Abstract
Purpose
To test the effect of different sodium channel blockers on the electrical activity of corneal nociceptors in intact and surgically injured corneas.
Methods
In anesthetized guinea pigs, a 4-mm diameter corneal flap was performed in one eye at a midstromal depth using a custom-made microkeratome. At different times after surgery (3 hours to 15 days), the electrical activity of corneal nociceptor fibers was recorded from ciliary nerve filaments in the superfused eye in vitro. Mechanical threshold was measured using calibrated von Frey hairs; chemical stimulation was performed applying 30-second CO2 gas pulses. The characteristics of the spontaneous and stimulus-evoked activity of corneal nociceptors recorded from intact and lesioned corneas, before and after treatment with the sodium channel blockers lidocaine, carbamazepine, and amitriptyline, were compared.
Results
No spontaneous or stimulus-evoked impulse activity was detected inside the flap at any of the studied time points. However, both were recorded from mechanonociceptor and polymodal nociceptors fibers in the surrounding corneal tissue, being significantly higher (sensitization) 24 to 48 hours after surgery. In these fibers, none of the tested drugs affected mechanical threshold, but they significantly reduced the CO2 response of polymodal nociceptors of intact and injured corneas. Likewise, they diminished significantly the transient increase in spontaneous and stimulus-evoked activity of sensitized polymodal nociceptors.
Conclusions
Na+ channel blockers decrease the excitability of intact and sensitized corneal nociceptor fibers, thus acting as potential tools to attenuate their abnormal activity, which underlies the spontaneous pain, hyperalgesia, and allodynia often accompanying surgical corneal lesions, as occurs after photorefractive surgery.
Keywords: injured cornea, sodium channel blockers, polymodal nociceptors, hyperexcitability, corneal pain
Peripheral axons of trigeminal ganglion neurons innervating the cornea and conjunctiva are functionally classified as polymodal nociceptors, mechanonociceptors, and cold thermoreceptors1–5 and their selective stimulation evokes qualitatively different conscious sensations.6 When mechanically stimulated, mechanonociceptors evoke acute, pricking pain, whereas the excitation of polymodal nociceptors by mechanical, thermal, or noxious chemical stimuli leads to a burning pain and neurogenic inflammation.7,8 Depending on the magnitude of temperature decrease, cold thermoreceptors evoke cooling or dryness sensations.1,6
Ocular nerves represent the first defensive barrier of the eye against potentially injuring threats (foreign bodies, accidental or surgical aggression, extreme environmental changes, pathologic disorders, infections). Damage to the eye surface immediately evokes a sustained discharge of nerve impulses in the nociceptor sensory nerve fibers. This information travels to higher level brain areas, leading ultimately to conscious unpleasant sensations and behavioral and autonomic protective responses. The activation of the peripheral sensory nerves by noxious stimuli is usually accompanied by an enhancement of their spontaneous activity (sensitization) and long-lasting, altered responsiveness to natural stimuli that are a consequence of the direct injury infringed to nerve terminals and the interaction of such terminals with locally released inflammatory mediators.2,9–11 The buildup of an abnormal impulse activity after corneal damage, such as occurs for instance after refractive surgery and other ocular surgeries, underlies the aberrant sensations (dysesthesias) often experienced by patients subjected to these procedures and usually described as ocular discomfort, eye dryness, and pain.12–15 Abnormal firing is mechanistically attributable to the development of corneal nerve membrane hyperexcitability16,17 caused by an altered expression of some of the ion channel types sustaining membrane potential, in particular voltage-gated sodium channels.18
To date, nine alpha subtypes of the voltage-gated sodium channel family (Nav 1.1 to Nav 1.9) have been reported to be expressed in peripheral sensory nerves. Of them, the expression of Nav 1.3, 1.7, 1.8, and 1.9 seem to be altered after peripheral nerve damage and sensitized and redistributed in uninjured axons by inflammatory mediators, suggesting their involvement in nociceptor hyperexcitability.19,20 Hence, they are being considered potential targets for novel analgesics.21,22
A plethora of sodium channel blockers have been tested clinically to treat different pathologies involving abnormal cell excitability.23 These blockers include local anesthetics as lidocaine, widely used as an injectable or topical agent to treat pain via neural blockade of Nav channels in the peripheral nervous system,24 or anticonvulsants such as carbamazepine, lamotrigine, phenytoin, gabapentin, and the antidepressant amitriptyline, all of them blockers of Nav channels used for systemic the treatment of neuropathic pain.25
In the present study, we analyzed the topical analgesic efficacy of a number of sodium channel blockers with well-defined action on membrane hyperactivity, measuring in surgically injured guinea pig corneas, their effect on the altered spontaneous and stimulus-evoked activity of polymodal nociceptor, and mechanonociceptor corneal nerve fibers.
Methods
Animals
Albino guinea pigs of both sexes weighing 200 to 400 g were used. The study was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, the NIH Guide for the Care and Use of Laboratory Animals, the European Union Directive (2010/63/EU), and the Spanish regulations on the protection of animals used for research, following a protocol approved and supervised by the Ethics Committee of the Universidad Miguel Hernández de Elche.
Microkeratome Lesion
In anesthetized guinea pigs (ketamine 50 mg/kg and xylazine 5 mg/kg, intraperitoneally), a corneal flap of 4-mm diameter was cut at the midstromal depth in one eye (Fig. 1A) using a custom-made microkeratome designed for the guinea pig eye. Animals were allowed to recover postoperatively and then housed individually under standard conditions in a certified animal facility. They were inspected daily for ocular inflammation, corneal epithelial defects, or infection as well as for abnormal behavior, and were treated accordingly. At different time points after surgery, both eyes (lesioned and contralateral) were evaluated biomicroscopically before killing of the animal for an ex vivo electrophysiologic recording of the corneal sensory nerve activity of the lesioned eyes.
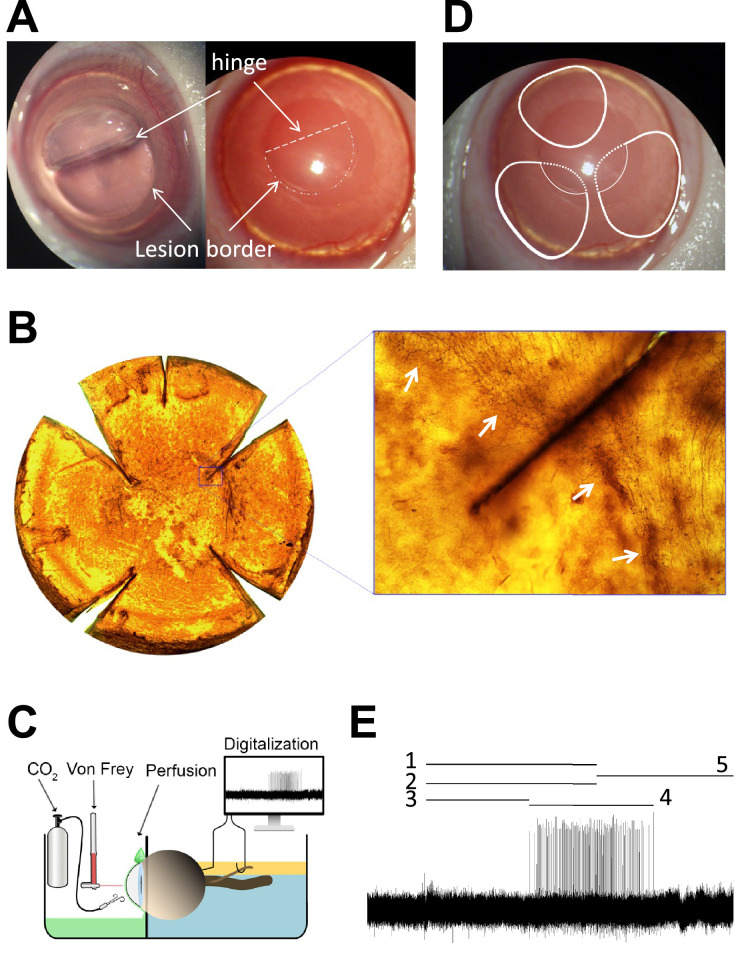
Figure 1.
(A) Guinea pig cornea showing the corneal flap, the border of the lesion and the hinge area, immediately after surgery. Arrows point the lesion border and the flap hinge (dashed line). (B) Flat-mounted cornea stained with Tuj-1 displaying the corneal flap absence of nerves inside the flap and the presence of Tuj1-positive subbasal nerve leashes outside the lesion. The inset shows how nerves bench at the border lesion without entering the flap. Arrows point the lesion border. (C) Schema of the ciliary nerve recording set-up. (D) Representation of the receptive field (RF) of three different nociceptive fibers. One is located at the peripheral cornea and the hinge area (up), that is, outside the lesion. The other two RFs covered the peripheral cornea (continuous lines) and presumably also part field inside the wound (dashed lines), but no activity was recorded when stimulating inside the lesion at any time point. (E) Sample recording of the nerve activity evoked in a polymodal nociceptor in response to CO2 pulse, showing the parameters analyzed to characterize chemical responsiveness: 1 is the duration of the CO2 pulse; 2 is the nerve activity during the pulse; 3 is the latency to the response; 4 is the response; 5 is the postdischarge (see Methods for further details).
Cornea Fixation and Whole Mount Staining
Fifteen days after surgery, four animals were killed with an overdose of sodium pentobarbitone and the eyes were enucleated and fixed in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) in 0.1 M phosphate buffer (PB) for 45 minutes at room temperature, followed by two washes in 0.1 M PB, for 10 minutes each. Eyes were cryoprotected with increasing concentrations of sucrose (Panreac Química S.L.U., Barcelona, Spain) in PB and kept overnight in 30% sucrose at room temperature. Then, eyes were rapidly frozen with liquid nitrogen-chilled isopentane and stored. After thawing, corneas were dissected and cut radially to flatten. Then, corneas were permeabilized by overnight incubation at 37 °C in 0.01% hyaluronidase type IV-S (Sigma-Aldrich) and 0.1% EDTA (Sigma Aldrich) in 0.1 M PB (pH 5.3), washed with 0.1 M PB, and incubated in 2.28% sodium (meta) periodate (NaIO4) (Sigma Aldrich) for 5 minutes and then in 0.02% sodium borohydride (NaBH4) (Panreac Química S.L.U.) for 5 additional minutes at room temperature. After washing with 0.1M PB, corneas were incubated in 10% normal goat serum (Jackson ImmunoResearch, West Grove, PA) in 0.1 M PB for 1 hour at 4 °C, followed by incubation for 2 days at 4 °C under agitation with the mouse monoclonal primary antibody, neuronal class III beta-tubulin (anti-TUJ 1; Covance Research Products, Berkeley, CA) diluted 1:200 in 0.1 M PB plus 1% TritonX-100 (Sigma Aldrich, Inc.). Then, the corneas were incubated for 1 day at 4 °C with the biotinylated horse anti-mouse IgG secondary antibody (Jackson ImmunoResearch Labs. Inc.) diluted 1:100 in 0.1 M PB plus 1% TritonX-100, washed with 0.1 M PB and incubated for 1 day at 4 °C with the avidin–biotin horseradish peroxidase complex (ABC reagent; Vector Laboratories, Burlingame, CA), followed by incubation in the dark under agitation for 15 minutes with 3,3’-diaminobenzidine tetrahydrochloride hydrate (Sigma-Aldrich) at 0.05% in 0.1 M PB, and then with fresh diaminobenzidine tetrahydrochloride hydrate solution with 0.01% H2O2 until stop the reaction by washes in cold distilled water. Then, the corneas were mounted in the mounting media Citifluor (Sigma-Aldrich) and coverslipped before taking bright field photomicrographs with the Leica DM 4000 B microscope (Leica Microsystems GmbH, Manheim, Germany) provided with 10× and 20× objectives to visualize the stained corneal nerves (stromal trunks, subbasal nerves, and intraepithelial nerves terminal) (Fig. 1B).
Electrophysiologic Recording
Guinea pigs were killed with an intraperitoneal injection of 100 mg/kg sodium pentobarbitone, and eyes were enucleated along with a short length of the optic nerve and surrounding tissues. Excised eyes were then pinned to the bottom of a silicone-coated chamber (Sylgard 184, Dow Corning, Midland, MI) filled with cold saline for further cleaning.
Connective tissue and extraocular muscles at the back of the eye were carefully removed to expose and isolate the ciliary nerves around the optic nerve. The excised eye was then placed in a chamber divided in two compartments by a Sylgard-coated plastic wall. The front of the eye was introduced into a round perforation made in the center of the dividing wall to which the bulbar conjunctiva was pinned, thereby isolating the front from the back of the eye. This maneuver prevented direct exposure of the ciliary nerves located in the back compartment to the chemical substances applied onto the corneal surface. The anterior compartment was continuously bathed with warmed (34 °C) physiologic saline solution of the following composition (in mM): NaCl, 133.4; KCl, 4.7; CaCl2, 2; MgCl2, 1.2; NaHCO3, 16.3; NaH2PO4, 1.3; glucose, 7.8, and gassed with 95% O2/5% CO2 to maintain a pH of 7.4. The rear compartment was filled with warm mineral oil (Fig. 1C).
Extracellular recordings were made in nerve filaments of the ciliary nerves dissected at the back of the excised eye.26 This preparation was particularly suitable for the detection of polymodal and mechanosensory afferent nerve fibers activity. Thin nerve filaments were teased apart from the ciliary nerve trunks and placed on an Ag–AgCl electrode for monopolar recording of single unit impulse activity, using conventional electrophysiologic equipment (DAM50 amplifier, WPI, Sarasota, FL). Electrical signals were fed into a PC through an acquisition system (CED Micro1401, Cambridge Electronic Design, Cambridge, UK) and analyzed with Spike 2 software (v8.0, Cambridge Electronic Design).
Receptive fields of afferent fibers innervating the corneoscleral surface were located using mechanical stimulation with a fine paint brush and mapped thereafter using a suprathreshold von Frey hair (5.88 mN) (Fig. 1D). Spontaneous activity was measured during 1 minute from recordings obtained before any stimulation was applied. Mechanical threshold was determined assessing the first impulse response evoked by calibrated von Frey hairs of increasing diameter (range, 0.25–4.00 mN; Bioseb, Vitrolles, France). For chemical stimulation, a gas jet of 98.5% CO2 was applied on the corneal receptive field for 30 seconds.26 To describe the characteristics of the response to chemical stimulation, the following parameters were measured: (a) the ongoing activity in imp/s (activity during the 30 seconds immediately before the CO2 pulse); (b) the latency of the response to CO2 stimulation, in seconds; (c) the mean discharge rate during the 30-second pulse, in imp/s; (d) the postdischarge (activity during the 30 seconds after the pulse, in imp/s); (e) the response duration, in seconds (duration of the impulse discharge evoked by the stimulus); and (f) the mean discharge rate during the response, in imp/s (Fig. 1E).
Drugs and Treatment Protocol
Drugs were purchased from Sigma-Aldrich and prepared in balanced saline solution at the desired concentration. A preliminary set of experiments was first performed to test different concentrations of the test drugs. According to the results, the following concentrations were used in the present experiments: lidocaine 0.01% (369 µM), carbamazepine 0.001% (42.32 µM), and amitriptyline 0.003% (100 µM). Drugs were tested applying a soaked piece of tissue (6 mm diameter) on the unit's receptive field for 10 minutes. After that, the piece of tissue was removed and the spontaneous activity recorded for 1 minute. Then, a 30-second duration CO2 pulse was applied to the unit's receptive field, and mechanical threshold was measured 2 minutes later. Afterwards, the receptive field was washed out for 10 minutes with a continuous perfusion of saline solution and the response to the chemical and mechanical stimulation was again tested.
Statistical Analysis
Data were collected and processed for statistical analysis using SigmaPlot software (SigmaPlot 11.0; Systat Software Inc, Point Richmond, CA). Data were expressed as mean ± SEM, with n being the number of explored nerve fibers. Differences in mechanical threshold, spontaneous activity, and firing frequency in response to CO2 pulses between control and lesioned corneas as well as before and after Na+ channel blocker treatment were compared using the independent t test or its nonparametric equivalent Mann–Whitney rank sum test. A P value of 0.05 or less was considered significant.
Results
In guinea pig corneas where a midstromal depth corneal flap had been performed (Fig. 1A), sub-basal and intraepithelial nerve fibers were absent within the flap during 14 days after surgery, in contrast with the surrounding, intact corneal region, where nerve fibers were present with a density roughly similar to nonoperated corneas. Fibers reaching the lesion border did not enter the flap at any of the studied time points following injury (Fig. 1B).
Surgical Lesion of the Cornea Altered the Electrical Activity of Mechanonociceptors and Polymodal Nociceptors
Ciliary nerve impulse activity in response to mechanical and CO2 stimulation was found only in fibers with a receptive field outside the flap. The shape parallel to the flat border of the RF of fibers located nearby the lesion area suggests that, presumably, some of their branches originally entered inside the flap (Fig. 1D), although no activity could be evoked when the mechanical or chemical stimulation was applied inside the flap at any of the recording time points after lesion.
Mechanonociceptors
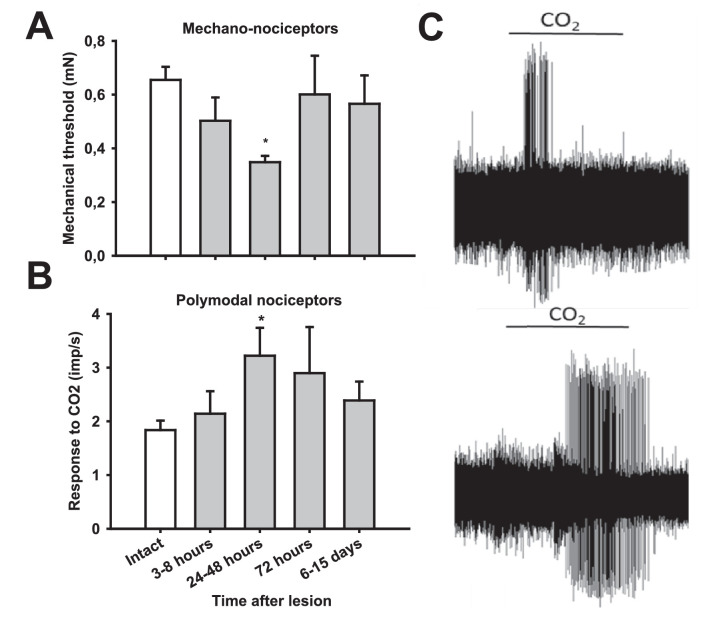
As previously described in intact corneas,3,6 corneal sensory nerve fibers responding to mechanical stimulation but not to CO2 were classified as mechanonociceptors. They were localized in the noninjured area of the cornea. Their receptive field was oval or round, with a mean maximal diameter of 3.54 ± 0.04 mm (n = 370; range, 2–6 mm). Mechanonociceptors showed a significantly lower mechanical threshold for 24 to 48 hours after lesion than mechanonociceptors of nonoperated corneas (Table 1, Fig. 2A), although their spontaneous activity was not modified significantly (Table 1).
Table 1.
General Properties of Mechanonociceptor Fibers Recorded From Intact and Injured Corneas at Different Times After Lesion With a Microkeratome
| Injured Corneas (Time After Lesion) | |||||
|---|---|---|---|---|---|
| Mechanonociceptors | Intact Corneas | 3–8 h | 24–48 h | 72 h | 6–15 Days |
| Spontaneous activity (imp/s) | 0.008 ± 0.004 (4/225) | 0.022 ± 0.022 (1/25) | 0 ± 0 (0/48) | 0 ± 0 (0/29) | 0 ± 0 (0/28) |
| Mechanical threshold (mN) | 0.66 ± 0.005 | 0.50 ± 0.09 | 0.35 ± 0.02* | 0.60 ± 0.14 | 0.57 ± 0.11 |
| n | 242 | 25 | 48 | 29 | 28 |
Data are mean ± SEM; n = number of recorded fibers; number in brackets indicate the number of fibers with spontaneous activity/number of recorded fibers. * P < 0.05, Mann–Whitney U test, compared with intact corneas.
Figure 2.
Responsiveness of corneal nociceptors to mechanical and chemical stimulation in intact and injured corneas at different time points after surgery. (A) Mechanical threshold values of corneal mechanonociceptor fibers measured with calibrated von Frey hairs. (B) Mean discharge rate during the 30-second CO2 pulse of polymodal nociceptive fibers. (C) Sample recordings of impulse response evoked by CO2 in polymodal nociceptors recorded from an intact cornea (top) and a lesioned cornea, 24 hours after surgery (bottom). *P < 0.05, t-test, compared with intact cornea.
Polymodal Nociceptors
Based on their response to acidic stimulation with CO2, part of the recorded fibers were classified as polymodal nociceptors. Polymodal nociceptors’ receptive field was also round or oval, with a mean maximal diameter of 3.5 ± 0.06 mm (n = 241; range, 2–6 mm). As indicated elsewhere in this article, polymodal nociceptor nerve activity was not detected when stimulating inside the flap. Similarly, the mechanical threshold of polymodal nociceptors was not significantly modified after lesion (Table 2). Consistent with previous studies in inflamed or damaged corneas,26–28 polymodal nociceptors from lesioned corneas displayed significantly augmented firing responses to CO2, including development of postdischarge (Table 3). The increased responsiveness to chemical stimulation in comparison with equivalent values measured in intact corneas was significant 24 to 48 hours after lesion (Fig. 2B, 2C; Table 3).
Table 2.
General Properties of Polymodal Nociceptor Fibers Recorded in Intact and Lesioned Corneas at Different Times After Lesion With Microkeratome
| Injured Corneas (Time After Lesion) | |||||
|---|---|---|---|---|---|
| Polymodal Nociceptors | Intact Corneas | 3–8 h | 24–48 h | 72 h | 6–15 Days |
| Spontaneous activity (imp/s) | 0.05 ± 0.024 (9/129) | 0 ± 0 (0/27) | 0.05 ± 0.03 (4/19) | 0.05 ± 0.05 (1/11) | 0.03 ± 0.02 (2/14) |
| Mechanical threshold (mN) | 0.38 ± 0.03 | 0.31 ± 0.03 | 0.53 ± 0.14 | 0.26 ± 0.01 | 0.37 ± 0.08 |
| n | 146 | 27 | 19 | 11 | 14 |
Data are mean ± SEM; n = number of recorded fibers; number in brackets indicate the number of fibers with spontaneous activity/number of recorded fibers. No significant differences between groups were found.
Table 3.
Characteristics of the Response to CO2 Pulses of Corneal Polymodal Nociceptors Recorded From Intact and Lesioned Corneas at Different Times After Lesion With Microkeratome
| Injured Corneas (Time After Lesion) | |||||
|---|---|---|---|---|---|
| Polymodal Nociceptors | Intact Corneas | 3–8 h | 24–48 h | 72 h | 6–15 days |
| Ongoing activity (imp/s) | 0.08 ± 0.03 | 0 ± 0 | 0.23 ± 0.08* | 0 ± 0 | 0.15 ± 0.05* |
| Latency (s) | 13.90 ± 0.83 | 14.01 ± 1.56 | 11.56 ± 1.35 | 11.52 ± 2.4 | 11.08 ± 1.64 |
| Activity during the pulse (imp/s) | 1.84 ± 0.18 | 2.14 ± 0.42 | 3.22 ± 0.52* | 2.9 ± 0.86 | 2.39 ± 0.35 |
| Postdischarge (imp/s) | 1.96 ± 0.55 | 2.11 ± 0.46* | 2.39 ± 0.69* | 1.31 ± 0.54 | 1.12 ± 0.25 |
| n | 96 | 23 | 33 | 8 | 20 |
Data are mean ± SEM; n = number of explored fibers. Activity during the pulse was significantly increased in lesioned corneas (ANOVA on ranks, P = 0.002; *P < 0.05, post hoc test, compared with intact corneas).
Taken together, these data confirm that, after mechanical injury to the cornea, both mechanonociceptor and polymodal nociceptor sensory fibers were transiently sensitized.
Sodium Channel Blockers Attenuated the Enhanced Chemical Response of Injured Polymodal Nociceptors Without Affecting Their Mechanical Sensitivity
The effects of the sodium channel blockers lidocaine, carbamazepine, and amitriptyline on the responsiveness to mechanical and chemical stimuli of sensory nerve fibers of injured corneas were tested 24 to 48 hours after performing the surgical lesion, the time period at which maximal sensitization was observed. The magnitude of the stimulus-evoked responses was compared with those obtained in corneal mechanonociceptor and polymodal nociceptor fibers of intact corneas.
Mechanonociceptors
As shown in Table 4, the mean frequency values of spontaneous activity or of mechanical threshold to stimulation with von Frey hairs of corneal mechanonociceptors recorded in intact and lesioned corneas, were not significantly modified by any of the sodium channel blockers tested (lidocaine, carbamazepine, and amitriptyline).
Table 4.
Effect of Lidocaine, Carbamazepine and Amitriptyline on the Spontaneous Activity and Mechanical Threshold Measured in Mechanonociceptor Fibers From Intact and Lesioned Corneas, 24 to 48 Hours After Lesion With Microkeratome
| Intact Corneas | Injured Corneas | |||
|---|---|---|---|---|
| Mechanonociceptors | Before | After | Before | After |
| Lidocaine | ||||
| Spontaneous activity (imp/s) | 0 ± 0 | 0 ± 0 | 0.02 ± 0.02 | 0.01 ± 0.01 |
| Mechanical threshold (mN) | 0.29 | 0.29 | 0.34 ± 0.03 | 0.33 ± 0.03 |
| n | 2 | 18 | ||
| Carbamazepine | ||||
| Spontaneous activity (imp/s) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Mechanical threshold (mN) | 0.41 ± 0.13 | 0.41 ± 0.13 | 0.7 ± 0.23 | 0.7 ± 0.23 |
| n | 9 | 14 | ||
| Amitriptyline | ||||
| Spontaneous activity (imp/s) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Mechanical threshold (mN) | 0.28 ± 0.05 | 0.28 ± 0.05 | 0.3 ± 0.05 | 0.3 ± 0.05 |
| n | 5 | 3-5 | ||
Data are mean ± SEM of the values obtained before and after topical application of the corresponding drug during 10 minutes; n = number of explored fibers. No significant differences between the groups were found.
Polymodal Nociceptors
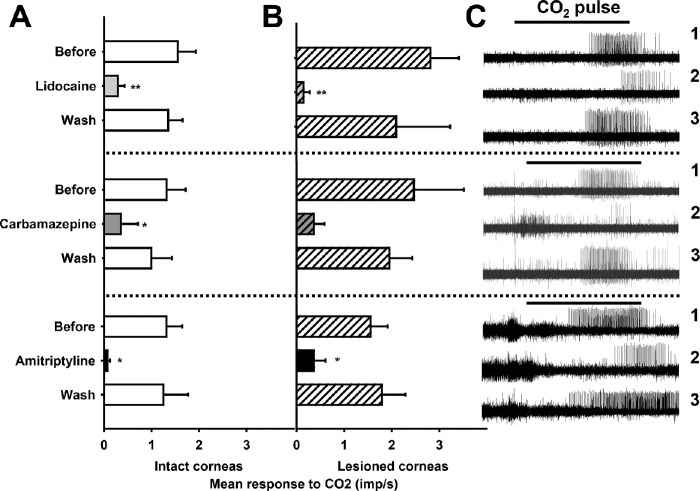
As in the case of mechanonociceptors, pretreatment with Na+ channel blockers did not significantly modify the mechanical threshold or the ongoing activity of corneal polymodal nociceptors (Table 5) innervating intact or operated corneas. In contrast, the sodium channel blockers lidocaine, carbamazepine, or amitriptyline significantly affected the response of polymodal nociceptor fibers to acidic stimulation with CO2 (Table 6, Fig. 3), both in control and lesioned corneas. Ten minutes after continuous perfusion with any of these Na+ channel blockers, a marked and reversible decrease of the CO2-evoked impulse discharge of polymodal nociceptors was observed (Table 6, Fig. 3), accompanied by a significantly longer duration of the latency of the response (Table 6). Both effects were reverted at 10 minutes after washing the drug by superfusion with saline alone.
Table 5.
Effect of Lidocaine, Carbamazepine, and Amitriptyline on the Spontaneous Activity and Mechanical Threshold Measured in Polymodal Nociceptor Fibers From Intact and Lesioned Corneas, 24 to 48 Hours After Lesion With Microkeratome
| Intact Corneas | Injured Corneas | |||
|---|---|---|---|---|
| Polymodal Nociceptors | Before | After | Before | After |
| Lidocaine | ||||
| Mechanical threshold (mN) | 0.39 ± 0.2 | 0.39 ± 0.2 | 0.57 ± 0.20 | 0.57 ± 0.20 |
| Spontaneous activity (imp/s) | 0.08 ± 0.05 | 0.09 ± 0.06 | 0.27 ± 0.25 | 0 ± 0 |
| n | 2 | 18 | ||
| Carbamazepine | ||||
| Mechanical threshold (mN) | 0.34 ± 0.21 | 0.43 ± 0.25 | 0.57 ± 0.20 | 0.57 ± 0.20 |
| Spontaneous activity (imp/s) | 0.16 ± 0.16 | 0.17 ± 017 | 0.02 ± 0.02 | 0.02 ± 0.02 |
| n | 9 | 14 | ||
| Amitriptyline | ||||
| Mechanical threshold (mN) | 0.42 ± 0.30 | 0.38 ± 0.31 | 0.54 ± 0.29 | 0.54 ± 0.29 |
| Spontaneous activity (imp/s) | 0.02 ± 0.02 | 0 ± 0 | 0.09 ± 0.05 | 0.09 ± 0.09 |
| n | 5 | 3–5 | ||
Data are mean ± SEM of the values obtained before and after topical application of the corresponding drug during 10 minutes; n = number of explored fibers. No significant differences between the groups were found.
Table 6.
Effects of Lidocaine, Carbamazepine, and Amitriptyline on the Characteristics of the Response to a 30-second Pulse of CO2 Applied to the Receptive Field of Corneal Polymodal Nociceptors Recorded in Intact and Lesioned Corneas (24, 48, or 72 Hours After Lesion With Microkeratome)
| Intact Corneas | Lesioned Corneas | |||||
|---|---|---|---|---|---|---|
| Before | After | Wash | Before | After | Wash | |
| Lidocaine | ||||||
| Ongoing activity (imp/s) | 0.08 ± 0.05 | 0.09 ± 0.06 | 0.10 ± 0.09 | 0.27 ± 0.25 | 0 ± 0 | 0.08 ± 0.06 |
| Latency (s) | 13.4 ± 2.49 | 35.06 ± 7.89* | 19.89 ± 3.01 | 13.03 ± 2.38 | 44.9 ± 10.28* | 16.6 ± 4.03 |
| Activity during the pulse (imp/s) | 1.56 ± 0.38 | 0.29 ± 0.14† | 1.35 ± 0.30 | 2.82 ± 0.60 | 0.16 ± 0.13† | 2.10 ± 1.14 |
| Postdischarge (imp/s) | 0.82 ± 0.19 | 0.14 ± 0.08† | 0.68 ± 0.27 | 1.25 ± 0.72 | 0.14 ± 0.13 | 0.77 ± 0.47 |
| Total response duration (s) | 26.6 ± 3.38 | 5.72 ± 3.36† | 30.59 ± 3.98 | 28.89 ± 5.72 | 2.21 ± 3.34* | 19.67 ± 5.28 |
| Mean discharge rate (imp/s) | 3.22 ± 0.49 | 0.28 ± 0.15† | 2.84 ± 0.31 | 3.84 ± 0.48 | 0.64 ± 0.40† | 3.69 ± 0.97 |
| n | 12 | 6 | ||||
| Carbamazepine | ||||||
| Ongoing activity (imp/s) | 0.16 ± 0.16 | 0.17 ± 017 | 0 ± 0 | 0.02 ± 0.02 | 0.02 ± 0.02 | 0.08 ± 0.08 |
| Latency (s) | 14.24 ± 3.96 | 51.43 ± 8.57† | 27.96 ± 8.33 | 16.7 ± 4.25 | 24.65 ± 9.43 | 15.40 ± 3.65 |
| Activity during the pulse (imp/s) | 1.32 ± 0.40 | 0.36 ± 0.36* | 1.00 ± 0.44 | 2.47 ± 1.05 | 0.46 ± 0.22 | 1.95 ± 0.48 |
| Postdischarge (imp/s) | 1.28 ± 0.42 | 0.24 ± 0.24† | 0.46 ± 0.17 | 1.25 ± 0.51 | 0.10 ± 0.06 | 1.49 ± 0.39 |
| Total response duration (s) | 20.35 ± 5.97 | 6.02 ± 6.02† | 38.4 ± 9.2 | 47.84 ± 19.10 | 4.84 ± 2.47 | 22.14 ± 3.43 |
| Mean discharge rate response (imp/s) | 2.6 ± 0.5 | 0.33 ± 0.33† | 2.55 ± 0.75 | 4.44 ± 0.87 | 1.88 ± 1.26 | 3.88 ± 0.89 |
| n | 7 | 6 | ||||
| Amitriptyline | ||||||
| Ongoing activity (imp/s) | 0.02 ± 0.02 | 0 ± 0 | 0.22 ± 0.15 | 0.09 ± 0.05 | 0.09 ± 0.09 | 0.09 ± 0.07 |
| Latency (s) | 13.80 ± 2.89 | 48.3 ± 7.40† | 28.03 ± 10.25 | 17.66 ± 3.29 | 39.65 ± 7.70 | 24.17 ± 6.24 |
| Activity during the pulse (imp/s) | 1.32 ± 0.33 | 0.07 ± 0.05* | 1.25 ± 0.52 | 1.56 ± 0.36 | 0.36 ± 0.25* | 1.79 ± 0.50 |
| Postdischarge (imp/s) | 0.51 ± 0.25 | 0.08 ± 0.07 | 0.52 ± 0.27 | 1.40 ± 0.39 | 0.17 ± 0.10* | 1.30 ± 0.44 |
| Total response duration (s) | 27.28 ± 6.59 | 4.52 ± 2.15* | 27.18 ± 2.87 | 23.92 ± 6.52 | 4.24 ± 2.10* | 17.92 ± 3.64 |
| Mean discharge rate response (imp/s) | 3.44 ± 1.31 | 0.46 ± 0.26* | 3.03 ± 0.53 | 3.50 ± 0.59 | 1.89 ± 0.75 | 3.90 ± 1.01 |
| n | 6 | 7 | ||||
Data are mean ± SEM of the values obtained before and after topical application of the corresponding drug during 10 minutes; n = number of tested fibers. * P < 0.05, †P < 0.01, paired t-test or Wilcoxon signed rank test, as appropriate.
Figure 3.
Activity during the pulse of CO2 of polymodal nociceptors from intact (A) and lesioned corneas (B) before, 1 minute after treating during 10 minutes with the corresponding Na+ blocker, and 10 minutes after washing the drug. Data are mean ± SEM, * P < 0.05, **P < 0.01, paired t-test, difference between before and after treatment. (C) Sample recordings of the electrical activity of three polymodal nociceptors in response to CO2 in lesioned corneas. Activity before (1), 1 minute after treatment with the Na+ channel blocker (2) and 10 minutes after washing the drug (3) is shown.
Use-Dependent Blockade of CO2 Evoked Response of Polymodal Nociceptor by Lidocaine, Carbamazepine, and Amitriptyline
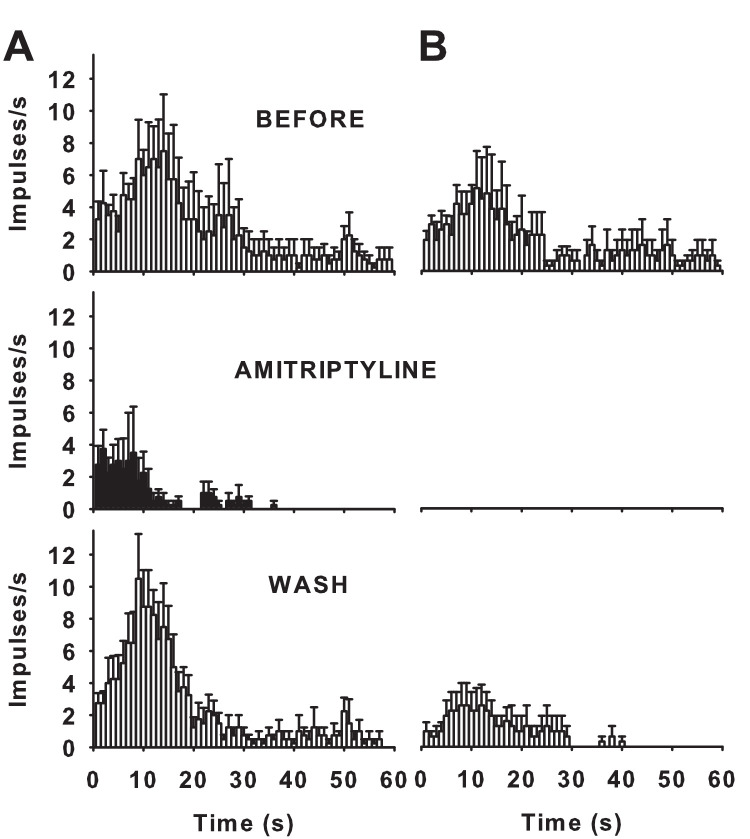
We next explored whether the blockade of polymodal nociceptor impulse activity by lidocaine, carbamazepine and amitriptyline was use dependent, that is, the block is enhanced by prolonged depolarization or repetitive depolarization.29 To address this question, the magnitude and time course of CO2-evoked response before and after treatment with each sodium channel blocker were compared. We observed that in two out of six units after lidocaine treatment, in three out of six units after carbamazepine treatment, and in four out of seven units after amitriptyline treatment, the response to CO2 at the beginning of the drug application was only partly blocked and fully inhibited afterward, consistent with a sodium channel blockade enhanced by frequent nerve terminal impulse discharge (Fig. 4A). Notably, the CO2-evoked response was fully inhibited in the remaining units in such a way that activity was not detected at any time point after treatment with any of the blockers (Fig. 4B), likely reflecting the occurrence of an additional mechanism of blockade. In this regard, resting or “tonic” blockade of sodium currents by local anesthetics and amitriptyline has been previously described.30–32 Moreover, the inhibition of the CO2-evoked response was reversible after washing out the three sodium channel blockers (Figs. 3–4). Overall, these results are consistent with a complex inhibition of voltage-gated sodium currents by the three compounds, which might imply tonic blockade in addition to phasic (use-dependent) blockade of polymodal nociceptor activity by which these sodium channel blockers preferentially inhibit Nav channels activity at open and inactivated states.
Figure 4.
Mean frequency curves evoked by CO2 pulses applied onto the receptive field of polymodal nociceptors of lesioned corneas before (top), 1 minute after treatment with amitriptyline during 10 minutes (middle graph), and 10 minutes after washing the corresponding the receptive field with saline solution (bottom). Data are mean ± SEM; n = 4 units with response after treatment (A), n = 3 units with no response after treatment (B).
Discussion
This study confirms that exposure of the corneal surface to a 98.5% CO2 gas jet locally forming carbonic acid and protons causing an immediate pH decrease, acts as a selective and reproducible chemical stimulation for corneal polymodal nociceptors, without affecting the corneal mechanonociceptor nerve terminals, which are exclusively recruited by mechanical stimulation.3 The production of a corneal flap with a microkeratome in guinea pigs, a surgical procedure routinely performed during LASIK photorefractive surgery in humans, produced a transient sensitization of mechanonociceptors and polymodal nociceptors in the corneal area surrounding the wound over 2 days. Lidocaine, amitriptyline, and carbamazepine, three drugs with well-known blocking effects on sodium channel currents,25,34 significantly decreased the response to CO2 in intact and lesioned corneas, without affecting the mechanical threshold.
In the present model of corneal surgical injury in the guinea pig, a corneal flap was obtained by means of a custom-made microkeratome that resembles closely the type of wound produced in humans in LASIK surgery. Currently, LASIK is the most common refractive surgery procedure worldwide.35,36 Although LASIK shows less postoperative pain in comparison with the first excimer laser technique introduced into refractive surgery, photorefractive keratectomy, early postoperative pain is still reported as a main symptom by LASIK patients.36–40 Consistent with the appearance of intense, acute postoperative pain in patients within the first days after corneal refractive surgery, we show that nociceptive corneal nerve terminal activity in guinea pigs is maximal at days 1 and 2 after surgery.
Corneal nerves were not seen within the flap at the time points at which nerve recordings were performed, strongly suggesting that only those corneal nociceptive nerve fibers with nerve terminals located outside the flap in the cornea and bulbar conjunctiva remain functional. The results shown in this work confirm that the chemical and mechanical responsiveness become potentiated (i.e., sensitized) as a consequence of corneal injury. Polymodal nociceptors’ receptive fields are quite large,2 so that a single afferent axon innervates a large extension of the corneal surface likely comprising areas inside and outside the flap, suggesting that at least a part of the branches of the majority of corneal nociceptor fibers were cut by the microkeratome wound. It is well established that release by damaged cells of the injured area of proinflammatory factors immediately after corneal injury contributes to sensitization of those corneal nociceptor terminals that remain intact.9,10 In addition, direct injury of peripheral axonal branches and nerve terminals of corneal nociceptor neurons lead to longer lasting, profound changes in the spontaneous and stimulus-evoked firing activity of the affected neurons.16–18
Nociceptive neurons express preferentially certain sodium channel isoforms that are critical for their particular neuronal excitability in health and disease. Their expression and functional properties of voltage-gated sodium channels are dynamically altered after axonal injury and inflammation. In this regard, several sodium channels have been proposed as potential targets for pain therapeutics.21,22 In particular, Nav 1.3 expression is augmented after sciatic nerve axotomy,20,41 whereas inflammatory modulators such as prostaglandin E2, adenosine, and 5-hydroxytryptamine increase tetrodotoxin-resistant sodium currents,42,43 and the intraplantar injection of carrageenan upregulates the expression of Nav 1.8 transcripts in rat nociceptive DRG neurons.44 Additionally, in transgenic mice lacking any of the three sodium channels that are expressed selectively in peripheral damage-sensing neurons from the trigeminal ganglia and dorsal root ganglia, namely, Nav 1.7, 1.8, and 1.9, thermal and mechanical hyperalgesia by the intraplantar injection of inflammatory agents is drastically decreased,45–47 suggesting a major role for these sodium channel isoforms in the nociceptor sensitization associated with peripheral inflammation at the injury site. Of note, lidocaine, carbamazepine, and amitriptyline have been reported to inhibit all the aforementioned Nav channel subtypes expressed by nociceptors.30,34,48–50 In addition, in corneal nociceptive nerve terminals, functional evidence suggests that tetrodotoxin-resistant sodium currents are the primary determinant of their excitability,51 which leads to the presumption that tetrodotoxin-resistant sodium channels are the main contributors to the onset of membrane hyperexcitability in corneal nociceptive terminals after injury, and in turn they are the main molecular targets of the sodium channel blockers assessed in this work.
The present experiments show that sodium channel blockers at the concentrations used in this work decrease the chemical responsiveness of polymodal nociceptors, but do not modify their mechanical threshold or the mechanical threshold of pure mechanonociceptor fibers. In the same way, previous works have reported that topical application in cat corneas of certain nonsteroidal anti-inflammatory drugs such as diclofenac, indomethacin, and flurbiprofen, as well as the calcium channel antagonist diltiazem, diminishes the response of corneal polymodal nociceptors to CO2 stimulation, although their mechanical threshold was not affected by any of these drugs.33,52,53
Lidocaine, carbamazepine, and amitriptyline are known to block voltage-gated Na channels by binding to specific amino acid residues that are located within the ion-conducting pore of the channel.48,54,55 Notably, the nature of this binding site suggests that it is highly unlikely that sodium channel blockers interact with this site in a Nav isoform-specific manner.30,45 Additionally, these drugs have been described to block preferentially at inactivated and open rather than resting channel states in a strong state-dependent manner. Consistently, it has been reported that the degree of inhibition of voltage-gated sodium channels by lidocaine, carbamazepine, and amitriptyline is modulated by the frequency of action potentials (use dependence), which might reflect a stronger binding of the drug to the inactivated and open states of the channel compared with the resting state.29,30,53,56 This pharmacologic feature is consistent with the faster buildup of inhibition evoked by sodium channel blockers when axons fire nerve impulses at higher frequencies, as occurs during CO2 stimulation, in contrast with the few impulses elicited with von Frey filaments to test mechanical threshold. Moreover, blocking in a use-dependent manner might imply a potent reduction of the injured nerve Na+ channel-mediated ectopic activity with no effects on nerve conduction.57
The systemic administration of analgesic adjuvants, compounds initially used for the treatment of medical conditions other than pain, are well-known to have analgesic properties in some instances when used as primary analgesics as well as adjuvants. Among them, the anticonvulsant carbamazepine, commonly used to control epileptic seizures, is useful in treating trigeminal neuralgia.58,59 The tricyclic antidepressant amitriptyline has pain-relieving properties that are independent of their effects on ameliorating depression.60,61 Similarly, several local anesthetics, including lidocaine, are used to treat neuropathic pain.62–64 However, the use of analgesic adjuvants systemically administered as primary analgesics is very limited owing to their potential neurologic and cardiac adverse effects.63,65–67 Therefore, local administration onto lesioned corneas evoking neuropathic pain might alleviate this condition more safely, allowing the use of drugs at higher doses and, thus, increasing drug efficacy. Indeed, topical ocular application of lidocaine has been shown to ameliorate ocular pain in patients with ophthalmic postherpetic neuralgia with the lack of systemic adverse effects.68 Nonetheless, it should be noted that local anesthetics are administered at much higher concentrations in clinical practice than in the present work, and at clinical concentrations may impair corneal epithelial wound healing in a dose-dependent manner.69 Further, the prolonged use of topical ophthalmic anesthetics, such as that associated with the abuse of these drugs, may lead to severe ocular complications.70
The findings of this work might be clinically relevant because the use of eyedrops containing low concentrations of lidocaine, carbamazepine, or amitriptyline would be suitable for a direct pharmacologic modulation of the enhanced corneal polymodal nociceptor activity in patients with neuropathic conditions, including postoperative ocular pain.
Acknowledgments
The authors are grateful for the contribution of Tansy Donovan-Rodriguez to the preliminary experiments performed to define the drug concentrations to be used in this study. They also kindly thank Manuel Bayonas for his expert technical assistance and Carlos M. Ramos for his help in the design of some figures of this manuscript.
Supported by the H2020 Program Grant Agreement No. 667400 from the European Commission (JG), and grants SAF-2017-83674-C2-1-R (JG) and -2R (MCA and CB) from the Agencia Estatal de Investigación and ERDF, Spain and EU. Support of the PROMETEO/2018/114 grant from the Generalitat Valenciana, Spain (JG), Fellowship BES-2015-072638 from AEI (SQ), and RTI-2018-100994-AI00 grant from AEI-ERDF (VM) is also acknowledged.
Disclosure: C. Luna, None; K. Mizerska, None; S. Quirce, None; C. Belmonte, None; J. Gallar, None; M.C. Acosta, None; V. Meseguer, None
References
- 1. Acosta MC, Tan ME, Belmonte C, Gallar J.. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001; 42: 2063. [PubMed] [Google Scholar]
- 2. Belmonte C, Carmen Acosta M, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004; 78: 513–525. [DOI] [PubMed] [Google Scholar]
- 3. Belmonte C, Gallar J, Pozo MA, Rebollo I.. Excitation by irritant chemical substances of sensory afferent units in the cat's cornea. J Physiol. 1991; 437: 709–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gallar J, Pozo MA, Tuckett RP, Belmonte C.. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat's cornea. J Physiol. 1993; 468: 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belmonte C, Giraldez F.. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol. 1981; 321: 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Acosta MC, Belmonte C, Gallar J.. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J Physiol. 2001; 534: 511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belmonte C, Acosta M, Merayo-Lloves J, Gallar J.. What causes eye pain? Curr Ophthalmol Rep. 2015; 3: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stapleton F, Tan ME, Papas EB, et al.. Corneal and conjunctival sensitivity to air stimuli. Br J Ophthalmol. 2004; 88: 1547–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J.. Nerves and sensations from the eye surface. Ocul Surf. 2004; 2: 248–253. [DOI] [PubMed] [Google Scholar]
- 10. Belmonte C. Pain, dryness, and itch sensations in eye surface disorders are defined by a balance between inflammation and sensory nerve injury. Cornea. 2019; 38(Suppl 1): S11–S24. [DOI] [PubMed] [Google Scholar]
- 11. Gallar J, Acosta MC, Gutierrez AR, Belmonte C.. Impulse activity in corneal sensory nerve fibers after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2007; 48: 4033. [DOI] [PubMed] [Google Scholar]
- 12. Stein R, Stein HA, Cheskes A, Symons S.. Photorefractive keratectomy and postoperative pain. Am J Ophthalmol. 1994; 117: 403–405. [DOI] [PubMed] [Google Scholar]
- 13. McCarty CA, Garrett SK, Aldred GF, Taylor HR.. Assessment of subjective pain following photorefractive keratectomy. Melbourne Excimer Laser Group. J Refract Surg. 1996; 12: 365–369. [DOI] [PubMed] [Google Scholar]
- 14. Hovanesian JA, Shah SS, Maloney RK.. Symptoms of dry eye and recurrent erosion syndrome after refractive surgery. J Cataract Refract Surg. 2001; 27: 577–584. [DOI] [PubMed] [Google Scholar]
- 15. Autrata R, Rehurek J.. Laser-assisted subepithelial keratectomy and photorefractive keratectomy for the correction of hyperopia: results of a 2-year follow-up. J Cataract Refract Surg. 2003; 29: 2105–2114. [DOI] [PubMed] [Google Scholar]
- 16. Kajander KC, Wakisaka S, Bennett GJ.. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992; 138: 225–228. [DOI] [PubMed] [Google Scholar]
- 17. Rivera L, Gallar J, Pozo MA, Belmonte C.. Responses of nerve fibres of the rat saphenous nerve neuroma to mechanical and chemical stimulation: an in vitro study. J Physiol. 2000; 527: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matzner O, Devor M.. Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol. 1994; 72: 349–359. [DOI] [PubMed] [Google Scholar]
- 19. Matzner O, Devor M.. Na + conductance and the threshold for repetitive neuronal firing. Brain Res. 1992; 597: 92–98. [DOI] [PubMed] [Google Scholar]
- 20. Black JA, Cummins TR, Plumpton C, et al.. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J Neurophysiol. 1999; 82: 2776–2785. [DOI] [PubMed] [Google Scholar]
- 21. Ma RSY, Kayani K, Oshodi DW, et al.. Voltage gated sodium channels as therapeutic targets for chronic pain. J Pain Res. 2019; 12: 2709–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tibbs GR, Posson DJ, Goldstein PA.. Voltage-gated ion channels in the PNS: novel therapies for neuropathic pain? Trends Pharmacol Sci. 2016; 37: 522–542. [DOI] [PubMed] [Google Scholar]
- 23. Nardi A, Damann N, Hertrampf T, Kless A.. Advances in targeting voltage-gated sodium channels with small molecules. ChemMedChem. 2012; 7: 1712–1740. [DOI] [PubMed] [Google Scholar]
- 24. Bagal SK, Marron BE, Owen RM, Storer RI, Swain NA.. Voltage gated sodium channels as drug discovery targets. Channels. 2015; 9: 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalso E. Sodium channel blockers in neuropathic pain. Curr Pharm Des. 2005; 11: 3005–3011. [DOI] [PubMed] [Google Scholar]
- 26. Acosta MC, Luna C, Quirce S, Belmonte C, Gallar J.. Changes in sensory activity of ocular surface sensory nerves during allergic keratoconjunctivitis. Pain. 2013; 154: 2353–2362. [DOI] [PubMed] [Google Scholar]
- 27. Acosta MC, Luna C, Quirce S, Belmonte C, Gallar J.. Corneal sensory nerve activity in an experimental model of UV keratitis. Invest Ophthalmol Vis Sci. 2014; 55: 3403–3412. [DOI] [PubMed] [Google Scholar]
- 28. Kovacs I, Luna C, Quirce S, et al.. Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease. Pain. 2016; 157: 399–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977; 69: 497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leffler A, Reiprich A, Mohapatra DP, Nau C.. Use-dependent block by lidocaine but not amitriptyline is more pronounced in tetrodotoxin (TTX)-Resistant Nav1.8 than in TTX-sensitive Na+ channels. J Pharmacol Exp Ther. 2007; 320: 354–364. [DOI] [PubMed] [Google Scholar]
- 31. Chernoff DM. Kinetic analysis of phasic inhibition of neuronal sodium currents by lidocaine and bupivacaine. Biophys J. 1990; 58: 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clarkson CW, Follmer CH, Ten Eick RE, Hondeghem LM, Yeh JZ. Evidence for two components of sodium channel block by lidocaine in isolated cardiac myocytes. Circ Res. 1988; 63: 869–878. [DOI] [PubMed] [Google Scholar]
- 33. Chen X, Gallar J, Belmonte C.. Reduction by antiinflammatory drugs of the response of corneal sensory nerve fibers to chemical irritation. Invest Ophthalmol Vis Sci. 1997; 38: 1944–1953. [PubMed] [Google Scholar]
- 34. Sheets PL, Heers C, Stoehr T, Cummins TR.. Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide], lidocaine, and carbamazepine. J Pharmacol Exp Ther. 2008; 326: 89–99. [DOI] [PubMed] [Google Scholar]
- 35. Sandoval HP, de Castro LE, Vroman DT, Solomon KD.. Refractive surgery survey 2004. J Cataract Refract Surg. 2005; 31: 221–233. [DOI] [PubMed] [Google Scholar]
- 36. Skevas C, Katz T, Wagenfeld L, Richard G, Linke S.. Subjective pain, visual recovery and visual quality after LASIK, EpiLASIK (flap off) and APRK - a consecutive, non-randomized study. Graefes Arch Clin Exp Ophthalmol. 2013; 251: 1175–1183. [DOI] [PubMed] [Google Scholar]
- 37. Torres LF, Sancho C, Tan B, Padilla K, Schanzlin DJ, Chayet AS.. Early postoperative pain following Epi-LASIK and photorefractive keratectomy: a prospective, comparative, bilateral study. J Refract Surg. 2007; 23: 126–132. [DOI] [PubMed] [Google Scholar]
- 38. O'Doherty M, Kirwan C, O'Keeffe M, O'Doherty J. Postoperative pain following epi-LASIK, LASEK, and PRK for myopia. J Refract Surg. 2007; 23: 133–138. [DOI] [PubMed] [Google Scholar]
- 39. Sharma N, Kaushal S, Jhanji V, Titiyal JS, Vajpayee RB.. Comparative evaluation of 'flap on' and 'flap off' techniques of Epi-LASIK in low-to-moderate myopia. Eye (Lond). 2009; 23: 1786–1789. [DOI] [PubMed] [Google Scholar]
- 40. Wang QM, Fu AC, Yu Y, et al.. Clinical investigation of off-flap epi-LASIK for moderate to high myopia. Invest Ophthalmol Vis Sci. 2008; 49: 2390–2394. [DOI] [PubMed] [Google Scholar]
- 41. Waxman SG, Kocsis JD, Black JA.. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol. 1994; 72: 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gold MS, Reichling DB, Shuster MJ, Levine JD.. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA. 1996; 93: 1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. England S, Bevan S, Docherty RJ.. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol. 1996; 495(Pt 2): 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG.. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport. 1998; 9: 967–972. [DOI] [PubMed] [Google Scholar]
- 45. Cummins TR, Sheets PL, Waxman SG.. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain. 2007; 131: 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baker MD, Wood JN.. Involvement of Na+ channels in pain pathways. Trends Pharmacol Sci. 2001; 22: 27–31. [DOI] [PubMed] [Google Scholar]
- 47. Habib AM, Wood JN, Cox JJ.. Sodium channels and pain. Handb Exp Pharmacol. 2015; 227: 39–56. [DOI] [PubMed] [Google Scholar]
- 48. Lin Z, Santos S, Padilla K, Printzenhoff D, Castle NA.. Biophysical and pharmacological characterization of Nav1.9 voltage dependent sodium channels stably expressed in HEK-293 cells. PLoS One. 2016; 11: e0161450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liang J, Liu X, Zheng J, Yu S.. Effect of amitriptyline on tetrodotoxin-resistant Nav1.9 currents in nociceptive trigeminal neurons. Mol Pain. 2013; 9: 31–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Horishita T, Yanagihara N, Ueno S, et al.. Antidepressants inhibit Nav1.3, Nav1.7, and Nav1.8 neuronal voltage-gated sodium channels more potently than Nav1.2 and Nav1.6 channels expressed in Xenopus oocytes. Naunyn Schmiedebergs Arch Pharmacol. 2017; 390: 1255–1270. [DOI] [PubMed] [Google Scholar]
- 51. Brock JA, McLachlan EM, Belmonte C.. Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea. J Physiol. 1998; 512(Pt 1): 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pozo MA, Gallego R, Gallar J, Belmonte C.. Blockade by calcium antagonists of chemical excitation and sensitization of polymodal nociceptors in the cat's cornea. J Physiol. 1992; 450: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Acosta MC, Berenguer-Ruiz L, Garcia-Galvez A, Perea-Tortosa D, Gallar J, Belmonte C.. Changes in mechanical, chemical, and thermal sensitivity of the cornea after topical application of nonsteroidal anti-inflammatory drugs. Invest Ophthalmol Vis Sci. 2005; 46: 282–286. [DOI] [PubMed] [Google Scholar]
- 54. Ragsdale DS, McPhee JC, Scheuer T, Catterall WA.. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994; 265: 1724–1728. [DOI] [PubMed] [Google Scholar]
- 55. Nau C, Wang GK.. Interactions of local anesthetics with voltage-gated Na+ channels. J Membrane Biol. 2004; 201: 1–8. [DOI] [PubMed] [Google Scholar]
- 56. Jo S, Bean BP.. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol. 2014; 85: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Devor M, Wall PD, Catalan N.. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain. 1992; 48: 261–268. [DOI] [PubMed] [Google Scholar]
- 58. BLOM S. Trigeminal neuralgia: its treatment with a new anticonvulsant drug (G-32883). Lancet. 1962; 1: 839–840. [DOI] [PubMed] [Google Scholar]
- 59. Taylor JC, Brauer S, Espir ML.. Long-term treatment of trigeminal neuralgia with carbamazepine. Postgrad Med J. 1981; 57: 16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Watson CP, Evans RJ, Reed K, Merskey H, Goldsmith L, Warsh J.. Amitriptyline versus placebo in postherpetic neuralgia. Neurology. 1982; 32: 671–673. [DOI] [PubMed] [Google Scholar]
- 61. Max MB, Culnane M, Schafer SC, et al.. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987; 37: 589–596. [DOI] [PubMed] [Google Scholar]
- 62. Boas RA, Covino BG, Shahnarian A.. Analgesic responses to i.v. lignocaine. Br J Anaesth. 1982; 54: 501–505. [DOI] [PubMed] [Google Scholar]
- 63. Backonja MM. Local anesthetics as adjuvant analgesics. J Pain Symptom Manage. 1994; 9: 491–499. [DOI] [PubMed] [Google Scholar]
- 64. Mao J, Chen LL.. Systemic lidocaine for neuropathic pain relief. Pain. 2000; 87: 7–17. [DOI] [PubMed] [Google Scholar]
- 65. Glazer S, Portenoy RK.. Systemic local anesthetics in pain control. J Pain Symptom Manage. 1991; 6: 30–39. [DOI] [PubMed] [Google Scholar]
- 66. Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R.. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992; 326: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 67. Knotkova H, Pappagallo M.. Adjuvant analgesics. Anesthesiol Clin. 2007; 25: 775–86, vi. [DOI] [PubMed] [Google Scholar]
- 68. Kanai A, Okamoto T, Suzuki K, Niki Y, Okamoto H.. Lidocaine eye drops attenuate pain associated with ophthalmic postherpetic neuralgia. Anesth Analg. 2010; 110: 1457–1460. [DOI] [PubMed] [Google Scholar]
- 69. Bisla K, Tanelian DL.. Concentration-dependent effects of lidocaine on corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1992; 33: 3029–3033. [PubMed] [Google Scholar]
- 70. Patel M, Fraunfelder FW.. Toxicity of topical ophthalmic anesthetics. Expert Opin Drug Metab Toxicol. 2013; 9: 983–988. [DOI] [PubMed] [Google Scholar]