for both groups of rats during the training and test phases. *P < 0.05.
In Vivo Hippocampal Long-Term Potentiation (LTP) Induction.
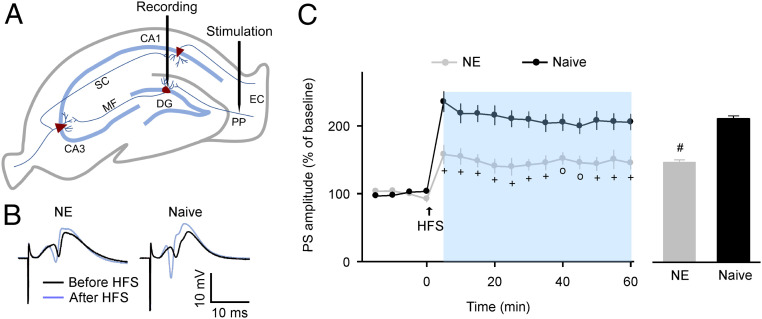
Hippocampal LTP, a persistent increase in the strength of synaptic connections between neurons, is a cellular mechanism that is widely considered to underlie the processes of learning and memory. The dentate gyrus (DG) has been proposed as the main gateway for multisensory (including acoustic) information into the hippocampus (32). Thus, it is plausible that the DG is one of the hippocampal subregions that is most vulnerable to sensory stimuli. We therefore assessed the effects of noise exposure on the induction of LTP at the perforant path (PP)-DG granule cell synapses (Fig. 4A). As shown in Fig. 4B, we successfully induced PP-DG LTP in both groups of rats after high-frequency stimulation (HFS) at the PP. However, average amplitudes of the population spike (PS) following HFS in the NE rats were significantly lower than those of the naïve rats (Fig. 4 C, Left; two-way ANOVA with Student-Newman-Keuls post hoc test, P < 0.01 to 0.001). Summarized data recorded 5 to 60 min after HFS were also lower for the NE rats than for the naïve controls (Fig. 4 C, Right; unpaired Student’s t test, P < 0.0001). We recorded reduced LTP in the NE rats 8 wk after cessation of noise exposure (SI Appendix, Fig. S5). Thus, early noise exposure enduringly reduces synaptic functional plasticity in the hippocampus of rats.
Fig. 4.
In vivo hippocampal LTP induction. (A) Schematic illustration of stimulating and recording electrode positions in the coronal plane of the hippocampus. EC, entorhinal cortex. MF, mossy fiber. SC, Schaffer collateral. (B) Sample PS evoked before and after the HFS recorded in the NE and naïve rats. (C) Time courses of PS amplitudes (Left) and average LTP amplitudes (Right) following HFS for the NE (n = 14) and naïve (n = 14) rats. Error bars represent SEM. o, P < 0.01; +, P < 0.001; #, P < 0.0001.
Expressions of LTP-Related Signaling Molecules.
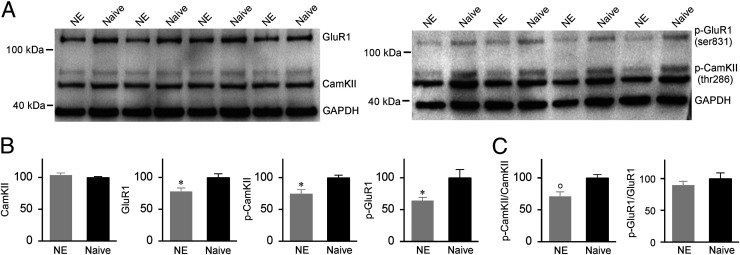
Multiple signaling cascades, particularly the calcium/calmodulin-dependent protein kinase II (CaMKII) cascade, have been implicated in the induction of hippocampal LTP (33–38). To detect the molecular mechanisms underlying the decrease in LTP amplitude after noise exposure, for both groups of rats, we determined the total and phosphorylated levels of the CaMKII and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit GluR1 (two LTP-related key signaling molecules within the CaMKII cascade) in synaptosome-enriched fractions that we prepared from the DG of the hippocampus after HFS.
As shown in Fig. 5 A and B, noise exposure significantly decreased the expression level of phosphorylated (p)-CaMKII (thr286) (unpaired two-tailed Student’s t test, P = 0.017) but had little effect on the level of CaMKII (unpaired Student’s t test, P = 0.24). As a result, the p-CaMKII/CaMKII ratio was significantly decreased in the NE rats compared to the naïve controls (Fig. 5 C, Left; unpaired Student’s t test, P = 0.0015), indicating dramatically reduced CaMKII phosphorylation. In addition, the expression levels of both GluR1 and p-GluR1 (ser831) were lower for the NE rats than for the naïve controls (Fig. 5 A and B; unpaired Student’s t test, P = 0.031 and 0.040). However, no significant difference in the p-GluR1/GluR1 ratio (i.e., GluR1 phosphorylation) was found between the NE and naïve rats (Fig. 5 C, Right; unpaired Student’s t test, P = 0.36).
Fig. 5.
Expressions of LTP-related signaling molecules. (A) Representative Western blots for the CaMKII, phosphorylated (p)-CaMKII (thr286), GluR1, and p-GluR1 (ser831). (B) Expression levels of the CaMKII, p-CaMKII, GluR1, and p-GluR1 in the NE (n = 12) and naïve (n = 12) rats. Error bars represent SEM. *P < 0.05. (C) Ratios of p-CaMKII/CaMKII and p-GluR1/GluR1. o, P < 0.01.
Discussion
In their daily lives, people are continuously bombarded by occupational and recreational noise with highly variable temporal patterns and intensities. Although most of this noise is at a moderate level, the majority of studies investigating the effects of noise on learning and memory have applied traumatic or threatening noise of intensity larger than 95 dB SPL that invoked stress-related mechanisms (12–16). In this study, we chronically exposed rat pups to structured noise delivered at a moderate level, ∼65 dB SPL, which is currently considered to be a safe level that does no structural damage to the cochlea and thus hardly affects peripheral hearing (in addition to the results of this study, see 2,4,26,39). We found that noise exposure had little apparent effect on normal locomotor activities and anxiety-related behaviors in the NE rat group. The serum corticosterone concentrations of the NE rats were also comparable to those of the naïve rats. Thus, exposure to moderate-level noise used in this study can be considered nonstressful. However, noise exposure significantly degraded hippocampus-related learning and memory abilities, decreased LTP induction in the hippocampus, and down-regulated certain LTP-related key signaling molecules in the hippocampal synapses. These results demonstrate that moderate-level, nonstressful noise has negative impacts on hippocampus-related behavior and synaptic processes. More recently, we have shown that a similar noise exposure regimen significantly and persistently degrades auditory cortical processing and auditory-related perceptual abilities (39–42). Taken together, these results in a rodent model provide direct evidence that even moderate-level noise with little effect on peripheral hearing and stress-related processes can substantially affect auditory and nonauditory functions. However, noise-induced auditory deficits plausibly do not interfere with the impaired behavioral performance for the Morris water maze, Y-maze test, and novel object recognition recorded in the NE rats, because these tests do not involve any auditory tasks.
Early experiences, particularly those in the critical period, have major and long-lasting impacts on brain processing (43–47). Previous studies have documented multiple feature-dependent critical periods for auditory cortical development in the rodent models, which extend to the second month after birth (48, 49). The critical period for development of the hippocampus, however, appears to be within the first month after birth (47). In the present study, noise exposure was applied between p9 and p56, which covers critical periods for development of both auditory cortex and hippocampus in rats. In addition, earlier studies have shown that auditory cortical plasticity is modulated by sound input dynamics (5, 39, 41, 50, 51). While structured noise exposure persistently degrades cortical processing and advances the closure of the critical period for cortical plasticity (39, 41), continuous noise exposure prolongs the duration of the critical period (50, 51). It remains to be investigated whether structured and continuous noise also differentially affects developmental plasticity of the hippocampus.
Learning and memory are widely believed to result from changes in connectivity within neuronal circuits due to synaptic plasticity. Thus, the LTP of synaptic transmission, which reflects enhancements in synaptic plasticity, is considered to be an important cellular model for learning and memory processes (36, 38, 52). Manipulations that facilitate or reduce the induction of LTP in the hippocampus are expected to enhance or impair learning and memory abilities (35, 37, 38, 53). For example, the overexpression of N-methyl-d-aspartate (NMDA) receptor 2B (NR2B) in the forebrains of transgenic mice leads to enhanced NMDA-dependent LTP in the cornu ammonis 1 (CA1) of the hippocampus and better performance in learning and memory tasks (54). Mice lacking the nociceptin receptor also show larger LTP in the hippocampal CA1 region and possess greater learning abilities compared to control mice (55). In contrast, knock-in mice with the point mutant T286A of CaMKII exhibit no NMDA receptor-dependent LTP in the hippocampus and show strong learning and memory impairments (56). These studies provide compelling evidence that synaptic plasticity is critical for hippocampus-related learning and memory. Consistent with these conclusions, we found that noise significantly decreased the induction of PP-DG LTP, which plausibly accounts for at least part of the impaired behavioral performance seen in the learning and memory tasks of the NE rats.
Multiple signaling cascades, particularly the CaMKII cascade, have been implicated in the hippocampal LTP elicited by various types of stimulation (33–38). CaMKII is highly enriched in the postsynaptic densities of excitatory synapses. It has been proposed that the CaMKII serves as a “molecular switch” that is essential for LTP induction and memory processes (34). After tetanic stimulation, CaMKII is activated by calcium influx through the NMDA receptor/channel. It then translocates to the synapse and binds to the NMDA receptors (e.g., the NR2B subunit), thereby producing postsynaptic potentiation via phosphorylating the principal and auxiliary subunits of AMPA receptors (e.g., the GluR1 subunit). In addition, CaMKII is also involved in regulating the insertion of additional AMPA receptors into postsynaptic densities (35, 37). Thus, alterations in the expression and activity of either CaMKII or its downstream molecules could have profound effects on synaptic plasticity and LTP induction (34, 57–59). In this study, we observed that noise exposure significantly down-regulated the expression level of p-CaMKII (thr286) but had little effect on the expression of CaMKII after HFS, resulting in reduced phosphorylation of CaMKII compared to the naïve controls. Noise exposure also reduced the expression levels of both GluR1 and p-GluR1 (ser831). These parallel changes after noise exposure in LTP-related key signaling molecules might translate into altered synaptic function, thereby resulting in LTP alteration in the hippocampus.
Interestingly, while both GluR1 and p-GluR1 were down-regulated after noise exposure, the phosphorylation level of GluR1 remained stable. Earlier studies have proposed that the phosphorylation of GluR1 is associated with increased AMPA conductance during LTP introduction (60, 61). This finding suggests that noise exposure hardly affects AMPA conductance after tetanic stimulation.
While some earlier studies have revealed that noise (particularly that with a high intensity) impacts learning and memory, the usual explanations for the origins of such effects have invoked stress-related mechanisms (15, 20–23). Indeed, it has been shown that noise-induced stress affects the induction of LTP in the hippocampus by mediating glutamatergic neurotransmission (62). Noise stress also triggers peroxidative damage along the lemniscal afferent pathway to the hippocampus, resulting in hyperphosphorylation of the tau protein and reduced neuronal density in the hippocampus (20, 23). In this study, we showed that chronic exposure to moderate-level noise significantly impaired the hippocampus-related learning and memory abilities of rats. However, we did not observe any significant changes in the plasmatic corticosterone concentrations of the NE rats when determined during or after noise exposure. Additionally, locomotor activities and anxiety-related behaviors were comparable between the NE and naïve rats. It has been shown that the hippocampus receives acoustic inputs from the auditory system through the lemniscal and nonlemniscal pathways (7–11). We propose that ongoing early noisy inputs via these connections into the hippocampus might derail the normal course of hippocampal development, thereby resulting in degraded hippocampal function as demonstrated by the present study. Interestingly, a recent study also reported that noise exposure at a high level (123 dB SPL) decreased neurogenesis in the hippocampus and impaired the spatial learning and memory of mice. These long-lasting effects were thought to be attributed to reduced auditory inputs as a result of noise-induced permanent hearing loss, rather than transient changes in initial oxidant stress (63). Nevertheless, to the extent to which a stress response might contribute to the modified hippocampus-related behaviors, LTP induction, and molecular expressions of the NE rats in this study remains an open question.
The DG of hippocampal formation receives multisensory (e.g., vestibular, olfactory, visual, auditory) inputs, mainly through the PP from the entorhinal cortex. This establishes it as the main gateway for sensory information into the hippocampus (32). The DG mediates a variety of mnemonic processes associated with multisensory inputs, and it could be one of the hippocampal subregions that is most vulnerable to sensory stimuli. Indeed, recent studies have shown that auditory learning specifically alters the parvalbumin expression of GABAergic interneurons in the DG rather than other subregions of the hippocampus (64). Thus, we primarily focused on the induction of LTP at the PP-DG granule cell synapses when investigating the effects of noise on synaptic plasticity. Further studies are needed to identify the roles of other pathways in the hippocampus (e.g., mossy fiber-CA3 and Schaffer collateral-CA1) in terms of how noise affects synaptic function and behavior.
It should be noted that responses to noise exposure might involve activation of a network of various brain regions other than the hippocampus, possibly including parts of the limbic structures (e.g., the amygdala and medial prefrontal cortex) (21, 23, 65–67). While the behavioral assessments we applied in the present study (the Morris water maze test, Y-maze test, and novel object recognition test) mostly reflect the function of the hippocampus (27–31), we certainly cannot exclude the possibility that neurobiological changes in these limbic structures following noise exposure might also contribute to the observed behavioral deficits in the NE rats.
Materials and Methods
All procedures were approved by the Institutional Animal Care and Use Committee and complied with NIH standards.
Noise Exposure.
Female offspring of timed-pregnant Sprague–Dawley rats were used in the experiments. Offspring were cross-fostered to achieve groups of 10 per litter on p1. Pups and their mothers were then placed in the sound-shielded chamber for noise exposure 10 h per day (8:00 to 18:00), from p9 to the weaning day (i.e., p21). These NE pups after weaning were thereafter housed in groups of 3 to 4 per cage (41 × 26 × 20 cm, length × width × height) and were continuously exposed to noise in the sound-shielded chamber until p56. During noise exposure, 50-ms noise pulses (5-ms ramps) delivered at ∼65 dB SPL were applied from a speaker placed ∼15 cm above rats at 6 pulses per second (pps). There was an interval of silence (randomly set at 0.5 or 1 ms) between every six noise pulses to minimize adaptation effects. Energy for pulsed noise stimuli was essentially flat across a broad frequency spectrum (0.8 to 30 kHz). Rats were given free access to food and water under a 12-h light/12-h dark cycle. The naïve rats were reared under the same conditions but without exposure to noise. Researchers were held blind to the group identity of the rats.
Open Field Test.
The apparatus for the open-field test was a rectangular box (42 × 42 × 37 cm, length × width × height), with side walls made of transparent plastic. During the test, the rats were placed at the center of box facing the wall, and were left to freely explore over a 10-min period. Total distance traveled and time spent in the central zone (i.e., central area of 28.9 × 28.9 cm) were analyzed using the True-Scan System (Coulbourn Instruments). The box was wiped clean with 75% ethanol after each session.
Elevated-Zero Maze Test.
The elevated-zero maze is an annular platform (5.5 cm width) with an outer diameter of 92 cm, divided into two open parts and two opposite closed parts (SI Appendix, Fig. S3A). While the closed parts were enclosed by side walls of 20 cm height, there were no walls for the open parts. The apparatus was elevated 50 cm above the floor.
During the test, the rats were placed into one of the open parts facing the closed part, and were allowed to freely explore the maze for 5 min. The behavior of each rat was recorded by a digital camera and was analyzed using the Any-maze system (Stoelting). The maze was wiped clean with 75% ethanol after each session.
Morris Water Maze Test.
The water maze consisted of a circular tank (150 cm diameter, 50 cm height) with a black inside wall, a digital camera, and a computer with a tracking system. The circular tank was elevated 30 cm off the ground on a secure platform and was filled with water. An escape platform (10 cm diameter) was placed at the center of the southeast quadrant, ∼2 cm below the water surface.
During the training phase, the rats were given 90 s to find the platform each trial. The rats were then allowed to rest at the platform for 20 s before the next trial. Each rat was trained four trials per day for four days. After four days’ training, the rats underwent a 90-s test period in the following day when the platform was removed. The behavior of each rat during the training and test phases was recorded by a digital camera and was analyzed using the Any-maze system (Stoelting).
Y-Maze Test.
The Y-maze apparatus is composed of three identical arms (45 × 15 × 35 cm, length × width × height; labeled as the start arm, the other arm, and the novel arm, respectively) arranged 120° apart around a central joining region (Fig. 2A).
During the training phase, the rats were placed at the start arm facing the wall, and were allowed to explore the maze for 10 min with one of the arms (i.e., the novel arm) closed. The rats were then returned to the home cage until the test phase 1 h later, during which the previously blocked arm was accessible and the rats were allowed to investigate all arms for 8 min. Behavior of each rat during the test was recorded by a digital camera and analyzed using the Any-maze system (Stoelting). The maze was wiped clean with 75% ethanol after each session.
Novel Object Recognition Test.
The apparatus for the novel object recognition test is a nontransparent plastic box (40 × 40 × 40 cm, length × width × height). The test consisted of a habituation phase on day 1 and 2, and a training phase followed by a test on day 3. For the habituation phase, the rats were placed in the box (with no stimulating objects inside) facing the wall, and were allowed to roam in the box for 10 min each day. During the training phase, the rats were again placed in the box and allowed to explore two identical objects inside for 5 min. The rats were returned to the home cage at the end of the training phase. After a 1-h interval, the rats were placed back into the box for the test phase, during which the rats were exposed to a new novel object and the familiar object, which are the same ones from the training phase, for an additional 5 min. The box and the objects inside were wiped clean with 75% ethanol after each session.
The exploration time of each object was defined as time during which the rat was touching the object with the nose and/or directing the nose to the object at a distance of no more than 2 cm. The preference index was calculated as the percentage of time spent at each object divided by the time spent exploring both objects. The discrimination ratio was then calculated as the difference between preference indices of two objects at both the training and test phases.
ABR Measurement.
ABR was measured in the shielded, double-walled sound chamber. The rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg body weight). Tone pips of 3, 10, 15, or 20 kHz at different intensities were generated using TDT System III (Tucker-Davis Technologies), and were delivered to the ear through a calibrated earphone with a sound tube positioned inside the external auditory meatus. ABRs were recorded by placing silver wires subdermally at the scalp midline, posterior to the stimulated ear, and on the midline of the back 1 to 2 cm posterior to the neck. During the recording, a state of areflexia was maintained with supplemental doses of 8 mg/mL diluted pentobarbital injected intraperitoneally. The animal’s body temperature was monitored using a rectal probe and maintained at ∼37 °C by a feedback-controlled heating blanket.
ABR signals were acquired, filtered, amplified, and analyzed using equipment and software (BioSig) manufactured by Tucker-Davis Technologies. The ABR threshold was defined as the lowest stimulus intensity capable of eliciting a response pattern characteristic of that seen at higher intensities (SI Appendix, Fig. S1A).
In Vivo LTP Induction.
The experiment was conducted in the shielded, double-walled sound chamber. Briefly, the rats were anesthetized with sodium pentobarbital (50 mg/kg body weight) and were fixed in a stereotaxic head holder. After making an incision along the midline of the head, the skin freed and the skull tissues were removed to make the sutures visible. A concentric bipolar-stimulating electrode (FHC) was then placed in the PP (AP: −7.8 mm from bregma; ML: +4.3 mm from bregma; DV: 3.2 mm from the skull surface), and a glass micropipette recording electrode (filled with 2 M NaCl with resistance of 1 to 3 MΩ) was lowered into the granular cell layer of the ipsilateral DG (AP: −3.8 mm from bregma; ML: +2.3 mm from bregma; DV: 2.7 to 3.2 mm from the skull surface) (Fig. 4A). Throughout the surgical procedures and during the recording session, a state of areflexia was maintained with supplemental doses of 8 mg/mL diluted pentobarbital injected intraperitoneally. The animal’s body temperature was monitored using a rectal probe and maintained at ∼37 °C by a feedback-controlled heating blanket.
The evoked potential was extracellularly recorded from the granule cell layer of the DG in response to electrical stimulation of the PP. The intensity of the test stimulation (0.15 ms duration at intervals of 30 s) was set to a level which evoked a PS of 40% of the maximum amplitude. After a 20-min stable baseline, HFS (8 pulses per burst at 100 Hz with bursts repeated at 5 Hz for a total of 10 bursts) was applied to the PP at the same stimulus intensity. LTP was evaluated by measuring changes in the amplitude of PS (recorded every 30 s for 60 min following the cessation of HFS).
The Master-8 stimulator (AMPI), MultiClamp 700B amplifier (Molecular Devices), and Clampex 9.0 Software (Molecular Devices) were used to generate the stimuli, amplify the signal, and acquire and store the data for offline analysis.
At the end of each recording session, small electrolytic lesions were made to permit histological verification of the tip position of the electrodes (SI Appendix, Fig. S6).
Corticosterone Analysis.
The rats were anesthetized with isoflurane and a lateral tail vein sampling was used for the blood collection. Blood samples were obtained between 9:00 to 10:00 for both the NE and naïve rats. Plasma was separated by centrifugation at 4 °C for 20 min at 1000 × g and stored at −80 °C. Corticosterone concentration was quantified using an enzyme-linked immunosorbent assay (ELISA) kit (X-Y Biotechnology).
Protein Isolation.
The rats were deeply anesthetized with sodium pentobarbital (80 mg/kg body weight). The hippocampus of each rat was removed and the DG was dissected out at 4 °C. As described previously (68), the tissue was homogenized in Buffer A containing 0.32 M sucrose, 1 mM MgCl2, a protease inhibitor mixture using a Teflon/glass homogenizer. The homogenates were centrifuged at 800 × g for 10 min to yield Pellet 1 (P1) and Supernatant 1 (S1) fractions. The S1 fraction was centrifuged at 10,000 × g for 10 min to yield the cytoplasmic S2 and the P2 that contains membranes and synaptosomes. The P2 pellet was resuspended in 3 mL of 0.32 M sucrose, layered onto 2.25 mL of 0.8 M sucrose, and centrifuged at 9,100 × g for 15 min using a swinging bucket rotor. Synaptosomes were collected from the 0.8 M sucrose layer and concentrated by centrifugation at 20,800 × g for 1 h. Synaptosomes were resuspended in Buffer B containing 30 mM Tris (pH 8.5), 5 mM magnesium acetate, 8 M urea, and 4% W/V CHAPS.
Western Blotting.
A portion of each sample (40 μg) was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and was transferred to nitrocellulose filter membranes (Millipore). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS) (150 mM NaCl, 20 mM Tris⋅HCl, pH 7.4) for 1 h at room temperature (RT) and then incubated overnight at 4 °C with primary antibodies. After washing with TBST (containing 0.1% Tween 20 in TBS) for 3 times, horseradish-peroxidase coupled secondary antibodies were applied for 1 h at RT. After additional washes, the membranes were analyzed by the ChemiDoc XRS+ System (Bio-Rad) and quantified by the Quantity One (Bio-Rad).
The following primary antibodies were used in this study: rabbit anti-GluR1 (1:2,000; Abcam), rabbit anti-CaMKII (1:2,000; Abcam), rabbit anti-phospho-GluR1 (ser831) (1:1,000; Cell Signaling Technology), rabbit anti-phospho-CaMKII (thr286) (1:1,000; Cell Signaling Technology), and rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:5,000; Abways).
Supplementary Material
Acknowledgments
We thank Dr. Robert Froemke for helpful comments on an earlier version of this paper. This work was supported by the National Natural Science Foundation of China (grant numbers 31771145 and 91632108), the Key Project of Shanghai Science and Technology Commission (grant number 18JC1412600), the Program of Introducing Talents of Discipline to Universities (grant number B16018), and the matching fund from the New York University-East China Normal University (NYU-ECNU) Institute of Brain and Cognitive Science at NYU Shanghai.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2017841117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Basner M., et al. , Auditory and non-auditory effects of noise on health. Lancet 383, 1325–1332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gourévitch B., Edeline J. M., Occelli F., Eggermont J. J., Is the din really harmless? Long-term effects of non-traumatic noise on the adult auditory system. Nat. Rev. Neurosci. 15, 483–491 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Ouda L., Burianová J., Balogová Z., Lu H. P., Syka J., Structural changes in the adult rat auditory system induced by brief postnatal noise exposure. Brain Struct. Funct. 221, 617–629 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Eggermont J. J., Effects of long-term non-traumatic noise exposure on the adult central auditory system. Hearing problems without hearing loss. Hear. Res. 352, 12–22 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Bureš Z., Popelář J., Syka J., The effect of noise exposure during the developmental period on the function of the auditory system. Hear. Res. 352, 1–11 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Shore S. E., Wu C., Mechanisms of noise-induced tinnitus: Insights from cellular studies. Neuron 103, 8–20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steward O., Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J. Comp. Neurol. 167, 285–314 (1976). [DOI] [PubMed] [Google Scholar]
- 8.Baisden R. H., Woodruff M. L., Hoover D. B., Cholinergic and non-cholinergic septo-hippocampal projections: A double-label horseradish peroxidase-acetylcholinesterase study in the rabbit. Brain Res. 290, 146–151 (1984). [DOI] [PubMed] [Google Scholar]
- 9.Germroth P., Schwerdtfeger W. K., Buhl E. H., Morphology of identified entorhinal neurons projecting to the hippocampus. A light microscopical study combining retrograde tracing and intracellular injection. Neuroscience 30, 683–691 (1989). [DOI] [PubMed] [Google Scholar]
- 10.Moxon K. A., et al. , Multiple single units and population responses during inhibitory gating of hippocampal auditory response in freely-moving rats. Brain Res. 825, 75–85 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Xiao C., Liu Y., Xu J., Gan X., Xiao Z., Septal and hippocampal neurons contribute to auditory relay and fear conditioning. Front. Cell. Neurosci. 12, 102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui B., Wu M., She X., Effects of chronic noise exposure on spatial learning and memory of rats in relation to neurotransmitters and NMDAR2B alteration in the hippocampus. J. Occup. Health 51, 152–158 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Kraus K. S., et al. , Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience 167, 1216–1226 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uran S. L., Aon-Bertolino M. L., Caceres L. G., Capani F., Guelman L. R., Rat hippocampal alterations could underlie behavioral abnormalities induced by exposure to moderate noise levels. Brain Res. 1471, 1–12 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Barzegar M., Sajjadi F. S., Talaei S. A., Hamidi G., Salami M., Prenatal exposure to noise stress: Anxiety, impaired spatial memory, and deteriorated hippocampal plasticity in postnatal life. Hippocampus 25, 187–196 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Hayes S. H., Manohar S., Majumdar A., Allman B. L., Salvi R., Noise-induced hearing loss alters hippocampal glucocorticoid receptor expression in rats. Hear. Res. 379, 43–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hygge S., Evans G. W., Bullinger M., A prospective study of some effects of aircraft noise on cognitive performance in schoolchildren. Psychol. Sci. 13, 469–474 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Lercher P., Evans G. W., Meis M., Ambient noise and cognitive processes among primary schoolchildren. Environ. Behav. 35, 725–735 (2003). [Google Scholar]
- 19.Stansfeld S. A.et al.; RANCH study team , Aircraft and road traffic noise and children’s cognition and health: A cross-national study. Lancet 365, 1942–1949 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Cheng L., Wang S. H., Chen Q. C., Liao X. M., Moderate noise induced cognition impairment of mice and its underlying mechanisms. Physiol. Behav. 104, 981–988 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Kraus K. S., Canlon B., Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear. Res. 288, 34–46 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Kim E. J., Pellman B., Kim J. J., Stress effects on the hippocampus: A critical review. Learn. Mem. 22, 411–416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jafari Z., Kolb B. E., Mohajerani M. H., Chronic traffic noise stress accelerates brain impairment and cognitive decline in mice. Exp. Neurol. 308, 1–12 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Moudon A. V., Real noise from the urban environment: How ambient community noise affects health and what can be done about it. Am. J. Prev. Med. 37, 167–171 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Kujala T., Brattico E., Detrimental noise effects on brain’s speech functions. Biol. Psychol. 81, 135–143 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Zhou X., Merzenich M. M., Environmental noise exposure degrades normal listening processes. Nat. Commun. 3, 843 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Morris R. G., Garrud P., Rawlins J. N., O’Keefe J., Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683 (1982). [DOI] [PubMed] [Google Scholar]
- 28.Morris R. G., Anderson E., Lynch G. S., Baudry M., Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319, 774–776 (1986). [DOI] [PubMed] [Google Scholar]
- 29.Moser E. I., Krobert K. A., Moser M. B., Morris R. G., Impaired spatial learning after saturation of long-term potentiation. Science 281, 2038–2042 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Conrad C. D., Galea L. A., Kuroda Y., McEwen B. S., Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 110, 1321–1334 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Heldt S. A., Stanek L., Chhatwal J. P., Ressler K. J., Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 12, 656–670 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolls E. T., A theory of hippocampal function in memory. Hippocampus 6, 601–620 (1996). [DOI] [PubMed] [Google Scholar]
- 33.Nicoll R. A., Malenka R. C., Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann. N. Y. Acad. Sci. 868, 515–525 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Lisman J., Schulman H., Cline H., The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 3, 175–190 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Lisman J., Yasuda R., Raghavachari S., Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13, 169–182 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shonesy B. C., Jalan-Sakrikar N., Cavener V. S., Colbran R. J., CaMKII: A molecular substrate for synaptic plasticity and memory. Prog. Mol. Biol. Transl. Sci. 122, 61–87 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Baudry M., et al. , Multiple cellular cascades participate in long-term potentiation and in hippocampus-dependent learning. Brain Res. 1621, 73–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicoll R. A., A brief history of long-term potentiation. Neuron 93, 281–290 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Zhou X., Merzenich M. M., Enduring effects of early structured noise exposure on temporal modulation in the primary auditory cortex. Proc. Natl. Acad. Sci. U.S.A. 105, 4423–4428 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X., Merzenich M. M., Intensive training in adults refines A1 representations degraded in an early postnatal critical period. Proc. Natl. Acad. Sci. U.S.A. 104, 15935–15940 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X., Merzenich M. M., Developmentally degraded cortical temporal processing restored by training. Nat. Neurosci. 12, 26–28 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu X., et al. , Environmental acoustic enrichment promotes recovery from developmentally degraded auditory cortical processing. J. Neurosci. 34, 5406–5415 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hensch T. K., Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Dahmen J. C., King A. J., Learning to hear: Plasticity of auditory cortical processing. Curr. Opin. Neurobiol. 17, 456–464 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Kral A., Auditory critical periods: A review from system’s perspective. Neuroscience 247, 117–133 (2013). [DOI] [PubMed] [Google Scholar]
- 46.de Villers-Sidani E., Merzenich M. M., Lifelong plasticity in the rat auditory cortex: Basic mechanisms and role of sensory experience. Prog. Brain Res. 191, 119–131 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Alberini C. M., Travaglia A., Infantile amnesia: A critical period of learning to learn and remember. J. Neurosci. 37, 5783–5795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Insanally M. N., Köver H., Kim H., Bao S., Feature-dependent sensitive periods in the development of complex sound representation. J. Neurosci. 29, 5456–5462 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhumika S., et al. , A late critical period for frequency modulated sweeps in the mouse auditory system. Cereb. Cortex 30, 2586–2599 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang E. F., Merzenich M. M., Environmental noise retards auditory cortical development. Science 300, 498–502 (2003). [DOI] [PubMed] [Google Scholar]
- 51.de Villers-Sidani E., Simpson K. L., Lu Y. F., Lin R. C., Merzenich M. M., Manipulating critical period closure across different sectors of the primary auditory cortex. Nat. Neurosci. 11, 957–965 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitlock J. R., Heynen A. J., Shuler M. G., Bear M. F., Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Lynch G., Cox C. D., Gall C. M., Pharmacological enhancement of memory or cognition in normal subjects. Front. Syst. Neurosci. 8, 90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang Y. P., et al. , Genetic enhancement of learning and memory in mice. Nature 401, 63–69 (1999). [DOI] [PubMed] [Google Scholar]
- 55.Manabe T., et al. , Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature 394, 577–581 (1998). [DOI] [PubMed] [Google Scholar]
- 56.Giese K. P., Fedorov N. B., Filipkowski R. K., Silva A. J., Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279, 870–873 (1998). [DOI] [PubMed] [Google Scholar]
- 57.Silva A. J., Stevens C. F., Tonegawa S., Wang Y., Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science 257, 201–206 (1992). [DOI] [PubMed] [Google Scholar]
- 58.Silva A. J., Paylor R., Wehner J. M., Tonegawa S., Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science 257, 206–211 (1992). [DOI] [PubMed] [Google Scholar]
- 59.Chen H. X., Otmakhov N., Strack S., Colbran R. J., Lisman J. E., Is persistent activity of calcium/calmodulin-dependent kinase required for the maintenance of LTP? J. Neurophysiol. 85, 1368–1376 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Lau C. G., Zukin R. S., NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 8, 413–426 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Gambrill A. C., Storey G. P., Barria A., Dynamic regulation of NMDA receptor transmission. J. Neurophysiol. 105, 162–171 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim J. J., Foy M. R., Thompson R. F., Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc. Natl. Acad. Sci. U.S.A. 93, 4750–4753 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu L., et al. , Noise induced hearing loss impairs spatial learning/memory and hippocampal neurogenesis in mice. Sci. Rep. 6, 20374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng Y., et al. , Positive impacts of early auditory training on cortical processing at an older age. Proc. Natl. Acad. Sci. U.S.A. 114, 6364–6369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Devilbiss D. M., Jenison R. L., Berridge C. W., Stress-induced impairment of a working memory task: Role of spiking rate and spiking history predicted discharge. PLoS Comput. Biol. 8, e1002681 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen G. D., Sheppard A., Salvi R., Noise trauma induced plastic changes in brain regions outside the classical auditory pathway. Neuroscience 315, 228–245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devilbiss D. M., Spencer R. C., Berridge C. W., Stress degrades prefrontal cortex neuronal coding of goal-directed behavior. Cereb. Cortex 27, 2970–2983 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin D. M., et al. , Reversal of behavioral deficits and synaptic dysfunction in mice overexpressing neuregulin 1. Neuron 78, 644–657 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.