Summary
Social insects exhibit extensive phenotypic diversities among the genetically similar individuals, suggesting a role for the epigenetic regulations beyond the genome level. The ADAR-mediated adenosine-to-inosine (A-to-I) RNA editing, an evolutionarily conserved mechanism, facilitates adaptive evolution by expanding proteomic diversities. Here, we characterize the A-to-I RNA editome of honeybees (Apis mellifera), identifying 407 high-confidence A-to-I editing sites. Editing is most abundant in the heads and shows signatures for positive selection. Editing behavior differs between foragers and nurses, suggesting a role for editing in caste differentiation. Although only five sites are conserved between bees and flies, an unexpectedly large number of genes exhibit editing in both species, albeit at different locations, including the nonsynonymous auto-editing of Adar. This convergent evolution, where the same target genes independently acquire recoding events in distant diverged clades, together with the signals of adaptation observed in honeybees alone, further supports the notion of recoding being adaptive.
Subject areas: Genetics, Evolutionary Biology, Genetic Engineering, Genomics
Graphical abstract

Highlights
-
•
Nonsynonymous editing sites in honeybees were under positive selection
-
•
Differential editing may contribute to the phenotypic diversity between sub-castes
-
•
Target genes acquire editing in different clades, suggesting convergent evolution
Genetics; Evolutionary Biology; Genetic Engineering; Genomics
Introduction
Adenosine (A)-to-inosine (A-to-I) RNA editing, catalyzed by enzymes of the ADAR (adenosine deaminase acting on RNA) family (Savva et al., 2012b), is an evolutionarily conserved mechanism that expands RNA diversity at the co-transcriptional or post-transcriptional level in metazoans (Bass 2002; Nishikura 2010; Eisenberg and Levanon 2018). Two catalytically active Adar genes are encoded in most metazoans. However, insects have lost ADAR1 and encode only a single Adar gene (Keegan et al., 2011). Due to the structural similarity between inosine (I) and guanosine (G), I is generally believed to be recognized as G in many cellular processes such as mRNA splicing (Rueter et al., 1999; Flomen et al., 2004; Jin et al., 2007; Lev-Maor et al., 2007), microRNA (miRNA) biogenesis or target recognition (Liang and Landweber 2007; Borchert et al., 2009; Alon et al., 2012), and mRNA translation (Basilio et al., 1962; Licht et al., 2019). Therefore, an A-to-I RNA editing usually has a similar effect as an A-to-G DNA substitution. A-to-I editing plays essential roles in many biological and physiological processes (Keegan et al., 2001; Nishikura 2010), and dysregulation of A-to-I editing might be associated with cancer, autoimmune disorders, or other human diseases (Gallo et al., 2017).
During the past two decades, A-to-I RNA editing sites have been systematically characterized in various metazoan species (Ramaswami and Li 2016). The majority of RNA editing sites are located in clusters in non-coding regions of humans (Athanasiadis et al., 2004; Blow et al., 2004; Kim et al., 2004; Levanon et al., 2004; Picardi et al., 2017), monkeys (Chen et al., 2014; Yang et al., 2015), mice (Neeman et al., 2006; Danecek et al., 2012), worms (Morse and Bass 1999; Zhao et al., 2015; Goldstein et al., 2017), corals (Porath et al., 2017b), and many other species (Porath et al., 2017a). In most species, only a minute fraction of the edits resides within the coding sequence. Notable exceptions are Drosophila (Graveley et al., 2011; Rodriguez et al., 2012; St Laurent et al., 2013; Mazloomian and Meyer 2015; Yu et al., 2016; Buchumenski et al., 2017; Duan et al., 2017; Zhang et al., 2017) and cephalopod species (Alon et al., 2015; Liscovitch-Brauer et al., 2017). Despite the deep conservation of A-to-I editing mechanism, the target landscapes of editing have considerably evolved during metazoan evolution. Only one editing site is known to be conserved across virtually all mammals, Drosophila, and cephalopods (Porath et al., 2019), and it thus seems that RNA editing modulates the diversity of the transcriptomes and proteomes in a lineage-specific manner.
The forces driving the evolution of A-to-I editing across species at the macro-evolutional scale are not well understood. RNA editing was hypothesized to facilitate adaptive evolution by increasing proteomic diversities temporally or spatially, in a manner more flexible than genomic mutations (Gommans et al., 2009; Nishikura 2010, 2016; Klironomos et al., 2013; Rosenthal 2015). However, in most species, the studied ratio of nonsynonymous (N) to synonymous (S) editing sites (N/S) is lower than expected for random sites, suggesting that recoding events may be overall non-adaptive (Xu and Zhang 2014). Here too Drosophila and cephalopods stand out as exceptions, exhibiting high N/S ratios indicating positive selection of recoding. Furthermore, hundreds of recoding sites were shown to be conserved across the Drosophila lineage (Graveley et al., 2011; Rodriguez et al., 2012; St Laurent et al., 2013; Mazloomian and Meyer 2015; Yu et al., 2016; Buchumenski et al., 2017; Duan et al., 2017; Zhang et al., 2017) and thousands in the behaviorally sophisticated cephalopods (Garrett and Rosenthal 2012; Alon et al., 2015; Liscovitch-Brauer et al., 2017), supporting the functional importance of these editing events. However, in only a few examples was the advantageous effect conferred by RNA editing explicitly demonstrated (see for example [Garrett and Rosenthal 2012]), and even it is not yet clear to what extent editing is indeed utilized for proteome diversification.
Social insects, including bees and ants, show extensive phenotypic plasticity. The morphologically and behaviorally differentiated social castes such as queens, workers, and drones have the same set of diploid or haploid genomes. The social insects provide us with model systems to study how phenotypic diversity is regulated (Page et al., 2012; Yan et al., 2014). RNA editing is well suited to contribute to the behavioral variation among genetically similar individuals. Indeed, differential editing was demonstrated for a few sites in leaf-cutting ants and in worker bumblebees (Li et al., 2014; Porath et al., 2019).
Here, we wish to study the contribution of RNA editing to caste differentiation in honeybee (Apis mellifera), an important pollinator and a model for complicated social behaviors in insects (Page et al., 2012). Unlike bumblebees, honeybees have a sharp task specialization, with distinct worker castes. Genetic mapping demonstrated that the phenotypic plasticity in honeybees is associated with complex epistatic and pleiotropic genetic networks that influence reproductive regulation and foraging behaviors (Page et al., 2012). We wish to check whether A-to-I RNA editing may contribute to the proteomic diversity underlying this phenotypic plasticity. We investigate the A-to-I RNA editome in different tissues of four honeybee drone individuals and detect over four hundred A-to-I sites in multiple tissues. Editing is enriched in the head and exhibits signs for positive selection and a particularly high N/S ratio. We show editing is elevated in foragers compared to nurses. Five editing sites are conserved between honeybee, bumblebee, and Drosophila. Interestingly, we find a significantly high number of cases where the same gene is edited in both bees and flies, even if not at the same position. One example is the auto-editing of Adar mRNA in bees and flies, which might play an auto-regulatory role in the two clades. This finding may further support possible convergent evolution and adaptation.
Results
Editome of honeybee
We deep sequenced genomic DNA and RNA from the head, thorax, and abdomen for each of four individual drones (12 RNA-seq samples) (Figure 1A). The transcriptome libraries of drones 1 and 2 were constructed by selecting polyA tailed mRNAs while the libraries of drones 3 and 4 were constructed by using the Ribo-Zero kit to deplete ribosomal RNAs (Transparent methods). The male drone is haploid, simplifying identification of its genomic single nucleotide polymorphisms (SNPs). DNA-Seq reads were mapped to the reference genome to identify 826,890-858,949 SNPs in each of the four drones and 1,303,225 unique sites combined (Transparent methods; Table S1). Median sequencing coverage at the SNP sites was 39, and in 96.6% of the sites, all DNA reads supported the SNP (Figure S1). The identified SNPs were then used to produce a masked genome version where the reference allele is substituted by the individual-specific alleles identified for each drone to facilitate accurate detection of RNA editing sites (Figure 1B; Transparent methods).
Figure 1.
Identification and annotation of the A-to-I RNA editing sites in honeybee
(A) Workflow of sample collection, dissection, and library construction.
(B) Identification of A-to-I RNA editing sites is facilitated by mapping to both the reference genome and the masked genome.
(C) Distribution of DNA-RNA mismatch types detected in honeybee.
(D) Distribution of the detected A-to-I editing sites over different genic regions.
(E) Editing levels at the detected sites as measured in three tissues of each of four individuals (D1-D4). Editing levels are higher in the head (head versus pooled thorax and abdomen, Wilcoxon rank-sum test; ∗∗∗: P < 0.001. Exact p values, left to right: 2.1 × 10−35, 5.7 × 10−20, 4.0 × 10−29, and 3.9 × 10−38).
(F) The editing profiles for the twelve tissues cluster according to their tissue of origin.
For each of the 12 RNA-Seq samples, 9.2-21.5 million reads were uniquely mapped to the reference genome, and similar numbers of reads were mapped to the masked genome sequences (Transparent methods; Table S2). Analyzing pooled data for each of the three tissues separately, we identified (false discovery rate [FDR] = 0.05) 376 (84.3%) A-to-G sites among 446 variations in heads, 106 (66.7%) A-to-G sites among 159 variations in thoraxes, and 137 (67.5%) A-to-G sites among 203 variations in abdomens (Figure S2). Combined, we obtained 407 (80.1%) unique A-to-G sites among 508 variations (Figure 1C). These 407 sites are regarded as A-to-I RNA editing sites (Table S3). The nucleotide context around these putative editing sites is consistent with the known ADAR binding motif (Figure S3). The median DNA coverage (over the 407 A-to-I editing sites) was 25 reads, sufficient to exclude possible SNPs (Figure S4).
To improve mapping accuracy, we discarded variants in repeat regions and required a variation site to be found by mapping to both the reference genome and the masked genome (Transparent methods). To test whether these criteria are too stringent, we checked the variation sites discarded by the two filtering steps. First, we looked at the variation sites located in the repeat regions. Without any filter, we found 19,573 unique variation sites that overlapped with repeat regions, only 3,694 (19%) of which were A-to-G variants. Following a binomial test and multiple testing correction (to remove random sequencing errors), 1,277 variation sites were maintained, only 324 (25%) of them A-to-G variations. Thus, filtering repetitive regions does contribute appreciably to the precision of our detection. Consistently, hyper-editing analysis did not reveal multiple sites in repetitive regions (Transparent methods).
As for the masked genome filter, only 38% (98/256) of the variation sites found by mapping to the masked genome but not to the reference genome are A-to-G mismatches. Following binomial test for sequencing error and multiple testing correction for p values, only 41 variation sites were maintained, none of them was an A-to-G variation. Conversely, only 31% (82/268) variation sites found by mapping to the reference genome but not to the masked genome are A-to-G mismatches. Of these 268 variation sites, 82 were maintained after multiple testing correction, 20 of them were A-to-G variations. Thus, most of the sites supported by only one method of mapping are likely not due to A-to-I RNA editing.
Among the 407 editing sites we identified, 199 sites are located in gene regions, including 111 nonsynonymous (N) sites, 9 synonymous (S) sites, and several sites in untranslated regions (UTRs) and introns (Figure 1D). The other 208 sites are annotated as intergenic. As expected from the different library construction strategies, the fraction of sites in coding regions is higher and the intergenic fraction is lower for sites observed in drones 1 and 2 compared with drones 3 and 4 (23.2 ± 2.6% in coding sequence (CDS) in drones 1 and 2 versus 14.5 ± 5.1% in CDS in drones 3 and 4; 54.9 ± 2.2% in intergenic regions in drones 1 and 2 versus 70.5 ± 5.4% in intergenic regions in drones 3 and 4). The number of sites for which editing is observed in each sample (per-sample editing level >0) varies considerably (40–278 sites per sample; Table S4). The partial overlap between these sets of sites is mostly due to sites being undetected at a given sample due to low per-sample coverage (Figure S5).
Interestingly, editing seems to be enriched in heads. The number of detected sites (Figure S6), the editing levels (Figure 1E), and the fraction of coding sites (Figure S7) are all higher in heads. Clustering the samples by their editing profile, the three tissue types form distinct clusters (Figure 1F). Consistently, Adar expression is also higher in heads than in other tissues (Figure S8). Looking at specific sites, 78 sites (out of 378 sites with sufficient coverage, see Transparent methods) are differentially edited in the head, compared to non-head tissues. In all of these 78 sites, editing is higher in the head (Table S3. Information of the candidate editing sites identified in this study, Figure 1).
Signals of adaptation
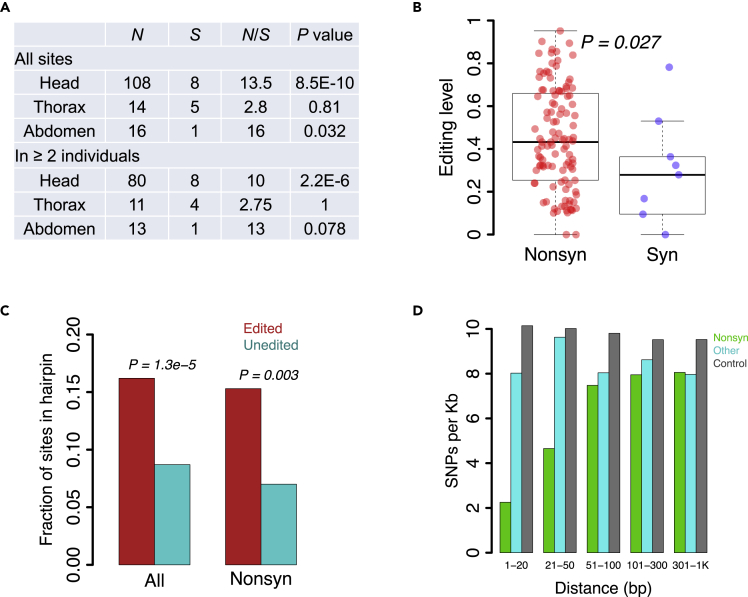
The nonsynonymous to synonymous (N/S) ratio for sites in the coding region is 111/9 = 12.3 (Table S4), compared to a ratio of 2.26 observed for random adenosine to guanosine substitutions (see Transparent methods). This strongly suggests that the nonsynonymous editing events in honeybees are overall adaptive. Notably, the N/S is exceptionally high, even compared to the ratios we previously found in the brains of Drosophila (Duan et al., 2017) or in cephalopods (Alon et al., 2015; Liscovitch-Brauer et al., 2017). Looking at each sample separately, at pooled tissue data, or focusing on sites that appear in at least two individuals per tissue all show the N/S ratio to be especially high in heads (Figure 2A and Table S4). Furthermore, the editing level at nonsynonymous sites is significantly higher than synonymous sites editing levels in heads of honeybees (Figure 2B).
Figure 2.
Signals of adaptation of A-to-I editing in honeybee
(A) The observed numbers of nonsynonymous (N) and synonymous (S) editing sites in each tissue. p values are obtained from Fisher's exact test, comparing the observed N and S counts to all adenosines in the coding sequence of in A. mel (N = 4,492,737 sites where A-to-G substitution is nonsynonymous and S = 1,986,130 for synonymous). “All sites” represents the numbers of sites that appear in at least one individual. Comparisons for sites appearing in at least two individuals are also shown.
(B) Editing levels of the nonsynonymous and synonymous sites in the head. Data from four drones were pooled to increase the statistical power. p values are calculated using Wilcoxon rank-sum test.
(C) The fraction of sites in predicted stable hairpin structures. The observed editing sites were compared to the unedited adenosines (p values using Fisher's exact test).
(D) The SNP density in the vicinity of nonsynonymous editing sites (green), other editing sites (cyan), or unedited adenosines (gray).
A previous study in cephalopods proposed that the coleoids massively edit their RNAs to diversify the transcriptome at the cost of constraining the evolution of genomic sequences (Liscovitch-Brauer et al., 2017). Maintaining beneficial editing requires genomic conservation of the genomic sequence encoding for the dsRNA structures that allow ADAR to bind and deaminate the adenosine. To test this, we first confirmed that the editing sites in honeybees are enriched in hairpin structures (Figure 2C). Then, we demonstrated that SNPs are depleted in the vicinity of nonsynonymous sites (Figure 2D), further supporting the notion of selective advantage for the nonsynonymous editing events that justifies the trade-off between transcriptome diversity and genome evolution.
Evolutionarily conserved and non-conserved editing sites
Editing at four nonsynonymous and one synonymous site is conserved between Drosophila and honeybees (Figure 3). Three of these sites are in the Shab transcript (shaker cognate b, two nonsynonymous and one synonymous) and the other two are in qvr (quiver). Editing at these sites is also observed in the bumblebee Bombus terrestris (Porath et al., 2019), and editing levels are high in all of these species (Figure 3). These findings suggest a potential functional role for these widely conserved editing sites. However, the majority of the editing sites are not shared between Drosophila and honeybees. For some sites, the editable adenosine is not conserved in the honeybee genome (Figure S9) and are thus clearly uneditable, while other adenosines are conserved but editing was not detected in RNA-Seq data (Figure S9). Overall, of the 639 Drosophila editing sites in CDS for which an orthologous site was identified in honeybees, 349 were conserved as A (55%), and 85, 127, and 78 (13%, 20%, and 12%) were mutated to C, G, and T, respectively.
Figure 3.
Editing sites conserved between bees and flies
(A) One synonymous and two nonsynonymous editing sites in Shab transcripts are edited in heads of two bee and four Drosophila species.
(B) Two nonsynonymous editing sites in qvr transcripts are edited in heads of two bee and four Drosophila species. The editing sites are colored red. Editing levels measured in male heads of flies (unpublished), pooled heads of honeybee drones, and brains of bumblebees (Porath et al., 2019) are shown. D. mel, Drosophila melanogaster; D. sim, Drosophila simulans; D. sec, Drosophila sechellia; D. ere, Drosophila erecta; D. pse, Drosophila pseudoobscura; D. wil, Drosophila willistoni; D. moj, Drosophila mojavensis; A. mel, Apis mellifera; B. ter, Bombus terrestris.
Similarly, most sites are not even conserved across the two bee species. The bumblebee study has reported 219 editing sites in CDS, 164 of which are nonsynonymous (Porath et al., 2019). Of these, we found 9 editing sites in coding regions conserved between honeybee and bumblebee (Table S5).
One of these conserved sites is a Ser > Gly site within the tipE (temperature-induced paralytic E; GB47375) transcript, editing levels of which correlate with task performance in bumblebees (nurses versus foragers) (Porath et al., 2019). Editing levels at this site in honeybee heads are ~0.7, similar to those observed in bumblebee brains. In the two other tissues, this gene is lowly expressed and poorly edited. The orthologous site in Drosophila genomes encodes the post-edit Gly codon GGC (Figure S10). Thus, tipE recoding seems to be bee specific, possibly related to social behavior and task performance.
Sex-dependent and caste-dependent editing
We further looked for potential sex-dependence and caste-dependent editing, comparing our honeybee drone head data with previously published brain RNA-seq data studying two sub-castes of female workers, foragers and reverted nurses (Herb et al., 2012) (Table S6). First, we compared the pooled editing levels between drones and workers for each well-covered (>10 reads in each pool) editing site separately. Twenty six sites exhibit differential editing (Wilcoxon rank-sum test; FDR = 0.05) between workers (females) and drones (males), including 4 recoding sites, 2 sites in 3′UTRs, 3 intronic sites, and 17 intergenic sites (Figure 4A and Table S7). In most cases, editing in drones is higher, but this may result from looking at sites identified in drone data to begin with. The difference in Adar expression was not statistically significant (Figure S11).
Figure 4.
Sex-dependent and caste-dependent editing
(A) Comparison of editing levels between drones (males) and workers (females). Statistically significant sites (FDR <0.05) are colored red.
(B) Adar expression level (reads count normalized by DESeq2) in nurses and foragers. Data are presented as mean ± SEM (standard error of mean). p value was calculated using Wilcoxon rank-sum test.
(C) Spearman's correlation between Adar expression and editing index (sum over all G alleles in all sites divided by sum of all the coverages in all sites) for all the nurse (orange) and forager (purple) samples.
(D) Comparison of editing levels between two sub-castes of workers: nurses and foragers. Statistically significant sites (FDR <0.3; see Results) are colored red.
(E and F) (E) PCA analysis of the editing profile shows a different behavior for nurses and foragers, while (F) PCA of the expression profile (genes with RPKM>1) does not lead to a clear separation.
Comparing the pooled editing levels between the two sub-castes (foragers and nurses), one finds 230 out of 407 sites with lower levels in nurses, compared to only 91 exhibiting higher levels in nurses (equal level, mostly zero, was observed in 84 sites), suggesting a globally higher editing activity (at the detected sites) in foragers (P = 1.3 × 10−14, proportion test). The surcharge of forager higher sites is maintained for various coverage and editing difference cutoffs (Figure S12). Furthermore, Adar expression (Figure 4B) and the editing index (Figure 4C) are both higher in foragers, and the editing index correlates with Adar expression. However, reliable detection of specific differentially edited sites with the available sample size (6 foragers and 6 nurses) is challenging, and not even a single site was identified with FDR = 0.05 (Wilcoxon rank-sum test). We have thus relaxed the statistical test, allowing for FDR = 0.3, and found 61 candidate sites, most of which are differentially edited (Figure 4D and Table S8). These include 10 nonsynonymous, 16 3′UTR, and 35 intergenic sites. Consistently, 55 of these sites exhibit higher levels of editing in foragers. Finally, PCA analysis of the editing profile across sites results in distinct clusters of drones, nurses, and foragers, a classification that is not achieved by the expression profile (Figures 4E and 4F). Taken together, these results show a global increase in editing in foragers compared to nurses.
Convergent adaptation of A-to-I editing?
One of the recoding sites conserved between the two bee species resides within Adar transcript (Table S5 and Figure 5A). In bumblebee, the recoding level of the conserved site, I482M (ATA to ATG), positively correlates with the global editing activity (Porath et al., 2019), suggesting a possible auto-regulation mechanism. Interestingly, Drosophila Adar is also auto-edited at a different position, where a Ser (AGT) to Gly (GGT) substitution leads to a less active ADAR protein, resulting in a negative feedback loop of editing activity (Palladino et al., 2000; Savva et al., 2012a). Intriguingly, fly-edited Ser amino acid is conserved in honeybee and bumblebee, but a different codon (TCA, uneditable at the first codon position) is used. Similarly, the bee-edited Ile is conserved in flies, but the edited adenosines (at the third position of ATA) is synonymously mutated to T, and the editable ATA bee codon is substituted by ATT codon in flies, which could not be edited at the third position (Figure 5A). Thus, while auto-editing S430G in flies is abolished in bees due to an uneditable Ser codon, and the auto-editing I482M in bees is abolished in flies due to an uneditable Ile codon. However, the mechanism of auto-editing, possibly used for global ADAR regulation, is shared by the two lineages, albeit at different positions and possibly with different effects on the protein. This might hint at the possibility of convergent evolution of ADAR auto-regulation strategy.
Figure 5.
Convergent evolution of editing
(A) Adar transcripts are auto-edited in Drosophila and bees. The Ser > Gly site is highly conserved across Drosophila species. The Drosophila-editable Ser codon AGT is changed to an uneditable Ser codon TCA in bees. On the other hand, the Ile > Met auto-editing site is conserved between honeybee and bumblebee. The bee-editable Ile codon ATA (edited at the third position) appears as an uneditable Ile codon ATT in flies. Editing sites are colored red. The editing levels from male heads of flies (unpublished), pooled heads of honeybee drones, and brains of bumblebee (Porath et al., 2019) are shown. D. mel, Drosophila melanogaster; D. sim, Drosophila simulans; D. sec, Drosophila sechellia; D. ere, Drosophila erecta; A. mel, Apis mellifera; B. ter, Bombus terrestris.
(B) Venn diagram demonstrating the overlap between orthologous genes with editing sites in coding regions.
(C) The observed number of orthologous genes with editing in coding regions, compared to the expected distribution. Genes with RPKM >1 in both species were chosen to calculate the expected numbers (results are essentially the same for other expression cutoffs, Figure S13). p values calculated by randomization test.
(D) The nonsynonymous to synonymous (N/S) ratios are significantly higher than expected, for both shared and species-specific sites (except for species-specific sites in B. terrestris). Here, the shared and species-specific sites refer to the sites in shared genes and species-specific genes as defined in the above Venn diagram in (B). The expected ratio is evaluated by calculating the effect of putative editing on all adenosines in the coding region. p values are obtained from Fisher's exact test.
Following this example, we wondered whether there are more genes for which editing is observed in both honeybees and Drosophila, even if the exact location of the editing site is not conserved. Excluding conserved sites, there are 53 genes exhibiting CDS editing in honeybee heads, 101 genes exhibiting CDS editing in bumblebee heads, and 312 genes edited in CDS in male brains of D. melanogaster. Of these, 21 genes exhibit editing in both bee species, 14 are edited in honeybee and Drosophila, 14 are edited in bumblebee and Drosophila, and six genes are found to be edited in all three species (Figure 5B and Table S9). These numbers are significantly higher than expected by random sampling, (p < 1 × 10−6, randomization test), even if one accounts for the expression profile (Transparent methods, Figures 5C and S13) (Table S9). We have repeated the analysis using stricter criteria to characterize sites, considering as edited only sites with an observed editing level >10% (all samples pooled), and a binomial P < 0.05 (excluding the null hypothesis of no editing), and considering a site to be not edited if the binomial P < 0.05 for the null hypothesis that the site is edited at 10%. Reassuringly, the results are robust to this change of definitions (Figures S14 and S15). These results suggest an interesting convergent evolution of A-to-I editing. Recoding sites are rarely conserved across clades, but the same genes in different lineages tend to acquire a recoding event, apparently independently, indicating a functional importance for recoding of this gene.
Discussion
Despite over fifteen years of developments, reliable detection of CDS editing remains a challenge. Systematic searches often lead to high false-positive rates (reflected by the fraction of non-AG mismatches observed), especially for mammalian transcriptomes where the scope of recoding is rather low. Important exceptions are Drosophila and cephalopods, where the recoding signal is much more pronounced and easier to detect. Applying a novel strict alignment approach, we were able to achieve here a high accuracy in CDS editing detection (84.3% in heads) despite the overall modest scope of recoding sites. Another similarity between honeybees Drosophila and cephalopods is related to the important question regarding the extent to which recoding is adaptive. This is often estimated by the N/S ratio, where Drosophila and cephalopods exhibit a pattern markedly different from the one seen in other species studied (including human). In most species, the N/S ratio observed is lower or similar to the one expected under neutrality, and it is significantly higher in Drosophila and cephalopods. Again, we find here that honeybees show a strikingly high N/S ratio, similar to the above two clades. We suggest that the two last points are interconnected. The apparently low N/S ratio observed in many species (human included) may reflect the low accuracy of the lists of CDS editing sites and not the (lack of) adaptive potential for recoding in these species.
The importance of post-transcriptional and post-translational mechanisms in generating the proteomic complexity of higher organisms has been emphasized in recent decades. These epigenetic mechanisms allow for diversification of the proteome and functional heterogeneity across tissues, developmental stages, brain regions, or even among individual cells.
Recoding by A-to-I RNA editing is an epigenetic mechanism capable of diversifying the proteome, creating a range of proteins from a single genomically encoded gene in a temporally regulated, tissue-specific, condition-dependent way and providing the organism with a new means for acclimation and adaptation. Indeed, several studies have demonstrated how recoding levels at specific sites do change as a function of the organism's condition (Garrett and Rosenthal 2012; Robinson et al., 2016; Gallo et al., 2017; Porath 2017; Terajima et al., 2017; Yablonovitch et al., 2017). Importantly, many studies have demonstrated altered editing of individual recoding targets in various disease states (Gallo et al., 2017). However, these interesting examples notwithstanding, the extent to which the recoding phenomenon is actually used as a means for proteome diversification is still under debate. In fact, several recent studies have raised the possibility that nonsynonymous editing may be used to compensate for otherwise deleterious G-to-A mutations, rather than allowing for the two alleles (A and G) to co-exist (Jiang and Zhang 2019; Mai and Chuang 2019; Popitsch et al., 2020). Thus, a high N/S ratio is not sufficient to prove that editing serves for adaptation through proteome diversification.
The behavioral, physiological, and morphological plasticity exhibited by social insects suggests an important role for epigenetic mechanisms. Differential RNA editing was previously shown for different castes of the highly social leaf-cutting ant Acromyrmex echinatior (Li et al., 2014), as well as for bumblebee workers (Porath et al., 2019). Our results expand these finding to another species, further supporting the notion of RNA editing as a source of proteomic complexity, that may be recruited to provide phenotypic variation among genetically identical individuals.
ADAR enzymes and the recoding phenomenon are widely conserved across metazoan. However, the repertoire of recoding sites varies considerably and seems to have developed almost independently in different clades. Here, we point out that the same gene targets are being edited in distant species, even if the exact locations of the editing sites within the transcript vary. A particularly interesting example is that of nonsynonymous auto-editing of Adar, shown to affect ADAR activity in bumblebees, flies, and even mammals (Rueter et al., 1999; Savva et al., 2012a; Porath et al., 2019), possibly serving as global editing autoregulation. It thus seems possible that the selective advantage in having multiple versions of the protein product is shared by the target genes, while there is more than one specific way to achieve this diversity through recoding. Clades developed along different evolutionary routes have converged on different implementations (in terms of the specific editing site) of the same diversifying solution. These ideas should be further tested in the future, using larger data sets and across additional species and clades. Moreover, as biochemical and functional understanding of the impact of the different edits are gained, it will be possible to look into a possible common effect for the different edits of the common targets.
Limitations of the study
The editomes of the bee species analyzed here are based on a limited number of samples. They probably represent a subset of the actual repertoire of editing sites. The results should be re-tested when more data are available. In particular, the honeybee editome was built using drone samples. Thus, some individual differential editing between the nurses and foragers sub-castes may have missed worker-specific sites. However, the global increased editing in foragers, supported by a higher Adar expression, is probably robust.
Resource availability
Lead contact
Further information and requests for resources should be directly and will be fulfilled by the Lead Contact, Jian Lu (luj@pku.edu.cn).
Materials availability
This study did not generate new unique materials.
Data and code availability
All deep-sequencing data generated in this study were deposited in the China National Genomics Data Center Genome Sequence Archive (GSA) under accession number CRA002262. Other deep-sequencing data analyzed were downloaded from SRA as follows. Brains of honeybee workers: accession numbers SRR445999 to SRR446004 (reverted nurse) and SRR446005 to SRR446010 (forager) (Herb et al., 2012). Bumblebee: SRP166322 (Porath et al., 2019). D. mel head: SRP067542 (Zhang et al., 2018). D. sim head: SRP074828 (Duan et al., 2017). D. pse: DRR055250 and DRR055251 (Nozawa et al., 2016). D. wil: SRR341127 and SRR341129 (Meisel et al., 2012). D. moj: SRR037508-SRR037518 (generated by the Drosophila modENCODE project). D. sec, and D. ere data are unpublished data generated in our own lab. The reference genomes versions used for Drosophila species are Dmel_r6.04 (http://flybase.org/) for D. mel, and versions droSim1, droSec1, droEre1, dp4, droWil1, and droMoj3 downloaded from UCSC Genome Browser (http://genome.ucsc.edu/) for the other fly species. For the Bombus terrestris, we used version Bter_1.0.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
We thank the National Center for Protein Sciences at Peking University for technical assistance. Part of the analysis was performed on the High Performance Computing Platform of the Center for Life Science at Peking University. This work was supported by the joint NSFC-ISF program (NSFC grant number 3201101147 to J.L. and ISF grant number 3371/20 to E.E.), as well as grants from the Ministry of Science and Technology of the People's Republic of China (2016YFA0500800) and National Natural Science Foundation of China (91731301) to J.L. and support from the Israel Science Foundation (1945/18) to E.E.
Author contributions
J.L. designed the research; J.X.H. contributed the honeybee materials; S.Q.D. performed the research; Y.G.D., H.T.P., E.E., and J.L. performed the bioinformatics analyses; E.E. and J.L. wrote the paper.
Declaration of interests
The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101983.
Contributor Information
Eli Eisenberg, Email: elieis@post.tau.ac.il.
Jian Lu, Email: luj@pku.edu.cn.
Supplemental information
References
- Alon S., Garrett S.C., Levanon E.Y., Olson S., Graveley B.R., Rosenthal J.J., Eisenberg E. The majority of transcripts in the squid nervous system are extensively recoded by A-to-I RNA editing. Elife. 2015;4:e05198. doi: 10.7554/eLife.05198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon S., Mor E., Vigneault F., Church G.M., Locatelli F., Galeano F., Gallo A., Shomron N., Eisenberg E. Systematic identification of edited microRNAs in the human brain. Genome Res. 2012;22:1533–1540. doi: 10.1101/gr.131573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiadis A., Rich A., Maas S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol. 2004;2:e391. doi: 10.1371/journal.pbio.0020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilio C., Wahba A.J., Lengyel P., Speyer J.F., Ochoa S. Synthetic polynucleotides and the amino acid code. Proc. Natl. Acad. Sci. U S A. 1962;48:613–616. doi: 10.1073/pnas.48.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass B.L. RNA editing by adenosine deaminases that act on RNA. Annu. Rev. Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow M., Futreal P.A., Wooster R., Stratton M.R. A survey of RNA editing in human brain. Genome Res. 2004;14:2379–2387. doi: 10.1101/gr.2951204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchert G.M., Gilmore B.L., Spengler R.M., Xing Y., Lanier W., Bhattacharya D., Davidson B.L. Adenosine deamination in human transcripts generates novel microRNA binding sites. Hum. Mol. Genet. 2009;18:4801–4807. doi: 10.1093/hmg/ddp443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchumenski I., Bartok O., Ashwal-Fluss R., Pandey V., Porath H.T., Levanon E.Y., Kadener S. Dynamic hyper-editing underlies temperature adaptation in Drosophila. PLoS Genet. 2017;13:e1006931. doi: 10.1371/journal.pgen.1006931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.-Y., Peng Z., Zhang R., Yang X.-Z., Tan B.C.-M., Fang H., Liu C.-J., Shi M., Ye Z.-Q., Zhang Y.E. RNA editome in rhesus macaque shaped by purifying selection. PLoS Genet. 2014;10:e1004274. doi: 10.1371/journal.pgen.1004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P., Nellaker C., McIntyre R.E., Buendia-Buendia J.E., Bumpstead S., Ponting C.P., Flint J., Durbin R., Keane T.M., Adams D.J. High levels of RNA-editing site conservation amongst 15 laboratory mouse strains. Genome Biol. 2012;13:26. doi: 10.1186/gb-2012-13-4-r26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Dou S., Luo S., Zhang H., Lu J. Adaptation of A-to-I RNA editing in Drosophila. PLoS Genet. 2017;13:e1006648. doi: 10.1371/journal.pgen.1006648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Levanon E.Y. A-to-I RNA editing — immune protector and transcriptome diversifier. Nat. Rev. Genet. 2018;19:473–490. doi: 10.1038/s41576-018-0006-1. [DOI] [PubMed] [Google Scholar]
- Flomen R., Knight J., Sham P., Kerwin R., Makoff A. Evidence that RNA editing modulates splice site selection in the 5-HT2C receptor gene. Nucleic Acids Res. 2004;32:2113–2122. doi: 10.1093/nar/gkh536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo A., Vukic D., Michalik D., O'Connell M.A., Keegan L.P. ADAR RNA editing in human disease; more to it than meets the I. Hum. Genet. 2017;136:1265–1278. doi: 10.1007/s00439-017-1837-0. [DOI] [PubMed] [Google Scholar]
- Garrett S., Rosenthal J.J. RNA editing underlies temperature adaptation in K+ channels from polar octopuses. Science. 2012;335:848–851. doi: 10.1126/science.1212795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B., Agranat-Tamir L., Light D., Ben-Naim Zgayer O., Fishman A., Lamm A.T. A-to-I RNA editing promotes developmental stage–specific gene and lncRNA expression. Genome Res. 2017;27:462–470. doi: 10.1101/gr.211169.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommans W.M., Mullen S.P., Maas S. RNA editing: a driving force for adaptive evolution? BioEssays. 2009;31:1137–1145. doi: 10.1002/bies.200900045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley B.R., Brooks A.N., Carlson J.W., Duff M.O., Landolin J.M., Yang L., Artieri C.G., van Baren M.J., Boley N., Booth B.W. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471:473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herb B.R., Wolschin F., Hansen K.D., Aryee M.J., Langmead B., Irizarry R., Amdam G.V., Feinberg A.P. Reversible switching between epigenetic states in honeybee behavioral subcastes. Nat. Neurosci. 2012;15:1371–1373. doi: 10.1038/nn.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Zhang J. The preponderance of nonsynonymous A-to-I RNA editing in coleoids is nonadaptive. Nat. Commun. 2019;10:5411. doi: 10.1038/s41467-019-13275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Tian N., Cao J., Liang J., Yang Z., Lv J. RNA editing and alternative splicing of the insect nAChR subunit alpha6 transcript: evolutionary conservation, divergence and regulation. BMC Evol. Biol. 2007;7:1–12. doi: 10.1186/1471-2148-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan L.P., Gallo A., O'Connell M.A. The many roles of an RNA editor. Nat. Rev. Genet. 2001;2:869–878. doi: 10.1038/35098584. [DOI] [PubMed] [Google Scholar]
- Keegan L.P., McGurk L., Palavicini J.P., Brindle J., Paro S., Li X., Rosenthal J.J., O'Connell M.A. Functional conservation in human and Drosophila of Metazoan ADAR2 involved in RNA editing: loss of ADAR1 in insects. Nucleic Acids Res. 2011;39:7249–7262. doi: 10.1093/nar/gkr423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.D., Kim T.T., Walsh T., Kobayashi Y., Matise T.C., Buyske S., Gabriel A. Widespread RNA editing of embedded alu elements in the human transcriptome. Genome Res. 2004;14:1719–1725. doi: 10.1101/gr.2855504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klironomos F.D., Berg J., Collins S. How epigenetic mutations can affect genetic evolution: model and mechanism. BioEssays. 2013;35:571–578. doi: 10.1002/bies.201200169. [DOI] [PubMed] [Google Scholar]
- Lev-Maor G., Sorek R., Levanon E.Y., Paz N., Eisenberg E., Ast G. RNA-editing-mediated exon evolution. Genome Biol. 2007;8:R29. doi: 10.1186/gb-2007-8-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon E.Y., Eisenberg E., Yelin R., Nemzer S., Hallegger M., Shemesh R., Fligelman Z.Y., Shoshan A., Pollock S.R., Sztybel D. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat. Biotech. 2004;22:1001–1005. doi: 10.1038/nbt996. [DOI] [PubMed] [Google Scholar]
- Li Q., Wang Z., Lian J., Schiott M., Jin L., Zhang P., Zhang Y., Nygaard S., Peng Z., Zhou Y. Caste-specific RNA editomes in the leaf-cutting ant Acromyrmex echinatior. Nat. Commun. 2014;5:4943. doi: 10.1038/ncomms5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Landweber L.F. Hypothesis: RNA editing of microRNA target sites in humans? RNA. 2007;13:463–467. doi: 10.1261/rna.296407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht K., Hartl M., Amman F., Anrather D., Janisiw M.P., Jantsch M.F. Inosine induces context-dependent recoding and translational stalling. Nucleic Acids Res. 2019;47:3–14. doi: 10.1093/nar/gky1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch-Brauer N., Alon S., Porath H.T., Elstein B., Unger R., Ziv T., Admon A., Levanon E.Y., Rosenthal J.J.C., Eisenberg E. Trade-off between transcriptome plasticity and genome evolution in cephalopods. Cell. 2017;169:191–202.e11. doi: 10.1016/j.cell.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai T.L., Chuang T.J. A-to-I RNA editing contributes to the persistence of predicted damaging mutations in populations. Genome Res. 2019;29:1766–1776. doi: 10.1101/gr.246033.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazloomian A., Meyer I.M. Genome-wide identification and characterization of tissue-specific RNA editing events in D. melanogaster and their potential role in regulating alternative splicing. RNA Biol. 2015;12:1391–1401. doi: 10.1080/15476286.2015.1107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel R.P., Malone J.H., Clark A.G. Disentangling the relationship between sex-biased gene expression and X-linkage. Genome Res. 2012;22:1255–1265. doi: 10.1101/gr.132100.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D.P., Bass B.L. Long RNA hairpins that contain inosine are present in Caenorhabditis elegans poly(A)+ RNA. Proc. Natl. Acad. Sci. U S A. 1999;96:6048–6053. doi: 10.1073/pnas.96.11.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman Y., Levanon E.Y., Jantsch M.F., Eisenberg E. RNA editing level in the mouse is determined by the genomic repeat repertoire. RNA. 2006;12:1802–1809. doi: 10.1261/rna.165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annu. Rev. Biochem. 2010;79:321–349. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol. 2016;17:83–96. doi: 10.1038/nrm.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa M., Onizuka K., Fujimi M., Ikeo K., Gojobori T. Accelerated pseudogenization on the neo-X chromosome in Drosophila miranda. Nat. Commun. 2016;7:13659. doi: 10.1038/ncomms13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R.E., Jr., Rueppell O., Amdam G.V. Genetics of reproduction and regulation of honeybee (Apis mellifera L.) social behavior. Annu. Rev. Genet. 2012;46:97–119. doi: 10.1146/annurev-genet-110711-155610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino M.J., Keegan L.P., O'Connell M.A., Reenan R.A. dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing. RNA. 2000;6:1004–1018. doi: 10.1017/s1355838200000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardi E., D'Erchia A.M., Lo Giudice C., Pesole G. REDIportal: a comprehensive database of A-to-I RNA editing events in humans. Nucleic Acids Res. 2017;45:D750–D757. doi: 10.1093/nar/gkw767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popitsch N., Huber C.D., Buchumenski I., Eisenberg E., Jantsch M., von Haeseler A., Gallach M. A-to-I RNA editing uncovers hidden signals of adaptive genome evolution in animals. Genome Biol. Evol. 2020;12:345–357. doi: 10.1093/gbe/evaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath H.T. A-To-I RNA editing in the earliest-diverging eumetazoan phyla. Mol. Biol. Evol. 2017;34:1890–1901. doi: 10.1093/molbev/msx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath H.T., Hazan E., Shpigler H., Cohen M., Band M., Ben-Shahar Y., Levanon E.Y., Eisenberg E., Bloch G. RNA editing is abundant and correlates with task performance in a social bumblebee. Nat. Commun. 2019;10:1605. doi: 10.1038/s41467-019-09543-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath H.T., Knisbacher B.A., Eisenberg E., Levanon E.Y. Massive A-to-I RNA editing is common across the Metazoa and correlates with dsRNA abundance. Genome Biol. 2017;18:185. doi: 10.1186/s13059-017-1315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath H.T., Schaffer A.A., Kaniewska P., Alon S., Eisenberg E., Rosenthal J., Levanon E.Y., Levy O. A-to-I RNA editing in the earliest-diverging eumetazoan phyla. Mol. Biol. Evol. 2017;34:1890–1901. doi: 10.1093/molbev/msx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami G., Li J.B. Identification of human RNA editing sites: a historical perspective. Methods. 2016;107:42–47. doi: 10.1016/j.ymeth.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.E., Paluch J., Dickman D.K., Joiner W.J. ADAR-mediated RNA editing suppresses sleep by acting as a brake on glutamatergic synaptic plasticity. Nat. Commun. 2016;7:10512. doi: 10.1038/ncomms10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J., Menet J.S., Rosbash M. Nascent-seq indicates widespread cotranscriptional RNA editing in Drosophila. Mol. Cell. 2012;47:27–37. doi: 10.1016/j.molcel.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J.J.C. The emerging role of RNA editing in plasticity. J. Exp. Biol. 2015;218:1812. doi: 10.1242/jeb.119065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter S.M., Dawson T.R., Emeson R.B. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- Savva Y.A., Jepson J.E., Sahin A., Sugden A.U., Dorsky J.S., Alpert L., Lawrence C., Reenan R.A. Auto-regulatory RNA editing fine-tunes mRNA re-coding and complex behaviour in Drosophila. Nat. Commun. 2012;3:790. doi: 10.1038/ncomms1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva Y.A., Rieder L.E., Reenan R.A. The ADAR protein family. Genome Biol. 2012;13:252. doi: 10.1186/gb-2012-13-12-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Laurent G., Tackett M.R., Nechkin S., Shtokalo D., Antonets D., Savva Y.A., Maloney R., Kapranov P., Lawrence C.E., Reenan R.A. Genome-wide analysis of A-to-I RNA editing by single-molecule sequencing in Drosophila. Nat. Struct. Mol. Biol. 2013;20:1333–1339. doi: 10.1038/nsmb.2675. [DOI] [PubMed] [Google Scholar]
- Terajima H., Yoshitane H., Ozaki H., Suzuki Y., Shimba S., Kuroda S., Iwasaki W., Fukada Y. ADARB1 catalyzes circadian A-to-I editing and regulates RNA rhythm. Nat. Genet. 2017;49:146–151. doi: 10.1038/ng.3731. [DOI] [PubMed] [Google Scholar]
- Xu G., Zhang J. Human coding RNA editing is generally nonadaptive. Proc. Natl. Acad. Sci. U S A. 2014;111:3769–3774. doi: 10.1073/pnas.1321745111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonovitch A.L., Fu J., Li K., Mahato S., Kang L., Rashkovetsky E., Korol A.B., Tang H., Michalak P., Zelhof A.C. Regulation of gene expression and RNA editing in Drosophila adapting to divergent microclimates. Nat. Commun. 2017;8:1570. doi: 10.1038/s41467-017-01658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Simola D.F., Bonasio R., Liebig J., Berger S.L., Reinberg D. Eusocial insects as emerging models for behavioural epigenetics. Nat. Rev. Genet. 2014;15:677–688. doi: 10.1038/nrg3787. [DOI] [PubMed] [Google Scholar]
- Yang X.-Z., Chen J.-Y., Liu C.-J., Peng J., Wee Y.R., Han X., Wang C., Zhong X., Shen Q.S., Liu H. Selectively constrained RNA editing regulation crosstalks with piRNA biogenesis in primates. Mol. Biol. Evol. 2015;32:3143–3157. doi: 10.1093/molbev/msv183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Zhou H., Kong Y., Pan B., Chen L., Wang H., Hao P., Li X. The landscape of A-to-I RNA editome is shaped by both positive and purifying selection. PLoS Genet. 2016;12:e1006191. doi: 10.1371/journal.pgen.1006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Dou S., He F., Luo J., Wei L., Lu J. Genome-wide maps of ribosomal occupancy provide insights into adaptive evolution and regulatory roles of uORFs during Drosophila development. PLoS Biol. 2018;16:e2003903. doi: 10.1371/journal.pbio.2003903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Deng P., Jacobson D., Li J.B. Evolutionary analysis reveals regulatory and functional landscape of coding and non-coding RNA editing. PLoS Genet. 2017;13:e1006563. doi: 10.1371/journal.pgen.1006563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.Q., Zhang P., Gao H., He X., Dou Y., Huang A.Y., Liu X.M., Ye A.Y., Dong M.Q., Wei L. Profiling the RNA editomes of wild-type C. elegans and ADAR mutants. Genome Res. 2015;25:66–75. doi: 10.1101/gr.176107.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All deep-sequencing data generated in this study were deposited in the China National Genomics Data Center Genome Sequence Archive (GSA) under accession number CRA002262. Other deep-sequencing data analyzed were downloaded from SRA as follows. Brains of honeybee workers: accession numbers SRR445999 to SRR446004 (reverted nurse) and SRR446005 to SRR446010 (forager) (Herb et al., 2012). Bumblebee: SRP166322 (Porath et al., 2019). D. mel head: SRP067542 (Zhang et al., 2018). D. sim head: SRP074828 (Duan et al., 2017). D. pse: DRR055250 and DRR055251 (Nozawa et al., 2016). D. wil: SRR341127 and SRR341129 (Meisel et al., 2012). D. moj: SRR037508-SRR037518 (generated by the Drosophila modENCODE project). D. sec, and D. ere data are unpublished data generated in our own lab. The reference genomes versions used for Drosophila species are Dmel_r6.04 (http://flybase.org/) for D. mel, and versions droSim1, droSec1, droEre1, dp4, droWil1, and droMoj3 downloaded from UCSC Genome Browser (http://genome.ucsc.edu/) for the other fly species. For the Bombus terrestris, we used version Bter_1.0.