Summary
Vitamin D is an essential steroid hormone that regulates systemic calcium homeostasis and cell fate decisions. The prostate gland is hormonally regulated, requiring steroids for proliferation and differentiation of secretory luminal cells. Vitamin D deficiency is associated with an increased risk of lethal prostate cancer, which exhibits a dedifferentiated pathology, linking vitamin D sufficiency to epithelial differentiation. To determine vitamin D regulation of prostatic epithelial differentiation, patient-derived benign prostate epithelial organoids were grown in vitamin D-deficient or -sufficient conditions. Organoids were assessed by phenotype and single-cell RNA sequencing. Mechanistic validation demonstrated that vitamin D sufficiency promoted organoid growth and accelerated differentiation by inhibiting canonical Wnt activity and suppressing Wnt family member DKK3. Wnt and DKK3 were also reduced by vitamin D in prostate tissue explants by spatial transcriptomics. Wnt dysregulation is a known contributor to aggressive prostate cancer, thus findings further link vitamin D deficiency to lethal disease.
Subject areas: Cell Biology, Developmental Biology, Transcriptomics
Graphical abstract
Highlights
-
•
Vitamin D sufficiency accelerates differentiation of patient-derived prostate organoids
-
•
Single-cell RNAseq of organoids reveals differentiation trajectory over time
-
•
Vitamin D inhibits canonical Wnt activity in human prostate organoids and explants
-
•
Inhibition of DKK3 by vitamin D promotes organoid growth and proliferation
Cell Biology; Developmental Biology; Transcriptomics
Introduction
Vitamin D is a misnomer as it is not a vitamin, but rather a vital steroid, synthesized in the skin following UV exposure. Circulating pro-hormone is further metabolized to the active hormone calcitriol (1,25-dihydroxyvitamin D [1,25D]) which controls systemic calcium homeostasis and locally regulates cell fate decisions. In target cells, 1,25D binds to the vitamin D receptor (VDR), a classical steroid hormone receptor that associates with vitamin D response elements on DNA to control gene expression (Feldman et al., 2014). Vitamin D regulates at least 3% of the genome (Bouillon et al., 2008) and chromatin immunoprecipitation (ChIP) sequencing for VDR-bound DNA in prostate epithelial cells revealed binding at more than 3,000 protein-coding genes, and over 1,000 noncoding sites (Baumann et al., 2019; Fleet et al., 2019). Genes regulated by vitamin D are involved in proliferation, differentiation, and apoptosis pathways (Feldman et al., 2014). Vitamin D metabolites are pro-differentiating in a variety of cell and tissue types, including keratinocytes (MacLaughlin et al., 1990), intestinal villi (Peregrina et al., 2015; Spielvogel et al., 1972), cardiomyocytes (Hlaing et al., 2014), odontoblasts (Mucuk et al., 2017), placenta (Hutabarat et al., 2018), and macrophages (Abe et al., 1981; James et al., 1997). Some of these studies explore 1,25D action in benign cells, whereas the majority of reports focus on vitamin D inhibition of cancer cell growth and tumor progression (Aguilera et al., 2007; Banks and Holick, 2015; Holick et al., 2007; Larriba et al., 2011; Tavera-Mendoza et al., 2017; Yang et al., 2017), and very little is known about the pro-differentiating activity of vitamin D in benign prostate.
Other hormones are essential for prostate differentiation; retinoic acid regulates prostate bud formation during development (Bryant et al., 2014) and androgen regulates terminal differentiation of luminal epithelial cells (Prins and Lindgren, 2015; Wright et al., 1996). The prostate has robust expression of VDR and the enzymes required for local production of 1,25D from the circulating pro-hormone, 25-hydroxyvitamin D (Peehl et al., 2004), indicating that the hormone vitamin D is active in the gland as well. The benign prostate of castrated rats supplemented with androgen and 1,25D showed increased number of secretory vesicles in regenerated luminal cells compared with androgen alone (Konety et al., 1996), supporting improved differentiation with vitamin D. In prostate cancer (PCa) cell lines, treatment with a vitamin D analog increased E-cadherin expression (Campbell et al., 1997). Although these two studies suggest that vitamin D enhanced prostatic differentiation by expression of a more “normal” phenotype, the mechanism was not determined.
Hormone dysregulation, such as androgen and androgen receptor, contributes to PCa initiation and progression (Karantanos et al., 2013). Similar observations have been made for vitamin D and VDR; well-differentiated prostate tumors have high VDR expression, whereas high Gleason grade, poorly differentiated tumors have low VDR expression (Hendrickson et al., 2011). The relationship between vitamin D dysregulation and aggressive disease is supported by rodent models, where prostatic VDR deletion within the TgAPT mouse model of prostate carcinogenesis increased adenocarcinoma foci number and area (Fleet et al., 2019). The relationship is also observed in patients, where vitamin D deficiency is associated with risk of aggressive PCa (Fang et al., 2011; Giovannucci et al., 2006; Murphy et al., 2014; Ramakrishnan et al., 2019; Studzinski and Moore, 1995). Vitamin D deficiency is especially pertinent to patients with PCa who are frequently deficient due to high skin melanin content, such as African Americans (Murphy et al., 2014), or lack of sun exposure (Gilbert et al., 2009), such as the elderly (Elshazly et al., 2017). This observation was originally identified as distance from the equator increasing the risk of PCa mortality (Hanchette and Schwartz, 1992; Studzinski and Moore, 1995) and diagnosis during summer improving PCa prognosis (Robsahm et al., 2004). Clinical trials using vitamin D supplementation in patients with existing PCa have had both promising (Marshall et al., 2012; Woo et al., 2005) and null results (Chandler et al., 2014). However, patients with PCa are known to have low expression of VDR (Hendrickson et al., 2011) supporting that vitamin D supplementation is more useful as a chemopreventive agent rather than as a PCa treatment. Recently, the VITAL study assessed 2000 IU/day vitamin D supplementation in healthy subjects to explore cancer incidence and found a decreased risk of cancer in the normal body mass index group (Manson et al., 2019).
Given the reliance of the prostate on hormone signaling for differentiation, the high activity of the hormone vitamin D in the gland, and the association of vitamin D deficiency with risk of aggressive PCa (Murphy et al., 2014), we hypothesized that vitamin D promotes prostatic epithelial differentiation and that its deficiency contributes to the loss of epithelial homeostasis observed in aggressive PCa. To explore the effects of deficient and sufficient 1,25D on prostate epithelium, patient-derived organoids were used to model epithelial differentiation (Drost et al., 2016) in physiologically relevant levels of the hormone. We previously optimized culture conditions for clonal growth of organoids from single cells (Richards et al., 2019) and characterized prostate organoids using single-cell RNA sequencing (scRNAseq) (McCray et al., 2019).
Results
Sufficient 1,25D supports growth of benign patient-derived prostate epithelial organoids
To determine the effect of 1,25D on organoid phenotype, we grew organoids derived from a benign region of radical prostatectomy tissue from a single patient in media supplemented with 10 nM 1,25D, 50 nM 1,25D (vitamin D-sufficient), or vehicle control (vitamin D-deficient, <0.01% EtOH) (Figure 1A). VDR was found to be expressed in all patient-derived PrE cells examined (Figure S1A), including patient AA2. The clonal origin of organoids within this model has been previously demonstrated by our group through time-lapse microscopy studies and lineage tracing with fluorescent proteins (Richards et al., 2019). Similar to other reports (Barros-Silva et al., 2018; Wang et al., 2015a), we observed organoids with heterogeneous morphologies that include solid spheres and acinar/tubule-like structures, with a small percentage of translucent spheres (Figure 1A). Both concentrations of 1,25D produced visibly larger organoid areas compared with the vitamin D-deficient control organoids (Figure 1A). The 10 nM dosage was selected for subsequent studies, as it strongly upregulated the VDR-response gene CYP24A1 (Chen and DeLuca, 1995; Zierold et al., 1995) (Figure S1B) and is a biologically relevant concentration, equivalent to ~4 ng/mL vitamin D (circulating levels of active and inactive forms in serum are 50 pg/mL and 40 ng/mL, respectively) (Richards et al., 2017). Studies were expanded to include a larger and more diverse group of 10 patient-derived PrE cells (Table 1). Vitamin D sufficiency increased the percentage of translucent and acinar organoids, which are considered more differentiated morphologies (Figure 1B). Organoid size was significantly increased by 1,25D (Figures 1C and 1D, Table 1). The magnitude of the 1,25D-induced size increase varied between patients, highlighting the model's preservation of patient heterogeneity (Figures 1C and 1D). Flow cytometry of day 14 organoids for luminal cell marker CD26 (Henry et al., 2018) and basal-progenitor colony-forming cell marker CD49f (Yamamoto et al., 2012) revealed a noticeable shift in CD49f cells at day 14 with 1,25D treatment (blue) when compared with control (red) (Figure 1E). Overall, sufficient 1,25D significantly increased organoid size and morphology and promoted differentiation away from a CD49f basal-progenitor phenotype, indicating improved growth with vitamin D.
Figure 1.
Sufficient 1,25D supports growth of benign patient-derived prostate epithelial organoids
(A) AA2 organoids grown in vehicle (Veh, <0.01 % EtOH) or 10 nM or 50 nM 1,25D until day 23. Three representative images are shown for each condition to illustrate heterogeneity of organoid morphology: solid (S), translucent (T), and acinar (A) structures were observed. Scale bar, 200 μm.
(B) Percentage of organoids with phenotype after culture in deficient 1,25D (V, vehicle), or sufficient 1,25D (D, 10 nM 1,25D). Solid (S), translucent (T), and acinar (A) organoids were counted per well in n ≥ 3 wells for 10 patients, and the percentage of each were calculated. Organoids derived from 7 patients were capable of forming all three types of morphologies; only those percentages were averaged and reported here. Error bars represent the standard error of the mean. (∗p < 0.1; ∗∗p < 0.05).
(C) Relative area of PrE organoids grown in vehicle (Veh) or 10 nM 1,25D (1,25D) for approximately 14 days, showing ≥3 replicate wells per patient for each condition, repeated 1–4 times per patient as listed in Table 1. Area is normalized to the mean of the vehicle per patient per experiment; each dot represents an organoid. Patients represent biological replicates. (AA, African American; EA, European American; O, Other, not African or European descent, ancestries were patient self-declared; ∗p < 0.1; ∗∗p < 0.05; ∗∗∗p < 0.01, ∗∗∗∗p < 0.001).
(D) Representative images of day 14 organoids grown in deficient (Veh, middle) or sufficient vitamin D (10 nM 1,25D, lower) and corresponding H&E staining for patient tissue from which organoids were derived (upper); scale bar: 200 μm for organoids and 100 μm for tissue.
(E) Overlay of flow histograms for luminal marker CD26 (right) and basal-progenitor marker CD49f (left) in AA1 organoids grown until day 14 in vehicle (red, Veh) or 10 nM 1,25D (blue, 1,25D).
See also Figure S1.
Table 1.
Patient characteristics
| Patienta | Age at RPb | Pathology | Fold change of organoid area with 1,25D | Significance of organoid area with 1,25Dc | # Of Aliquots tested per patient | # Of replicate wells per condition |
|---|---|---|---|---|---|---|
| EA1 | 59 | Benign | NAd | NA | ||
| EA2 | ? | Benign | 1.41 | 0.0313 | 1 | 3 |
| EA3 | 57 | Benign | 1.345 | 0.0011 | 2 | 6 |
| EA4 | 60 | Benign | 1.708 | <0.0001 | 3 | 9 |
| AA1 | 71 | Benign | 1.359 | <0.0001 | 4 | 12 |
| AA2 | 68 | Benign | 1.22 | 0.0142 | 1 | 3 |
| AA3 | 72 | Benign | NA | NA | ||
| AA4 | 58 | Benign | 1.1119 | 0.0251 | 2 | 6 |
| AA5 | 60 | Benign | 1.182 | 0.0019 | 2 | 6 |
| AA6 | 50 | Benign | 1.47 | 0.0007 | 2 | 6 |
| AA8 | 58 | Benign | 1.189 | 0.0536 | 1 | 3 |
| AA9 | ? | Benign | NA | NA | ||
| Other1 | 58 | Benign | 0.9680 | 0.4616 | 1 | 3 |
Significant values are bold and italics.
EA, European American; AA, African American; Other, non-European or African descent.
RP, radical prostatectomy.
Non-parametric one-tailed Mann-Whitney test.
NA, patient not used for organoid area assay.
Epithelial populations in primary prostate cell organoids by scRNAseq
Having established a 1,25D-induced phenotype of increased size across multiple patient-derived organoids (Figure 1), we next determined how 1,25D affected the epithelial populations within the organoids over time. Control and 1,25D-sufficent organoids from patient AA2 were collected for scRNAseq at two time points along the differentiation spectrum: day 8 and day 14 (Figure 2A, left). AA2 was selected due to availability of cells from this patient, capacity for these cells to form acinar organoids, and response to 1,25D. Target 1,25D genes and pathways identified by the scRNAseq in AA2 were later validated in additional patients’ cells. Seurat version 3 was used to integrate datasets, align similar cells found in each sample, generate clusters, and perform differential expression analysis (Butler et al., 2018; Satija et al., 2015); the resulting UMAP of the integrated dataset and distribution of epithelial clusters found in each sample are shown (Figure 2A, middle and right). Cluster markers were determined by unbiased identification of genes that had uniquely high or low expression in each cluster (Figures 2B and S2A) and were input into Ingenuity Pathway Analysis (IPA) software to identify canonical pathways enriched in each population (Kramer et al., 2014) (Figure S2B), as described below. To name the clusters, the expression of specific previously identified epithelial markers was interrogated and visualized by dot plot (Figure 2C), which shows the percentage of cells in each cluster that express the marker of interest, and how high or low that expression is relative to the other cells in the dataset.
Figure 2.
Epithelial populations in primary prostate cell organoids by scRNAseq
(A) Representative images of the AA2 organoids grown in vehicle (Veh) or 10 nM 1,25D (1,25D) collected at day 8 and day 14 for scRNAseq (left). Scale bar, 100 μm. UMAP of integrated scRNAseq data for vehicle and 1,25D samples at day 8 and day 14 (middle), showing clustering (upper) and distribution of samples (lower). Bar chart depicting the percentage of cells per cluster in each sample, vehicle (V) and 1,25D (D), at each time point (right); percentage of total dividing (%Div.) cells is shown in red text.
(B) Heatmap of cluster markers for integrated scRNAseq dataset shown in (A). Cluster markers were identified by Seurat as genes having uniquely high or low expression in each cluster, compared with all other cells in the dataset.
(C) Dot plot showing expression of specific epithelial markers in each cell cluster. The size of dots represents the percentage of cells in each cluster that express the gene, and the color represents relative expression of the cells in that cluster compared with all remaining cells.
(D) Immunostaining of formalin-fixed, paraffin-embedded organoids (upper middle and right) and whole-mounted organoids (remaining panels). Staining for luminal makers CK8 and AR; basal markers CK5 and P63; stem cell marker CK13; polarization markers ITGα2β1, ITGα2, and ITGβ1; and incorporation of EdU are shown. Black and white arrow heads indicate AR+/P63- luminal cells. Green arrowhead indicates P63+ cell. Scale bar, 50 μm. Single channels for each condition included in Figures S3.
(E) Overlay of flow cytometry histogram for proliferation marker Ki67 in AA1 organoids grown until day 7 in vehicle (red, Veh) or 10 nM 1,25D (blue, 1,25D).
See also Figures S2–S4.
Resident, quiescent stem cells were identified by high expression of KRT13 (Henry et al., 2018; Hu et al., 2017) (Figures 2B and 2C). This cluster had the most distinct expression profile with the highest number of markers (Figure S2A). By IPA, the KRT13 stem cell population had specific activation of the Wnt/β-catenin and VDR/RXR pathways, similar to what has been reported in the gut and skin stem cells (Bikle, 2004; Peregrina et al., 2015) (Figure S2B, red asterisks). Downstream progenitors were marked by modest KRT13 expression but high expression of the progenitor marker KRT6A (Schmelz et al., 2005), along with expression of basal markers KRT5/KRT14, intermediate marker KRT19 ((Wang et al., 2001)), and TACSTD2, which codes for the stem cell marker Trop2 (Goldstein et al., 2008) (Figures 2B and 2C). A luminal phenotype was distinguished by expression of KRT8/KRT18 and DPP4 (CD26) (Figure 2C). However, transcripts for DPP4 were low and do not reflect the protein expression of CD26 observed by flow cytometry. Two basal populations were identified by KRT5, KRT14, TP63, and PDPN (Henry et al., 2018) (Figure 2C). Dividing cells were recognized by cell division markers CENPF, MKI67, TOP2A, PCNA, MCM7, CCNB1, and HIST1H4C (Figure 2C). The “IntegrinHigh” population was identified based on integrin expression (ITGB6, ITGB4), expression of integrin-binding partner FN1, and other adhesion junction proteins (DST, CD44, Figures 2B and 2C) (Ryan et al., 2012; Sneath and Mangham, 1998). Markers for the IntegrinHigh cluster were enriched for the HIPPO pathway, which regulates contact inhibition of polarized cell types (Genevet and Tapon, 2011) (Figure S2, red asterisks). These cells may be starting to polarize through integrin interactions with the basement-membrane-protein ligands found in the Matrigel.
Immunostaining of organoids confirmed protein expression for major cluster markers (Figure 2D, single channels shown in Figure S3). Basal proteins CK5 and p63 were expressed along the periphery of the organoid (Figure 2D, upper left and right, green arrowhead). Although AR mRNA transcripts were low in the dataset, AR+ luminal cells were observed by immunohistochemistry (IHC) (Figure 2D, upper middle, black arrowhead). The luminal marker CK8/18 was highly expressed by most cells in the organoids, but there were p63-/CK8+ cells indicating a continuum of basal-intermediate-luminal cells in the organoids (Figure 2D, upper left and right, white and green arrowheads). This was also observed in the scRNAseq data where 100% of cells in every clusters express KRT8 (Figure 2C), but at different relative levels between the clusters. A CK13+ stem cell identified in an organoid was negative for EdU incorporation after briefly pulsing overnight with EdU before staining (Figure 2D, lower middle), supporting its quiescence. Integrin subunits α2 and β1 and heterodimer integrin α2β1 were observed at the organoid-Matrigel interface where they are likely binding collagen and laminins found in the Matrigel to direct cell polarity (Figure 2D, lower left and right). CK13+ cells were negative for integrin α2/β1, demonstrating separation between the stem and IntegrinHigh clusters. In sum, these results confirm differentiation of a heterogeneous population of epithelial cells sharing markers identified by scRNAseq.
Once clusters were identified, population shifts over time and with vitamin D were explored using Fisher's exact test (Figure S4, p value shown inset in the heatmap). Early-stage day 8 organoids consisted primarily of dividing cell types, indicative of rapid cellular expansion in culture and significantly different when compared with day 14 organoids (Figure 2A, red text, Figure S4A). After differentiation at day 14, there was significant enrichment in the IntegrinHigh polarized cells compared with day 8. Culture in sufficient 1,25D increased the percentage of dividing cells at day 8 from 59% to 67% of the cell population (Figure 2A, red text), which was significant (Figure S4B) and validated by flow cytometry for KI67 (Figure 2E). At day 14, population shifts with 1,25D were modest but significant (Figure S4C), with a slight increase in the number of polarized and basal cells and a decrease in the number of progenitor and intermediate cells (Figure 2A, red asterisks). The data support that 1,25D promotes both growth and differentiation through enrichment of dividing cells at day 8 and IntegrinHigh cells at day 14.
1,25D modulates the Wnt pathway in organoids
Like most hormones, vitamin D is pleiotropic and regulates many pathways in prostate cells. To determine the action of 1,25D in organoids, differentially expressed genes (DEGs) after culture in 1,25D compared with vehicle in each cluster at each time point were explored (Figure S5A). Of note, the stem cells had the fewest DEGs with 1,25D, indicating a stable transcriptional program. DEGs were input into IPA software to identify enriched Canonical Pathways (Figures 3A and S5B), Upstream Regulators (Figure S6), and their Downstream Effects (Figure S7) (Kramer et al., 2014).
Figure 3.
1,25D modulates the Wnt pathway in organoids
(A) Enriched pathways related to “Organismal Growth and Development” with 1,25D treatment in each cluster at each time point. Differentially expressed genes (DEGs) with 1,25D treatment per cluster were input into IPA Canonical Pathway analysis; pathways related to “Organismal Growth & Development” are shown. Scale represents significance, –log(p value), for enrichment of each pathway with 1,25D. Dark purple is significant at p < 0.05. Red box shows DEGs related to Wnt/β-catenin signaling with 1,25D treatment in each cluster. Scale represents the log ratio with 1,25D compared with control.
(B) RT-qPCR array for Wnt pathway gene expression in flow-sorted day 17 AA3 organoids grown in vehicle or 10 nM 1,25D. Cells were sorted by CD49High (CD49fH) or CD49Low (CD49fL). Log2 of RQ values are shown, normalized to average of housekeeper genes. Hierarchical clustering was performed by Pearson correlation for row and by Spearman for column. Red astericks indicate Wnt ligands and DKK family members.
(C) β-catenin protein localization in 1,25D-treated prostate epithelial cells. Monolayer PrE from two patients (middle and right panel) and benign 957E (left panel) cells grown for 48 or 96 h, respectively, with vehicle or 10 nM 1,25D. Cells were treated with 9 μM Chiron for 5 h, followed by immunostaining for β-catenin (red) and counterstaining with DAPI (blue). Scale bar, 50 μm. Quantification of the percentage of positive nuclei is shown (right); error bars represent the standard deviation of two fields of view. The average of the −Chiron controls (CON, treated with DMSO) is shown to illustrate significant nuclear β-catenin induction upon Chiron treatment (AA8 CON was not collected). p value represents outcome of paired two-way ANOVA with uncorrected Fisher's comparison by row for vehicle versus 1,25D, and for CON versus 957E Chiron Veh. (ns = not significant, ∗p < 0.05, ∗∗∗∗p < 0.0001).
(D) RT-qPCR of AXIN2 expression in monolayer PrE cells and benign 957E cells grown and treated as in (C). Relative quantitation is normalized to HPRT1. Error bars represent standard deviation of technical RT-qPCR pipetting replicates. Cells from three sources serve as biological replicates. p value represents outcome of paired two-way ANOVA with uncorrected Fisher's comparison by row for vehicle versus 1,25D (∗p < 0.05; ∗∗∗p < 0.001).
(E) RT-qPCR of AXIN2 expression (left) in monolayer AA1 PrE cells transfected with siCTL (solid bars) or siVDR RNA (hatched bars) and grown as described in (C). Relative quantitation is normalized to HPRT1. Error bars represent standard deviation of technical RT-qPCR pipetting replicates. p value represents outcome of paired two-way ANOVA with uncorrected Fisher's comparison by row for vehicle versus 1,25D. Western for VDR in siCTL and siVDR samples (right) with housekeeper cyclophilin-B (cyclo); full Western blot included in Figure S8C.
See also Figures S4–S8.
Significantly enriched canonical pathways in all clusters included VDR/RXR activation (Figure S5B), driven by the highly 1,25D-regulated genes CYP24A1 and IGFBP3 (Figure S5B, upper red box) (Martin and Pattison, 2000; Zierold et al., 1995). Examination of pathways related to “Organismal Growth & Development” (Figure 3A) showed enrichment of the BMP and Wnt pathways in 1,25D-sufficient organoids, known regulators of prostate development and differentiation (Toivanen and Shen, 2017). DEGs within the Wnt/β-catenin signaling pathway included upregulation of DKK1 at day 8 and downregulation of DKK3 at day 14 (Figure 3A, lower red box). Stem cells showed the fewest number of DEGs with 1,25D culture (Figure S5A), and this cluster showed no enrichment for pathways related to “Organismal Growth & Development.” Vitamin D status did not alter the stem population viability as shown by three passages of self-renewal assays in four patients (Figure S5C). Together, this indicates that 1,25D mainly regulates lineage-committed cells on the path to differentiation and does not alter the stable transcriptional program in the stem cells, although these findings were not formally demonstrated by following the progeny of flow-isolated CK13+ cells.
Upstream Regulator analysis predicted active transcription factors, which may have influenced DEG expression. Activation of Vitamin D3-VDR-RXR and cell-type-specific regulation by CTNNB1 (β-catenin) were among the top regulators (Figure S6, red asterisks), confirming vitamin D function and further supporting Wnt/β-catenin involvement. To understand the net effect of the Pathway and Regulator analysis, downstream Disease & Function analysis was performed (Figure S7). Consistent with the hypothesis, there was enrichment for “Differentiation of Epithelial Tissue” at both time points with sufficient 1,25D (Figure S7, red box). The Wnt pathway was selected for additional investigation in the primary prostate cells because previous studies in the RWPE1 prostate cell line, gut, skin, and heart have shown 1,25D inhibition of canonical Wnt signaling (Aguilera et al., 2007; Hlaing et al., 2014; Kovalenko et al., 2010; Larriba et al., 2011; Muralidhar et al., 2019).
Wnt regulation by 1,25D was validated with a targeted RT-qPCR array (Figure 3B). Before RNA isolation, organoids cultured with 10 nM 1,25D or vehicle were sorted by high or low CD49f (Guo et al., 2012; Yamamoto et al., 2012) in an attempt to preserve differences in transcription between progenitor versus lineage-committed cells or basal versus luminal cells, respectively. The expression of multiple canonical and non-canonical Wnts were altered by 1,25D, in some cases in a CD49f-cell-type-specific manner, including WNT7A, WNT9A, WNT2B, WNT5B, WNT7B, WNT3, WNT5A, and WNT4. Notably, DKK3 was downregulated with 1,25D treatment in both high (CD49fH) and low cells (CD49fL) (Figure 3B, red asterisks).
To determine overall directionality of Wnt regulation by 1,25D, β-catenin translocation and AXIN2 induction were assessed. The GSK3β-inhibitor, Chiron, was used for β-catenin stabilization and Wnt pathway induction. PrE cells from two patients (AA8, AA9) and the benign 957E cell line grown with 1,25D had decreased nuclear β-catenin after Chiron treatment compared with vehicle controls (Figure 3C). Consistent with reduced β-catenin activity, 1,25D abrogated Chiron-induced activation of the Wnt response gene AXIN2 (Figure 3D). Knockdown of VDR in AA1 monolayer cells (Figures 3E and S8A–S8C) blocked 1,25D's ability to inhibit AXIN2 after Chiron treatment (Figure 3E). Overall, these data support an inhibitory effect of 1,25D on the canonical Wnt pathway and implicate direct regulation via VDR.
1,25D inhibits Dickkopf family member 3
Dickkopf family member 3 (DKK3) emerged as a 1,25D target in both the scRNAseq dataset and the RT-qPCR array, so its expression was profiled in a panel of prostate cell lines, monolayer PrE cells, and organoids (Figure 4A). All the cell lines, both immortalized benign (RWPE1 and 957E) and PCa (LAPC4 and PC3), had very low or undetectable DKK3 expression. DKK3 is also known as “Reduced Expression in Immortalized Cells” (Hsieh et al., 2004), thus our findings are consistent with previous reports of limited expression in cell lines. Across PrE cells grown as monolayers or organoids, 1,25D inhibited DKK3 expression (Figure 4A).
Figure 4.
1,25D inhibits Dickkopf family member 3 (DKK3)
(A) RT-qPCR of DKK3 expression in prostate cell lines (LAPC4, PC3, RWPE1, 957E), monolayer prostate epithelial cells (PrE 2D), and organoid PrE cells (3D) grown in vehicle or 10 nM 1,25D. Monolayer cells were treated for 24–72 h, and organoids were treated for 2–3.5 weeks. Relative quantitation is normalized to HPRT1; error bars represent standard deviation of technical RT-qPCR pipetting replicates. Cells from multiple sources represent biological replicates. p value represents interaction outcome of grouped two-way ANOVA comparison of vehicle versus 1,25D in PrE cells using different patients as biological replicates; individual p values are shown (∗p < 0.1; ∗∗p < 0.05; ∗∗∗∗p < 0.001).
(B) Immunostaining for DKK3 (green) in a whole-mounted day 17 AA3 organoid counterstained with DAPI (blue) and phalloidin/F-actin (pink). Scale bar, 50 μm.
(C) ELISA quantification of secreted DKK3 in media collected from monolayer PrE cells grown in vehicle or 10 nM 1,25D for 72 h, normalized to the number of cells. Error bars represent the standard error of triplicate ELISA technical samples. Cells from multiple sources present biological replicates. p value represents interaction outcome of grouped two-way ANOVA comparison of vehicle versus 1,25D using different patients as biological replicates, individual p values are shown (∗∗p < 0.05; ∗∗∗∗p < 0.001).
(D) Western blot of DKK3 expression in cell lines and in monolayer and organoid PrE cells grown in vehicle or 10 nM 1,25D (sDKK3, secreted DKK3 variants; DKK3b, intracellular DKK3 variant); molecular weight marker shown (M.W.).
(E) VDR-ChIP sequencing peak near DKK3 in PrE cells treated with 1,25D for 3 h; IGV track showing 1,25D-treated minus vehicle-treated coverage (data from NCBI GEO accession number GSE124576).
(F) Western blot of monolayer AA1 prostate epithelial (PrE) cells transduced with control virus (siGFP) or siDKK3 virus and grown in vehicle, 10 nM 1,25D, or 50 ng/mL rDKK3 (upper left); molecular weight marker shown (M.W.). Representative images of siGFP- or siDKK3-treated AA1 PrE organoids grown in vehicle or 10 nM 1,25D for 2 weeks (lower left), with quantification of relative area (right). Area was normalized to the mean of the vehicle, showing ≥3 replicate wells for each condition; each dot represents an organoid. (∗p < 0.1;∗∗∗p < 0.01; ∗∗∗∗p < 0.001. Scale bar, 200 μm).
(G) Relative area of PrE organoids grown in vehicle or 10 nM 1,25D combined with 50 ng/mL rDKK3 treatment for 2 weeks. Area was normalized to the mean of the vehicle, showing ≥3 replicate wells for each condition, each dot represents an organoid. (∗∗p < 0.05; ∗∗∗p < 0.01; ∗∗∗∗p < 0.001).
Immunofluorescence for DKK3 revealed a vesicular and perinuclear staining pattern (Figure 4B, white box), similar to other secreted members of the Dickkopf family (Glinka et al., 1998; Inoue et al., 2017). ELISA showed that culture in 1,25D reduced DKK3 secretion in media (Figure 4C), and western blots of cell lysates showed reduced intracellular protein levels with 1,25D treatment (Figure 4D). Western blot of DKK3 detected several sized bands, agreeing with preceding reports describing many variants: a heavy secreted-form (sDKK3), a lighter intracellular variant (DKK3b), and a 50-kDa secreted form (sDKK3) (Abarzua et al., 2005; Hsieh et al., 2004; Kawano et al., 2006; Leonard et al., 2017; Zenzmaier et al., 2008; Zhang et al., 2010). The 957E cell line had no detectable expression of sDKK3 and faint expression of DKK3b, uniform with the low RNA expression observed. PrE cells grown as monolayers and organoids showed reductions in all variants of DKK3 with sufficient 1,25D compared with controls (Figure 4D). Analysis of our previously published VDR-ChIP-seq dataset (Baumann et al., 2019) in PrE cells showed a peak 20 kb upstream of DKK3 after 1,25D treatment (Figure 4E), supporting potential genomic regulation by VDR.
To emulate 1,25D actions, PrE cells were transduced with lentivirus containing an siDKK3 sequence and GFP tag and grown as organoids in the presence of vehicle or 10 nM 1,25D (Figure 4F, left). Knockdown alone did not recapitulate the effect of 1,25D treatment, but combination of siDKK3 with 1,25D significantly enhanced the effect of vitamin D on organoid area (Figure 4F, right). To rescue 1,25D inhibition of DKK3, exogenous recombinant DKK3 (rDKK3) was added to culture. Treatment with rDKK3 enhanced all DKK3 variants within cell lysates (Figure 4F, left). PrE organoids from three patients were grown in media supplemented with vehicle, 1,25D, or 1,25D in combination with 50 ng/mL rDKK3 for 2 weeks. rDKK3 did not affect organoid area under vehicle conditions, but blocked the increase in area seen in 1,25D-treated organoids (Figure 4G). In sum, these data support that inhibition of DKK3 by 1,25D serves to promote organoid growth.
1,25D suppresses DKK3
The function of DKK3 in the Wnt pathway is not well understood, but it has been shown to inhibit proliferation in prostate and breast cancer cell lines (Leonard et al., 2017). Expression of DKK3 and KRT13 was mutually exclusive in the scRNAseq dataset, suggesting that DKK3 is expressed only in lineage-committed cells (Figure 5A, lower). To observe lineage-committed cells and separate them from progenitor and stem cells, the scRNAseq data was subset into three groups based on KRT13 expression (Figure 5B, left). DKK3 was expressed at both time points and most visibly inhibited by 1,25D at day 14 (Figure 5B, right), indicating stronger inhibition with increased length of exposure. Immunofluorescence for CK13 and DKK3 in organoids showed no co-localization (Figure 5C). To observe expression across organoid differentiation trajectories, day 8 and day 14 vehicle scRNAseq datasets were plotted together in pseudotime using Monocle 3 (Figure 5D). KRT13 was expressed at the beginning of pseudotime, with cells from day 8 and day 14 samples found at this stage, showing the resident stem cells in organoid culture (Hu et al., 2017). Day 8 cells clustered halfway through the differentiation trajectory where MKI67 expression is high, and day 14 cells clustered at the end. A fork was seen in day 14 cells, possibly where basal and luminal lineages start to diverge. DKK3 expression increased across pseudotime, consistent with expression in lineage-committed cells and indicating its upregulation in senescence (Untergasser et al., 2002) (Figure 5D, bottom right).
Figure 5.
1,25D suppresses DKK3
(A) UMAP of integrated scRNAseq dataset shown in Figure 2A (upper); blended UMAP of integrated scRNAseq data showing expression of KRT13 (green) and DKK3 (red) (lower).
(B) Violin plot of integrated dataset shown in (A), subset by KRT13 expression into lineage-committed, progenitor, or stem populations (left). Violin plots of integrated dataset shown in (A), separated by time point, showing DKK3 expression (right) in vitamin D (pink) and vehicle (teal) samples.
(C) DKK3 (green) and CK13 (red) expression in whole-mounted AA3 organoid counterstained with DAPI (blue). Scale bar, 50 μm.
(D) Pseudotime of integrated scRNAseq data for day 8 and day 14 vehicle samples (upper left). Sample distribution across pseudotime for day 8 (dark blue) and day 14 (light blue) cells (upper right). KRT13 (lower left), MKI67 (lower middle), and DKK3 (lower right) expression across pseudotime.
Vitamin D regulates the Wnt pathway and DKK3 in prostate tissue slice explants
To determine if the Wnt pathway and DKK3 are targets of vitamin D in situ, fresh benign prostate tissue slices from a patient undergoing radical prostatectomy were cultured ex vivo for 8 h in vehicle and vitamin D using precision-cut fresh tissue sections (Maund et al., 2014). Gene expression was determined by spatial transcriptomics with RNA sequencing (Stahl et al., 2016). DKK3 was expressed in both prostate stroma and epithelium (Figure 6A), compared with the epithelial marker NKX3.1 and stromal marker FGF7 (Figure 6A). Due to spot size of 55 μm, the resolution of spatial transcriptomics is not at the single-cell level, so the captured areas are not completely pure for one distinct cell type (Stahl et al., 2016). As such, spots enriched in pooled epithelial markers were selected to compare deficient (veh) and sufficient vitamin D conditions in prostate epithelium.
Figure 6.
Vitamin D inhibits DKK3 in prostate tissue slice explants by spatial transcriptional profiling
(A) H&E of vehicle-treated tissue slice with spatial mapping of mRNAs for DKK3 (red), NKX3.1 (epithelial marker, blue), and FGF7 (stromal marker, magenta). Gene expression determined by 10X Visium spatial profiling and shown as log2 relative expression.
(B) Heatmap depicting expression of genes upregulated by Wnt pathway activation (Nusse, 2020) and the vitamin D pathway in epithelium of prostate tissue slices cultured in vehicle control (veh) or 25D (vitamin D) (Nusse, 2020). Color scale shows log2 fold change; number inset shows average expression calculated by Loupe software. p values are adjusted using the Benjamini-Hochberg correction for multiple tests, ∗∗∗∗p < 0.001, ∗∗∗p < 0.01, ∗∗ p <0.05.
(C) Violin plot of the average log2 counts for DKK3 in control and 25D (vitamin D)-treated epithelium. Horizontal bar = mean, box = 95% confidence interval.
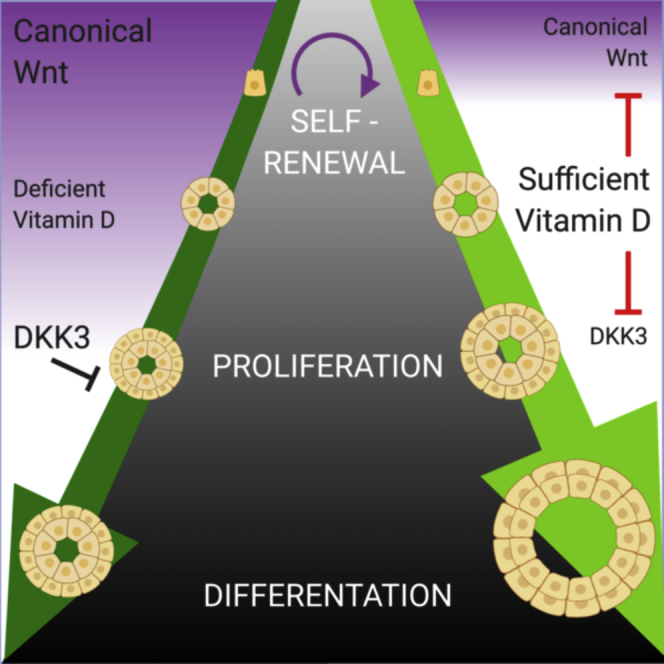
(D) Diagram summarizing the effects of 1,25D on prostate epithelial organoids. Under vehicle conditions “DEFICIENT 1,25D,” a self-renewing stem cell with high Wnt activity undergoes asymmetric division to produce progenitor cells that will rapidly expand. As differentiation occurs, downstream cells will have reduced Wnt activity. Under sufficient conditions, “SUFFICIENT 1.25D,” proliferation is enhanced through inhibition of DKK3, resulting in a larger organoid. Wnt signaling is also inhibited in lineage-committed cells through β-catenin sequestration, to promote differentiation away from a stem cell phenotype.
The expression of genes known to be upregulated by Wnt signaling was queried in the spatial dataset (Figure 6B). The 19 genes are per the curated list from Roel Nusse Lab at Stanford University (Nusse, 2020), selecting only genes that are direct Wnt targets. More than half of the genes were downregulated with vitamin D, suggesting a net suppression of Wnt activity. Specifically, FST, MYC, MMP7, TCF4, and JUN were significantly lower. AXIN2 was not significantly decreased, but it had an extremely low base level of detection, which hindered its analysis. Similarly, whereas DKK3 was markedly lower in the vitamin D-sufficient tissue (Figure 6C), the means did not achieve a significant difference due to the limited number of spots captured in the vitamin D sample. Together these findings are consistent with in vitro data and support the regulation of Wnt activity and DKK3 by vitamin D in intact prostate tissue
Discussion
The prostate is a hormonally regulated gland that requires steroids for development, and dysregulation of hormones occurs during carcinogenesis and late-stage PCa. Vitamin D is a steroid hormone that promotes differentiation in many cell types, yet its role has not been studied in the differentiation of benign human prostate epithelium. Using primary epithelial organoids from multiple patients, we found that continuous culture in physiologically relevant concentrations of 1,25D promoted differentiation and increased organoid area compared with vitamin D-deficient conditions (Figures 1 and 2). The dominant mechanisms were inhibition of the canonical Wnt pathway (Figures 3 and 6) and reduction of Wnt pathway member DKK3 (Figures 4, 5, and 6), to promote epithelial growth (Figure 6D). These findings were corroborated in fresh patient tissue explants in which vitamin D also reduced Wnt target gene expression and DKK3 (Figure 6).
The Wnt pathway is known to be highly active in prostate stem cells compared with differentiated cells (Blum et al., 2009). In kidney organoids and prostate organogenesis, Wnt activity is critical early on to promote progenitor outgrowth but then decreases to allow for differentiation (Prins and Putz, 2008; Simons et al., 2012; Takasato et al., 2016). Similarly, in snake venom gland organoids, Wnt agonists must be removed to allow for differentiation and secretory function (Post et al., 2020). Consistently, we observed that lineage-committed cells had lower Wnt activity and selective Wnt inhibition by 1,25D was observed in the lineage-committed cells, but not in KRT13+ stem cells, resulting in enhanced epithelial differentiation in the organoids (Figure 6D). This effect of vitamin D complements the reports of Wnt pathway regulation in other epithelial models. Kovalenko et al. reported that 1,25D inhibited Wnt and promoted genes that are “induced during differentiation” from Gene Set Analysis in the benign prostate RWPE1 cell line (Kovalenko et al., 2010). In colon cancer cell lines and mouse colon tissue, 1,25D inhibited the Wnt pathway by decreased nuclear β-catenin and increased cellular differentiation (Aguilera et al., 2007; Groschel et al., 2016; Larriba et al., 2013). Similar results of β-catenin by vitamin D metabolites were observed in Kaposi sarcoma (Tapia et al., 2020) and renal cell carcinoma (Xu et al., 2020).
In PCa, Wnt signaling has been shown to promote resistance to androgen deprivation therapy (Yokoyama et al., 2014). PCa stem cells show increased nuclear β-catenin, and TCF/LEF activity (Zhang et al., 2017) and Lef-1 identifies an androgen-insensitive population of basal progenitors in mouse prostate maturation (Wu et al., 2011). As PCa progresses, APC and CTNNB1 become mutated in 22% castration-resistant PCa to drive Wnt pathway activation (Murillo-Garzon and Kypta, 2017). In PCa cell lines, disruption of e-cadherin enhanced Wnt signaling and increased tumor growth (Davies et al., 2000) and WNT5A has been shown to promote cancer cell invasion (Wang et al., 2020). Our results indicate that vitamin D sufficiency could provide negative pressure on the Wnt pathway to complement other therapies and improve PCa patient outcome.
DKK3 emerged as potently and consistently inhibited by 1,25D in the prostate organoids and monolayer cells. Our findings suggest an anti-proliferative role for DKK3 in prostate organoids, and this adds to the literature that is unclear about the downstream target of this protein in prostate cells. In general, the Dickkopf family members are inhibitors of Wnt signaling, such as DKK1 (Glinka et al., 1998; Kruithof-De Julio et al., 2013). However, DKK3 is the most structurally divergent member of the Dickkopf family (Krupnik et al., 1999) and has varied effects on Wnt, ranging from no effect (Krupnik et al., 1999; Pinho Christof Niehrs, 2007; Romero et al., 2013) to promoting (Ferrari et al., 2019; Nakamura and Hackam, 2010; Yin et al., 2018) or inhibiting Wnt activity (Bhattacharyya et al., 2017; Leonard et al., 2017; Liu et al., 2019; Sharma Das et al., 2013). DKK3 also functions as both a positive and negative regulator of TGFβ signaling, depending on the model (Al Shareef et al., 2018; Busceti et al., 2017; Kardooni et al., 2018; Li et al., 2017; Pinho Christof Niehrs, 2007; Romero et al., 2013; Wang et al., 2015b), but DKK3 did not impact TGFβ in this study (data not shown). Despite possibly conflicting reports of DKK3's signaling targets, it has consistently been shown to restrain cell proliferation (Kawano et al., 2006; Leonard et al., 2017). It acts as a cell cycle inhibitor whose expression is reduced in immortalized cells (Kawano et al., 2006), upregulated in senescent PrE cells at passage 10 compared with passage 3 (Untergasser et al., 2002) and upregulated during aging (Yin et al., 2018). In developing mouse prostate, addition of exogenous DKK3 blunted proliferation, preventing luminal differentiation, Nkx3.1 expression, and epithelial bud formation (Kruithof-De Julio et al., 2013). Our findings that 1,25D reduction of DKK3 resulted in increased organoid size is similar to the DKK3-deficient mice, which are viable with normal prostate glands, but have increased Ki-67+ proliferating cells (Romero et al., 2013). We observed that DKK3 was not expressed by the stem cells in organoids, similar to patient tissue (Henry et al., 2018), indicating that DKK3 acts downstream to regulate cell growth (Figure 6D). Notably, DKK3 is reported highly expressed in the stroma of the prostate, which indicates a multifaceted role for this protein that can be explored in the future using co-culture models (Al Shareef et al., 2018; Henry et al., 2018; Zenzmaier et al., 2013). The increase in organoid size induced by 1,25D may seem contradictory to the traditional anti-proliferative role of vitamin D metabolites (Feldman et al., 2014; Murphy et al., 2017; Wagner et al., 2013). However, the organoid model was optimized to create a permissive environment for expansion and differentiation of stem/progenitor cells and is supplemented with androgen to drive cell division.
In summary, we report two complementary mechanisms by which vitamin D sufficiency promotes prostate epithelial differentiation—inhibition of canonical Wnt signaling and the protein DKK3. This is the first report, to our knowledge, of the hormone vitamin D regulating DKK3 expression in the prostate. These findings are particularly impactful for patients who are frequently deficient in vitamin D, such as African Americans and adults older than 70 years, who have significantly high rates of PCa (Jacques et al., 1997; Murphy et al., 2014).
Limitations of the study
Access to primary cells from multiple, ancestrally diverse patients; fresh tissue explants; and the use of scRNAseq and spatial transcriptomics are the major strengths of this study, yet they are not without limitations. Patient-derived cells have inherent inter-patient heterogeneity and epithelial content in the tissue collected; as a result the aliquots available differ between patients. To address this, we used as many patients as possible for each endpoint and supplemented with cell lines if necessary. scRNAseq and spatial transcriptomics are powerful tools to analyze the transcriptomes of thousands of cells from heterogenous samples, but it only captures a subset of the transcripts in each cell (~30%–32% of transcripts for the 10x Chromium Single cell v3 kit). Thus, the lack of a transcript does not mean that protein is absent; for example, DPP4 and AR were low in the scRNAseq data, but CD26 was detected by flow cytometry, nuclear AR was detected by IHC, and CK8+/p63- cells were observed (Figures 1E and 2D). As well, because the organoids were completely epithelial without other cell types, the data lack extremely different clusters. However, our clusters were transcriptionally distinct and similar to those observed in scRNAseq data from the epithelial clustering of whole patient prostate tissue (Henry et al., 2018). When assigning cluster markers and interpreting scRNAseq data, the use of the terms “lineage-committed” and “stem” were guided by previously reported findings established in the literature. Our experiments did not utilize flow cytometry to isolate stem cells and demonstrate that organoids initiated exclusively from CK13/PRAC1 populations. Because of this, organoids may have initiated from cells at various points along the stem and progenitor hierarchy, although it is widely believed that terminally differentiated epithelial cells are unable to form organoids in culture. These assumptions do not impact the conclusions that vitamin D inhibits canonical Wnt activity and DKK3 in prostate epithelial cells, but caution should be used when interpreting terms “lineage-committed” and “stem.” Despite these limitations, the approach successfully identified vitamin D-regulated genes and pathways that were validated by multiple assays.
Resource availability
Lead contact
Lead Contact, Larisa Nonn (lnonn@uic.edu).
Materials availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Larisa Nonn (lnonn@uic.edu). This study did not generate new unique reagents.
Data and code availability
ScRNAseq data has been submitted to NCBI Gene Expression Omnibus and can be found under accession number GSE142489. ChIP sequencing data can be found under accession number GSE124576. Spatial transcriptomics data can be found under accession number GSE159697.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
We thank UIC Biorepository (Alex Susma), and urologists (Drs. Daniel Moreira, and Simone Crivallero) for facilitation of tissue acquisition for patient-derived cultures. We thank UIC Urology patients for donating their tissue—without their participation, none of this research would be possible. We thank Dr. Ke Ma with UIC Fluorescence Imaging Core for assistance with confocal. We thank Dr. Matt McDougall of the Merrill laboratory for his counsel with the Wnt pathway. We thank Dr. Alvaro Hernandez, Chris Wright, Jenny Zadeh, and Jessica Holmes and the UIUC DNA services team for sequencing support and the CellRanger pipeline. We thank Magdalena Rogozinska and Dr. Gayatry Mohapatra for their help with TapeStation quantification. We thank UIC Pathology Department members Joseph Marsili, Morgan Zenner, Dr. Larischa DeWet, and Lenny Hong for their thoughts and troubleshooting. We thank Junbin Huang for his participation on the project through ResearcHStart. Graphical abstract was created with www.BioRender.com. This work was funded, in part, by the Department of Defense Prostate Cancer Research Program Health Disparities Idea Award PC121923 (Nonn) and the UIC Center for Clinical and Translation Science Pre-Doctoral Education for Clinical and Translational Scientists (PECTS) Program (McCray). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Department of Defense.
Author contributions
Conceptualization, T.M. and L.N.; Formal analysis, T.M.; Investigation, T.M., J.P., C.L., J.G., B.B., and M.J.S.; Resources, L.N., K.V.-N., and M.R.A.; Writing, T.M., B.B., and L.N.; Visualization, T.M.; Supervision, L.N.; Funding acquisition, L.N.
Declaration of interests
The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101974.
Supplemental information
Document S1. Transparent methods and Figures S1–S8
References
- Abarzua F., Sakaguchi M., Takaishi M., Nasu Y., Kurose K., Ebara S., Miyazaki M., Namba M., Kumon H., Huh N.-h. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res. 2005;65:9617–9622. doi: 10.1158/0008-5472.CAN-05-0829. [DOI] [PubMed] [Google Scholar]
- Abe E., Miyaura C., Sakagami H., Takeda M., Konno K., Yamazaki T., Yoshiki S., Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. U S A. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera O., Pena C., Garcia J.M., Larriba M.J., Ordonez-Moran P., Navarro D., Barbachano A., Lopez de Silanes I., Ballestar E., Fraga M.F. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–1884. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- Al Shareef Z., Kardooni H., Murillo-Garzón V., Domenici G., Stylianakis E., Steel J.H., Rabano M., Gorroño-Etxebarria I., Zabalza I., dM Vivanco M. Protective effect of stromal Dickkopf-3 in prostate cancer: opposing roles for TGFBI and ECM-1. Oncogene. 2018;37:5305–5324. doi: 10.1038/s41388-018-0294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks M., Holick M.F. Molecular mechanism(s) involved in 25-Hydroxyvitamin D's antiproliferative effects in CYP27B1-transfected LNCaP cells. Anticancer Res. 2015;35:3773–3779. [PubMed] [Google Scholar]
- Barros-Silva J.D., Linn D.E., Steiner I., Guo G., Ali A., Pakula H., Ashton G., Peset I., Brown M., Clarke N.W. Single-Cell analysis identifies LY6D as a marker linking castration-resistant prostate luminal cells to prostate progenitors and cancer. Cell Rep. 2018;25:3504–3518. doi: 10.1016/j.celrep.2018.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B., Lugli G., Gao S., Zenner M., Nonn L. High levels of PIWI-interacting RNAs are present in the small RNA landscape of prostate epithelium from vitamin D clinical trial specimens. Prostate. 2019;79:840–855. doi: 10.1002/pros.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Feferman L., Tobacman J.K., Bhattacharyya S., Feferman L., Tobacman J.K. Chondroitin sulfatases differentially regulate Wnt signaling in prostate stem cells through effects on SHP2, phospho-ERK1/2, and Dickkopf Wnt signaling pathway inhibitor (DKK3) Oncotarget. 2017;8:100242–100260. doi: 10.18632/oncotarget.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D.D. Vitamin D regulated keratinocyte differentiation. J. Cell. Biochem. 2004;92:436–444. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- Blum R., Gupta R., Burger P.E., Ontiveros C.S., Salm S.N., Xiong X., Kamb A., Wesche H., Marshall L., Cutler G. Molecular signatures of prostate stem cells reveal novel signaling pathways and provide insights into prostate cancer. PLoS One. 2009;4:e5722. doi: 10.1371/journal.pone.0005722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R., Carmeliet G., Verlinden L., van Etten E., Verstuyf A., Luderer H.F., Lieben L., Mathieu C., Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr. Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant S.L., Francis J.C., Lokody I.B., Wang H., Risbridger G.P., Loveland K.L., Swain A. Sex specific retinoic acid signaling is required for the initiation of urogenital sinus bud development. Dev. Biol. 2014;395:209–217. doi: 10.1016/j.ydbio.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busceti C.L., Marchitti S., Bianchi F., Di Pietro P., Riozzi B., Stanzione R., Cannella M., Battaglia G., Bruno V., Volpe M. Dickkopf-3 upregulates VEGF in cultured human endothelial cells by activating activin receptor-like kinase 1 (ALK1) pathway. Front. Pharmacol. 2017;8:111. doi: 10.3389/fphar.2017.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A., Hoffman P., Smibert P., Papalexi E., Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotech. 2018;36:411. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M.J., Elstner E., Holden S., Uskokovic M., Koeffler H.P. Inhibition of proliferation of prostate cancer cells by a 19-nor-hexafluoride vitamin D3 analogue involves the induction of p21waf1, p27kip1 and E-cadherin. J. Mol. Endocrinol. 1997;19:15–27. doi: 10.1677/jme.0.0190015. [DOI] [PubMed] [Google Scholar]
- Chandler P.D., Giovannucci E.L., Scott J.B., Bennett G.G., Ng K., Chan A.T., Hollis B.W., Emmons K.M., Fuchs C.S., Drake B.F. Null association between vitamin D and PSA levels among black men in a vitamin D supplementation trial. Cancer Epidemiol. Biomarkers Prev. 2014;23:1944–1947. doi: 10.1158/1055-9965.EPI-14-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.S., DeLuca H.F. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim. Biophys. Acta. 1995;1263:1–9. doi: 10.1016/0167-4781(95)00060-t. [DOI] [PubMed] [Google Scholar]
- Davies G., Jiang W.G., Mason M.D. Cell-cell adhesion molecules and signaling intermediates and their role in the invasive potential of prostate cancer cells. J. Urol. 2000;163:985–992. [PubMed] [Google Scholar]
- Drost J., Karthaus W.R., Gao D., Driehuis E., Sawyers C.L., Chen Y., Clevers H. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 2016;11:347–358. doi: 10.1038/nprot.2016.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshazly M.A., Sultan M.F., Aboutaleb H.A., Salem S.M., Aziz M.S., Abd Elbaky T.M., Elsherif E.A., Gawish M.M., Alajrawi F.T., Elgadi F.A.A. Vitamin D deficiency and lower urinary tract symptoms in males above 50 years of age. Urol. Ann. 2017;9:170–173. doi: 10.4103/0974-7796.204192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Kasperzyk J.L., Shui I., Hendrickson W., Hollis B.W., Fall K., Ma J., Gaziano J.M., Stampfer M.J., Mucci L.A. Prediagnostic plasma vitamin D metabolites and mortality among patients with prostate cancer. PLoS One. 2011;6:e18625. doi: 10.1371/journal.pone.0018625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- Ferrari N., Ranftl R., Chicherova I., Slaven N.D., Moeendarbary E., Farrugia A.J., Lam M., Semiannikova M., Westergaard M.C.W., Tchou J. Dickkopf-3 links HSF1 and YAP/TAZ signalling to control aggressive behaviours in cancer-associated fibroblasts. Nat. Commun. 2019;10:130. doi: 10.1038/s41467-018-07987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet J.C., Kovalenko P.L., Li Y., Smolinski J., Spees C., Yu J.G., Thomas-Ahner J.M., Cui M., Neme A., Carlberg C. Vitamin D signaling suppresses early prostate carcinogenesis in TgAPT121 mice. Cancer Prev. Res. (Phila) 2019;12:343–356. doi: 10.1158/1940-6207.CAPR-18-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A., Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem. J. 2011;436:213–224. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- Gilbert R., Metcalfe C., Oliver S.E., Whiteman D.C., Bain C., Ness A., Donovan J., Hamdy F., Neal D.E., Lane J.A. Life course sun exposure and risk of prostate cancer: population-based nested case-control study and meta-analysis. Int. J. Cancer. 2009;125:1414–1423. doi: 10.1002/ijc.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E., Liu Y., Rimm E.B., Hollis B.W., Fuchs C.S., Stampfer M.J., Willett W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- Glinka A., Wu W., Delius H., Monaghan A.P., Blumenstock C., Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Goldstein A.S., Lawson D.A., Cheng D., Sun W., Garraway I.P., Witte O.N. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc. Natl. Acad. Sci. U S A. 2008;105:20882–20887. doi: 10.1073/pnas.0811411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschel C., Aggarwal A., Tennakoon S., Hobaus J., Prinz-Wohlgenannt M., Marian B., Heffeter P., Berger W., Kallay E. Effect of 1,25-dihydroxyvitamin D3 on the Wnt pathway in non-malignant colonic cells. J. Steroid Biochem. Mol. Biol. 2016;155:224–230. doi: 10.1016/j.jsbmb.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Guo C., Liu H., Zhang B.H., Cadaneanu R.M., Mayle A.M., Garraway I.P. Epcam, cd44, and cd49f distinguish sphere-forming human prostate basal cells from a subpopulation with predominant tubule initiation capability. PLoS One. 2012;7:e34219. doi: 10.1371/journal.pone.0034219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchette C.L., Schwartz G.G. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–2869. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hendrickson W.K., Flavin R., Kasperzyk J.L., Fiorentino M., Fang F., Lis R., Fiore C., Penney K.L., Ma J., Kantoff P.W. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J. Clin. Oncol. 2011;29:2378–2385. doi: 10.1200/JCO.2010.30.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G.H., Malewska A., Joseph D.B., Malladi V.S., Lee J., Torrealba J., Mauck R.J., Gahan J.C., Raj G.V., Roehrborn C.G. A cellular anatomy of the normal adult human prostate and prostatic urethra. Cell Rep. 2018;25:3530–3542. doi: 10.1016/j.celrep.2018.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlaing S.M., Garcia L.A., Contreras J.R., Norris K.C., Ferrini M.G., Artaza J.N. 1,25-Vitamin D3 promotes cardiac differentiation through modulation of the WNT signaling pathway. J. Mol. Endocrinol. 2014;53:303–317. doi: 10.1530/JME-14-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick C.N., Stanford J.L., Kwon E.M., Ostrander E.A., Nejentsev S., Peters U. Comprehensive association analysis of the vitamin D pathway genes, VDR, CYP27B1, and CYP24A1, in prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2007;16:1990–1999. doi: 10.1158/1055-9965.EPI-07-0487. [DOI] [PubMed] [Google Scholar]
- Hsieh S.-Y., Hsieh P.-S., Chiu C.-T., Chen W.-Y. Dickkopf-3/REIC functions as a suppressor gene of tumor growth. Oncogene. 2004;23:9183–9189. doi: 10.1038/sj.onc.1208138. [DOI] [PubMed] [Google Scholar]
- Hu W.-Y., Hu D.-P., Xie L., Li Y., Majumdar S., Nonn L., Hu H., Shioda T., Prins G.S. Isolation and functional interrogation of adult human prostate epithelial stem cells at single cell resolution. Stem Cell Res. 2017;23:1–12. doi: 10.1016/j.scr.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutabarat M., Wibowo N., Obermayer-Pietsch B., Huppertz B. Impact of vitamin D and vitamin D receptor on the trophoblast survival capacity in preeclampsia. PLoS One. 2018;13:e0206725. doi: 10.1371/journal.pone.0206725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Fujita H., Bando T., Kondo Y., Kumon H., Ohuchi H. Expression analysis of Dickkopf-related protein 3 (Dkk3) suggests its pleiotropic roles for a secretory glycoprotein in adult mouse. J. Mol. Histol. 2017;48:29–39. doi: 10.1007/s10735-016-9703-2. [DOI] [PubMed] [Google Scholar]
- Jacques P.F., Felson D.T., Tucker K.L., Mahnken B., Wilson P.W., Rosenberg I.H., Rush D. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am. J. Clin. Nutr. 1997;66:929–936. doi: 10.1093/ajcn/66.4.929. [DOI] [PubMed] [Google Scholar]
- James S.Y., Williams M.A., Kelsey S.M., Newland A.C., Colston K.W. Interaction of vitamin D derivatives and granulocyte-macrophage colony-stimulating factor in leukaemic cell differentiation. Leukemia. 1997;11:1017–1025. doi: 10.1038/sj.leu.2400676. [DOI] [PubMed] [Google Scholar]
- Karantanos T., Corn P.G., Thompson T.C. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–5511. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardooni H., Gonzalez-Gualda E., Stylianakis E., Saffaran S., Waxman J., Kypta R.M. CRISPR-mediated reactivation of DKK3 expression attenuates TGF-β signaling in prostate cancer. Cancers. 2018;10:1–19. doi: 10.3390/cancers10060165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y., Kitaoka M., Hamada Y., Walker M., Waxman J., Kypta R. Regulation of prostate cell growth and morphogenesis by Dickkopf-3. Oncogene. 2006;25:6528–6537. doi: 10.1038/sj.onc.1209661. [DOI] [PubMed] [Google Scholar]
- Konety B.R., Schwartz G.G., Acierno J.S., Jr., Becich M.J., Getzenberg R.H. The role of vitamin D in normal prostate growth and differentiation. Cell Growth Differ. 1996;7:1563–1570. [PubMed] [Google Scholar]
- Kovalenko P.L., Zhang Z., Cui M., Clinton S.K., Fleet J.C. 1,25 dihydroxyvitamin D-mediated orchestration of anticancer, transcript-level effects in the immortalized, non-transformed prostate epithelial cell line, RWPE1. BMC Genomics. 2010;11:26. doi: 10.1186/1471-2164-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A., Green J., Pollard J., Jr., Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof-De Julio M., Shibata M., Desai N., Reynon M., Halili M.V., Hu Y.-P., Price S.M., Abate-Shen C., Shen M.M. Canonical Wnt signaling regulates Nkx3.1 expression and luminal epithelial differentiation during prostate organogenesis. Dev. Dyn. 2013;242:1160–1171. doi: 10.1002/dvdy.24008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnik V.E., Sharp J.D., Jiang C., Robison K., Chickering T.W., Amaravadi L., Brown D.E., Guyot D., Mays G., Leiby K. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- Larriba M.J., González-Sancho J.M., Barbáchano A., Niell N., Ferrer-Mayorga G., Muñoz A. Vitamin D is a multilevel repressor of Wnt/β-catenin signaling in cancer cells. Cancers (Basel) 2013;5:1242–1260. doi: 10.3390/cancers5041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larriba M.J., Ordonez-Moran P., Chicote I., Martin-Fernandez G., Puig I., Munoz A., Palmer H.G. Vitamin D receptor deficiency enhances Wnt/beta-catenin signaling and tumor burden in colon cancer. PLoS One. 2011;6:e23524. doi: 10.1371/journal.pone.0023524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J.L., Leonard D.M., Wolfe S.A., Liu J., Rivera J., Yang M., Leonard R.T., Johnson J.P.S., Kumar P., Liebmann K.L. The Dkk3 gene encodes a vital intracellular regulator of cell proliferation. PLoS One. 2017;12:e0181724. doi: 10.1371/journal.pone.0181724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu H., Liang Y., Peng P., Ma X., Zhang X. DKK3 regulates cell proliferation, apoptosis and collagen synthesis in keloid fibroblasts via TGF-β1/Smad signaling pathway. Biomed. Pharmacother. 2017;91:174–180. doi: 10.1016/j.biopha.2017.03.044. [DOI] [PubMed] [Google Scholar]
- Liu B., Zhou W., Jiang H., Xiang Z., Wang L. miR-1303 promotes the proliferation, migration and invasion of prostate cancer cells through regulating the Wnt/beta-catenin pathway by targeting DKK3. Exp. Ther. Med. 2019;18:4747–4757. doi: 10.3892/etm.2019.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaughlin J.A., Cantley L.C., Holick M.F. 1,25(OH)2D3 increases calcium and phosphatidylinositol metabolism in differentiating cultured human keratinocytes. J. Nutr. Biochem. 1990;1:81–87. doi: 10.1016/0955-2863(90)90054-o. [DOI] [PubMed] [Google Scholar]
- Manson J.E., Cook N.R., Lee I.-M., Christen W., Bassuk S.S., Mora S., Gibson H., Gordon D., Copeland T., D’Agostino D. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall D.T., Savage S.J., Garrett-Mayer E., Keane T.E., Hollis B.W., Horst R.L., Ambrose L.H., Kindy M.S., Gattoni-Celli S. Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J. Clin. Endocrinol. Metab. 2012;97:2315–2324. doi: 10.1210/jc.2012-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J.L., Pattison S.L. Insulin-like growth factor binding protein-3 is regulated by dihydrotestosterone and stimulates deoxyribonucleic acid synthesis and cell proliferation in LNCaP prostate carcinoma cells. Endocrinology. 2000;141:2401–2409. doi: 10.1210/endo.141.7.7534. [DOI] [PubMed] [Google Scholar]
- Maund S.L., Nolley R., Peehl D.M. Optimization and comprehensive characterization of a faithful tissue culture model of the benign and malignant human prostate. Lab. Invest. 2014;94:208–221. doi: 10.1038/labinvest.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray T., Moline D., Baumann B., Vander Griend D.J., Nonn L. Single-cell RNA-Seq analysis identifies a putative epithelial stem cell population in human primary prostate cells in monolayer and organoid culture conditions. Am. J. Clin. Exp. Urol. 2019;7:123–138. [PMC free article] [PubMed] [Google Scholar]
- Mucuk G., Sepet E., Erguven M., Ekmekci O., Bilir A. 1,25-Dihydroxyvitamin D3 stimulates odontoblastic differentiation of human dental pulp-stem cells in vitro. Connect. Tissue Res. 2017;58:531–541. doi: 10.1080/03008207.2016.1264395. [DOI] [PubMed] [Google Scholar]
- Muralidhar S., Filia A., Nsengimana J., Pozniak J., O'Shea S.J., Diaz J.M., Harland M., Randerson-Moor J.A., Reichrath J., Laye J.P. Vitamin D-VDR signaling inhibits Wnt/beta-catenin-mediated melanoma progression and promotes antitumor immunity. Cancer Res. 2019;79:5986–5998. doi: 10.1158/0008-5472.CAN-18-3927. [DOI] [PubMed] [Google Scholar]
- Murillo-Garzon V., Kypta R. WNT signalling in prostate cancer. Nat. Rev. Urol. 2017;14:683–696. doi: 10.1038/nrurol.2017.144. [DOI] [PubMed] [Google Scholar]
- Murphy A.B., Nyame Y., Martin I.K., Catalona W.J., Hollowell C.M., Nadler R.B., Kozlowski J.M., Perry K.T., Kajdacsy-Balla A., Kittles R. Vitamin D deficiency predicts prostate biopsy outcomes. Clin. Cancer Res. 2014;20:2289–2299. doi: 10.1158/1078-0432.CCR-13-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A.B., Nyame Y.A., Batai K., Kalu R., Khan A., Gogana P., Dixon M., Macias V., Kajdacsy-Balla A., Hollowell C.M. Does prostate volume correlate with vitamin D deficiency among men undergoing prostate biopsy? Prostate Cancer Prostatic Dis. 2017;20:55–60. doi: 10.1038/pcan.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R.E.I., Hackam A.S. Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors. 2010;28:232–242. doi: 10.3109/08977191003738832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R.A.L.X. Wnt target genes. 2020. https://web.stanford.edu/group/nusselab/cgi-bin/wnt/target_genes
- Peehl D.M., Shinghal R., Nonn L., Seto E., Krishnan A.V., Brooks J.D., Feldman D. Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J. Steroid Biochem. Mol. Biol. 2004;92:131–141. doi: 10.1016/j.jsbmb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Peregrina K., Houston M., Daroqui C., Dhima E., Sellers R.S., Augenlicht L.H. Vitamin D is a determinant of mouse intestinal Lgr5 stem cell functions. Carcinogenesis. 2015;36:25–31. doi: 10.1093/carcin/bgu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho Christof Niehrs S. Dkk3 is required for TGF-b signaling during Xenopus mesoderm induction. Differentiation. 2007;75:957–967. doi: 10.1111/j.1432-0436.2007.00185.x. [DOI] [PubMed] [Google Scholar]
- Post Y., Puschhof J., Beumer J., Kerkkamp H.M., de Bakker M.A.G., Slagboom J., de Barbanson B., Wevers N.R., Spijkers X.M., Olivier T. Snake Venom Gland Organoids. Cell. 2020;180:233–247 e221. doi: 10.1016/j.cell.2019.11.038. [DOI] [PubMed] [Google Scholar]
- Prins G.S., Lindgren M. Accessory sex glands in the male. In: Plant T.M., Zeleznik A.J., editors. Knobil and Neill's Physiology of Reproduction. Fourth Edition. Elsevier; 2015. pp. 773–797. [Google Scholar]
- Prins G.S., Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan S., Steck S.E., Arab L., Zhang H., Bensen J.T., Fontham E.T.H., Johnson C.S., Mohler J.L., Smith G.J., Su L.J. Association among plasma 1,25(OH)2 D, ratio of 1,25(OH)2 D to 25(OH)D, and prostate cancer aggressiveness. Prostate. 2019;79:1117–1124. doi: 10.1002/pros.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards Z., Batai K., Farhat R., Shah E., Makowski A., Gann P.H., Kittles R., Nonn L. Prostatic compensation of the vitamin D axis in African American men. JCI Insight. 2017;2:e91054. doi: 10.1172/jci.insight.91054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards Z., McCray T., Marsili J., Zenner M.L., Garcia J., Manlucu J.T., Voisine C., Murphy A.B., Prins G.S., Murray M. Prostate stroma increases the viability and maintains the branching phenotype of human prostate organoids. iScience. 2019;12:304–317. doi: 10.1016/j.isci.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robsahm T.E., Tretli S., Dahlback A., Moan J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway) Cancer Causes Control. 2004;15:149–158. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- Romero D., Kawano Y., Bengoa N., Walker M.M., Maltry N., Niehrs C., Waxman J., Kypta R. Downregulation of Dickkopf-3 disrupts prostate acinar morphogenesis through TGF-b/Smad signalling. J. Cell Sci. 2013;126:1858–1867. doi: 10.1242/jcs.119388. [DOI] [PubMed] [Google Scholar]
- Ryan S.D., Ferrier A., Kothary R. A novel role for the cytoskeletal linker protein dystonin in the maintenance of microtubule stability and the regulation of ER-Golgi transport. Bioarchitecture. 2012;2:2–5. doi: 10.4161/bioa.20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satija R., Farrell J.A., Gennert D., Schier A.F., Regev A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015;33:495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M., Moll R., Hesse U., Prasad A.R., Gandolfi J.A., Hasan S.R., Bartholdi M., Cress A.E. Identification of a stem cell candidate in the normal human prostate gland. Eur. J. Cell Biol. 2005;84:341–354. doi: 10.1016/j.ejcb.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Das D., Wadhwa N., Kunj N., Sarda K., Shankar Pradhan B., Majumdar S.S. Dickkopf homolog 3 (DKK3) plays a crucial role upstream of WNT/b-CATENIN signaling for sertoli cell mediated regulation of spermatogenesis. PLoS One. 2013;8:e63603. doi: 10.1371/journal.pone.0063603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons B.W., Hurley P.J., Huang Z., Ross A.E., Miller R., Marchionni L., Berman D.M., Schaeffer E.M. Wnt signaling though beta-catenin is required for prostate lineage specification. Dev. Biol. 2012;371:246–255. doi: 10.1016/j.ydbio.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneath R.J., Mangham D.C. The normal structure and function of CD44 and its role in neoplasia. Mol. Pathol. 1998;51:191–200. doi: 10.1136/mp.51.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielvogel A.M., Farley R.D., Norman A.W. Studies on the mechanism of action of calciferol. V. Turnover time of chick intestinal epithelial cells in relation to the intestinal action of vitamin D. Exp. Cell Res. 1972;74:359–366. doi: 10.1016/0014-4827(72)90388-6. [DOI] [PubMed] [Google Scholar]
- Stahl P.L., Salmen F., Vickovic S., Lundmark A., Navarro J.F., Magnusson J., Giacomello S., Asp M., Westholm J.O., Huss M. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353:78–82. doi: 10.1126/science.aaf2403. [DOI] [PubMed] [Google Scholar]
- Studzinski G.P., Moore D.C. Sunlight--can it prevent as well as cause cancer? Cancer Res. 1995;55:4014–4022. [PubMed] [Google Scholar]
- Takasato M., Er P.X., Chiu H.S., Little M.H. Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc. 2016;11:1681–1692. doi: 10.1038/nprot.2016.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia C., Suares A., De Genaro P., Gonzalez-Pardo V. In vitro studies revealed a downregulation of Wnt/beta-catenin cascade by active vitamin D and TX 527 analog in a Kaposi's sarcoma cellular model. Toxicol. In Vitro. 2020;63:104748. doi: 10.1016/j.tiv.2019.104748. [DOI] [PubMed] [Google Scholar]
- Tavera-Mendoza L.E., Westerling T., Libby E., Marusyk A., Cato L., Cassani R., Cameron L.A., Ficarro S.B., Marto J.A., Klawitter J. Vitamin D receptor regulates autophagy in the normal mammary gland and in luminal breast cancer cells. Proc. Natl. Acad. Sci. U S A. 2017;114:E2186–E2194. doi: 10.1073/pnas.1615015114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivanen R., Shen M.M. Prostate organogenesis: tissue induction, hormonal regulation and cell type specification. Development. 2017;144:1382–1398. doi: 10.1242/dev.148270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser G., Koch H.B., Menssen A., Hermeking H. Characterization of epithelial senescence by serial analysis of gene expression: identification of genes potentially involved in prostate cancer. Cancer Res. 2002;62:6255–6262. [PubMed] [Google Scholar]
- Wagner D., Trudel D., Van der Kwast T., Nonn L., Giangreco A.A., Li D., Dias A., Cardoza M., Laszlo S., Hersey K. Randomized clinical trial of vitamin D 3 doses on prostatic vitamin D metabolite levels and Ki67 labeling in prostate cancer patients. J. Clin. Endocrinol. Metab. 2013;98:1498–1507. doi: 10.1210/jc.2012-4019. [DOI] [PubMed] [Google Scholar]
- Wang H.H., Wang L., Jerde T.J., Chan B.D., Savran C.A., Burcham G.N., Crist S., Ratliff T.L. Characterization of autoimmune inflammation induced prostate stem cell expansion. Prostate. 2015;75:1620–1631. doi: 10.1002/pros.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Singhal U., Qiao Y., Kasputis T., Chung J.S., Zhao H., Chammaa F., Belardo J.A., Roth T.M., Zhang H. Wnt signaling drives prostate cancer bone metastatic tropism and invasion. Transl. Oncol. 2020;13:100747. doi: 10.1016/j.tranon.2020.100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hayward S., Cao M., Thayer K., Cunha G. Cell differentiation lineage in the prostate. Differentiation. 2001;68:270–279. doi: 10.1046/j.1432-0436.2001.680414.x. [DOI] [PubMed] [Google Scholar]
- Wang Z., Lin L., Thomas D.G., Nadal E., Chang A.C., Beer D.G., Lin J. The role of dickkopf-3 overexpression in esophageal adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2015;25:368–379. doi: 10.1016/j.jtcvs.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo T.C., Choo R., Jamieson M., Chander S., Vieth R. Pilot study: potential role of vitamin D (Cholecalciferol) in patients with PSA relapse after definitive therapy. Nutr. Cancer. 2005;51:32–36. doi: 10.1207/s15327914nc5101_5. [DOI] [PubMed] [Google Scholar]
- Wright A.S., Thomas L.N., Douglas R.C., Lazier C.B., Rittmaster R.S. Relative potency of testosterone and dihydrotestosterone in preventing atrophy and apoptosis in the prostate of the castrated rat. J. Clin. Invest. 1996;98:2558–2563. doi: 10.1172/JCI119074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Daniels G., Shapiro E., Xu K., Huang H., Li Y., Logan S., Greco M.A., Peng Y., Monaco M.E. LEF1 identifies androgen-independent epithelium in the developing prostate. Mol. Endocrinol. 2011;25:1018–1026. doi: 10.1210/me.2010-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Zhang Z.H., Fu L., Song J., Xie D.D., Yu D.X., Xu D.X., Sun G.P. Calcitriol inhibits migration and invasion of renal cell carcinoma cells by suppressing Smad2/3-, STAT3- and beta-catenin-mediated epithelial-mesenchymal transition. Cancer Sci. 2020;111:59–71. doi: 10.1111/cas.14237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Masters J.R., Dasgupta P., Chandra A., Popert R., Freeman A., Ahmed A. CD49f is an efficient marker of monolayer- and spheroid colony-forming cells of the benign and malignant human prostate. PLoS One. 2012;7:1–11. doi: 10.1371/journal.pone.0046979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhu S., Lin G., Song C., He Z. Vitamin D enhances omega-3 polyunsaturated fatty acids-induced apoptosis in breast cancer cells. Cell Biol. Int. 2017;41:890–897. doi: 10.1002/cbin.10806. [DOI] [PubMed] [Google Scholar]
- Yin J., Yang L., Xie Y., Liu Y., Li S., Yang W., Xu B., Ji H., Ding L., Wang K. Dkk3 dependent transcriptional regulation controls age related skeletal muscle atrophy. Nat. Commun. 2018;9:1752. doi: 10.1038/s41467-018-04038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama N.N., Shao S., Hoang B.H., Mercola D., Zi X. Wnt signaling in castration-resistant prostate cancer: implications for therapy. Am. J. Clin. Exp. Urol. 2014;2:27–44. [PMC free article] [PubMed] [Google Scholar]
- Zenzmaier C., Sampson N., Plas E., Berger P. Dickkopf-related protein 3 promotes pathogenic stromal remodeling in benign prostatic hyperplasia and prostate cancer. Prostate. 2013;73:1441–1452. doi: 10.1002/pros.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenzmaier C., Untergasser G., Hermann M., Dirnhofer S., Sampson N., Berger P. Dysregulation of Dkk-3 expression in benign and malignant prostatic tissue. Prostate. 2008;68:540–547. doi: 10.1002/pros.20711. [DOI] [PubMed] [Google Scholar]
- Zhang K., Guo Y., Wang X., Zhao H., Ji Z., Cheng C., Li L., Fang Y., Xu D., Zhu H.H. WNT/beta-Catenin directs self-renewal symmetric cell division of hTERT(high) prostate cancer stem cells. Cancer Res. 2017;77:2534–2547. doi: 10.1158/0008-5472.CAN-16-1887. [DOI] [PubMed] [Google Scholar]
- Zhang K., Watanabe M., Kashiwakura Y., Li S.A., Edamura K., Huang P., Yamaguchi K., Nasu Y., Kobayashi Y., Sakaguchi M. Expression pattern of REIC/Dkk-3 in various cell types and the implications of the soluble form in prostatic acinar development. Int. J. Oncol. 2010;151:414–420. doi: 10.3892/ijo_00000802. [DOI] [PubMed] [Google Scholar]
- Zierold C., Darwish H.M., DeLuca H.F. Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter. J. Biol. Chem. 1995;270:1675–1678. doi: 10.1074/jbc.270.4.1675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Document S1. Transparent methods and Figures S1–S8
Data Availability Statement
ScRNAseq data has been submitted to NCBI Gene Expression Omnibus and can be found under accession number GSE142489. ChIP sequencing data can be found under accession number GSE124576. Spatial transcriptomics data can be found under accession number GSE159697.








