Abstract
Background
Takotsubo Syndrome (TTS) is diagnosed in 1–2% of all patients presenting with acute coronary syndrome. Next to the typical apical manifestation, other locations of left ventricular contraction abnormality are possible, but their relationship to patient characteristics, clinical correlates as well as long-term outcome are poorly understood.
Methods & results
We retrospectively analyzed 126 patients presenting TTS. Cases were categorized according to left ventricular contraction abnormality patterns: typical apical pattern (71%, n = 89) vs. atypical patterns (29%, n = 37). Cases with typical TTS showed significantly higher levels of troponin I (3.12 ng/ml vs. 1.32 ng/ml, p = 0.013) and creatin kinase (CK) on admission (461 (±1207)U/l vs. 173 (±177) U/l, p = 0.03) as well as peak CK (973 (±2860)U/l vs. 301 (±328) U/l, p = 0.03) and more often ischemia related ECG changes (p = 0.02). Follow-up data was available for 85% of the patients. Median FU time was 4.4 years (IQR 1.4–7.7 years). All-cause mortality during follow-up was 39%, with no significant difference between patients with typical or atypical TTS (43% vs. 29%, p = 0.17). In multivariate logistic regression analysis, only anemia was predictive for long-term mortality (OR 3.93, 95%CI 1.02–2.08, p = 0.015). The majority of surviving patients (69%) reported good quality of life, even though only 56% reported being symptom-free.
Conclusion
Patients with TTS have poor long-term prognosis with an overall mortality of 39.1% within 4 years and nearly half of all patients report persisting symptoms. Even though the apical contraction pattern is associated with higher elevation of serum markers for myocardial damage, it was not associated with higher long-term mortality.
Keywords: Takotsubo Syndrome, Takotsubo cardiomyopathy, Broken heart syndrome, Stress cardiomyopathy, Contraction pattern
1. Background
Takotsubo Syndrome (TTS) is defined by the presence of a left ventricular wall motion abnormality which extends beyond a single epicardial coronary artery perfusion territory and is transient in most cases, the presence of new electrocardiographic abnormalities or elevation in cardiac troponin levels, and the absence of pheochromocytoma or myocarditis [1]. Thus, TTS is a potential diagnosis of patients presenting with acute myocardial infarction (AMI) or acute heart failure [2], [3]. Contrary to patients with non-obstructive coronary artery disease, TTS can either cause AMI or can be caused by AMI [2], [3]. TTS is diagnosed in 1–2% of all patients presenting with AMI [4], [5].
The short-term prognosis of TTS is comparable to other patients presenting with acute coronary syndrome (ACS); the reported intra-hospital death rate is 4% [5]. Available data on long-term prognosis after TTS are inhomogeneous. While the largest registry reports a relatively high mortality rate of 5.6% per patient-year of follow-up, data in a smaller population showed a very good long-term prognosis regarding survival (94% over 3.6 years) and recovery of systolic left ventricular (LV)-function in all surviving patients to a LV-EF > 50% [5], [6]. The differences in these results might be explained by differences in triggers in the populations. Lately data of a cohort of 1613 patients with TTS showed a worse long-term outcome for patients with physical trigger [7].
While most patients show the typical apical contraction abnormality pattern, atypical contraction patterns (midventricular, basal, or focal contraction abnormalities) can also be present [5]. Apical Ballooning is associated with a lower left ventricular ejection fraction on admission and with a higher prevalence of cardiogenic shock. Lately, patients with a typical contraction abnormality pattern are known to present more frequently with cardiogenic shock [8], [9], [10]. Until now, data on the influence of contraction pattern on mortality is inhomogeneous. While the largest study failed to reveal significant differences, a smaller study reports a significantly higher 6-months mortality for patients presenting with the apical ballooning pattern [8], [9].
In our study, we, analyze the relationship between contraction pattern and clinical characteristics, cardiac enzymes, long-term outcome with a focus on mortality, rehospitalisation, repeated coronary angiograms and persisting symptoms in a single-center all-comer group.
2. Methods
We retrospectively analyzed 126 consecutive patients presenting with their first episode of within the years 2008–2019 in our department. Patients were included in the registry on the basis of the Mayo Clinic diagnostic criteria for TTS as follows: [1]
-
1.
Transient hypokinesia, akinesia, or dyskinesia of the left ventricular mid segments with or without apical involvement; the regional wall motion abnormalities extend beyond a single epicardial vascular distribution.
-
2.
Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture.
-
3.
New electrocardiographic abnormalities (either ST-segment elevation and/or T-wave inversion) or elevation in cardiac troponin.
-
4.
No sign or evidence for pheochromocytoma or myocarditis
2.1. Types of TTS
Cases were categorized according to typical and atypical contraction abnormalities either in the LV angiogram or in echocardiography. A “typical” pattern was defined in the presence of an apical contraction abnormality, whereas TTS was classified as “atypical” in patients with midventricular, basal and focal contraction abnormalities. The apical type was characterized by hypo-, a- or dyskinesia of apical and midventricular segments of the anterior, septal, inferior and lateral wall of the left ventricle, associated with hyperkinesia of basal segments. The “midventricular” type was classified in the presence of a cuff-like hypo-, a- or dyskinesia of midventricular segments, with normo- or hyperkinesia of basal and apical segments. The patients were classified as the “basal” type in the presence of a hypo-, a- or dyskinesia of basal segments and normo- or hyperkinesia of midventricular and apical segments. Whereas all these patterns imply left ventricular wall motion abnormalities beyond a single epicardial coronary artery perfusion territory, the “focal” type is diagnosed with the presence of only a focal hypo-, a- or dyskinesia of any segment of the left ventricle the laboratory, electrocardiographic and clinical criteria.
2.2. Laboratory and hemodynamic measurements
Troponin was measured with the Beckman Coulter troponin I assay (99th percentile = 0.04 ng/ml for the European population age 40–99 years).
Creatin-kinase (CK) was measured with the Beckman Coulter assay for the quantitative determination of creatine kinase with a cut-off of <=170U/l. Anemia was defined by hemoglobin values < 12 g/dl for women and <14 g/dl for men according to the WHO definition.
In case of a LV angiogram the pressure curve of the LV was documented and the LV end-diastolic pressure (LVEDP) as a correlate for LV volume load was measured.
2.3. Follow-up
Patients were systemically followed up by written or telephone interview as well as review of medical records. Written informed consent was provided by all patients with follow-up data. Follow-up data comprised death, rehospitalisation due to heart failure or ACS, coronary angiography as well as quality of life, symptoms and echocardiographic data if available. In case of a reported relapse, the primary episode was chosen for the start of the follow-up period. The study conformed to the principles outlined in the declaration of Helsinki. The study was approved by the ethics committee of the Friedrich Alexander University of Erlangen-Nürnberg.
2.4. Statistical analysis
Chi-square and t-test were used to compare categorical and continuous variables. We used Kaplan-Meier method to provide survival estimates, which were assessed with a log-rank test. Binary logistic regression analysis was performed for association with mortality for all variables with a significance of <0.05 in the univariate analysis.
3. Results
We identified 126 patients (86% female, mean age 68 ± 11 years) at our hospital between 2008 and 2019. In only 14% (n = 17) an emotional trigger could be determined, whereas 50% (n = 63) showed a physical trigger such as stroke, thrombosis or infection. If a trigger was present, male patients more frequently reported a physical trigger (male 67% vs. female 47%); no male showed an emotional trigger (vs. 16% in female, p = 0.04). 72% of all patients showed ECG abnormalities including a complete left or right bundle branch block of unknown onset, 65% showed ischemia related changes. 79% had elevated troponin I and 41% elevated CK levels on admission. During the course of hospitalization, elevated troponin and CK levels were observed in 89% and 64% of all patients. All patients underwent coronary angiography, revealing significant coronary artery disease (coronary artery stenosis > 50%) in 16%. Intrahospital death occurred in 9% (n = 11). 36% (n = 4) patients died from cardiovascular causes (cardiogenic shock caused by heart failure (n = 2), aortic stenosis (n = 1), resuscitation due to heart failure (n = 1). The other 64% (died due to septic shock (n = 2), end-stage cancer (n = 2) or cerebral causes (n = 3).
3.1. Typical vs. Atypical contraction patterns
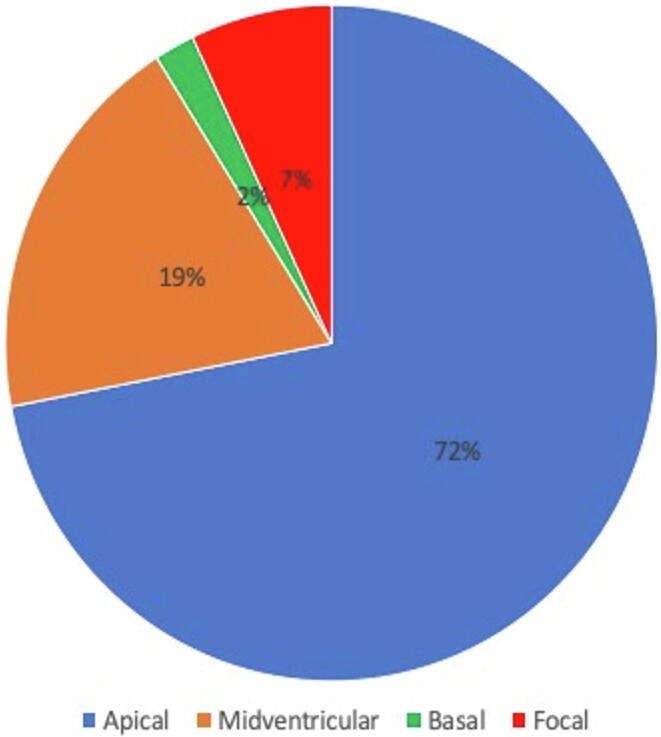
71% of the cases (n = 89) presented with a typical apical contraction abnormality. Atypical patterns were present in 29% (n = 37): a midventricular type was present in 25 (20%), while the basal type was present in 2 (2%) and a focal type in 10 (8%) patients (Fig. 1). Among all cases, 19% (n = 24) presented the ECG pattern of an acute ST-elevation myocardial infarction, 68% (n = 85) with NSTE-ACS, 14% (n = 18) in cardiogenic shock including cardiopulmonary resuscitation (n = 8;6%) and heart failure NYHA IV (n = 10; 8%).
Fig. 1.
Prevalence of the different contraction abnormality patterns of TTS.
Baseline, clinical and laboratory characteristics of the cases with a typical and atypical contraction abnormality pattern are shown in Table 1. Cases with typical vs. atypical location of the contraction abnormality showed no differences in cardiovascular risk factors or clinical parameters such as sex, age, global systolic left ventricular function on admission and prior to discharge (typical 43% (+−11) vs. atypical 46%(+−12); p = 0.29). or triggering factors for TTS. Coronary artery disease was infrequent in both groups (57% vs.49%; p = 0.35). Cases with typical TTS showed significantly a higher rate of ischemia related ECG changes (67% vs. 51%; p = 0.04). Patients with a typical contraction pattern had a trend to present more often as STEMI and showed higher levels of troponin I (3.12 ng/ml vs. 1.32 ng/ml, p = 0.013), CK on admission (461 (±1207)U/l vs. 173 (±177) U/l, p = 0.03) as well as peak CK (973 (±2860)U/l vs. 01 (±328) U/l, p = 0.03) and a intrahospital death did not differ between the two groups (Table 1).
Table 1.
Typical and atypical TTS.
| Typical | Atypical | P | |
|---|---|---|---|
| N = 89 | N = 37 | ||
| Female | 85% (76) | 86% (32) | 0.87 |
| Age (years) | 68 (11) | 65 (12.0) | 0.19 |
| BMI (kg/m2) | 24.7 (4.1) | 24.7 (4.9) | 0.94 |
| Hypertension | 66% (59) | 54% (20) | 0.15 |
| Diabetes | 16% (14) | 5% (2) | 0.10 |
| Prior PCI | 6% (5) | 0% (0) | 0.14 |
| Prior CABG | 1% (1) | 0% (0) | 0.52 |
| Clinical presentation | |||
| Identifyable trigger | 63% (56) | 65% (24) | 0.95 |
|
13% (12) | 14% (5) | 0.98 |
|
49% (44) | 51% (19) | |
| Heart Rate* (bpm) | 83 (21) | 84 (17) | 0.70 |
| Heart Rate > 90 bpm | 36% (32) | 35% (13) | 0.98 |
| RR systolic < 90 mmHg | 3% (3) | 11% (4) | 0.12 |
| Echocardiography | |||
| EF on admission * (%) | 33 (11) | 36(12) | 0.25 |
| EF prior discharge* (%) | 43 (12) | 46 (11) | 0.29 |
| EF discharge – EF on admission (%) | 10 (2) | 13 (14) | 0.27 |
| Improving LV-EF prior discharge (yes) | 65% | 68% | 0.81 |
| ECG: Ischemia related changes | 67% (63) | 51% (19) | 0.04 |
|
20% (18) | 16% (6) | 0.006 |
|
2% (2) | 8% (3) | |
|
48% (43) | 27% (10) | |
| Clinical presentation | 0.05 | ||
|
20% (18) | 16% (6) | |
|
52% (46) | 65% (24) | |
|
17% (15) | 0% (0) | |
|
6% (5) | 14% (5) | |
|
7% (6) | 5% (2) | |
| Coronary angiography | |||
| LVEDP* (mmHg) | 21 (8) | 19 (9) | 0.48 |
| LVEDP > 15 mmHg | 81% | 60% | 0.06 |
| Coronary Arteries | 0.34 | ||
|
43% (38) | 51% (19) | |
|
38% (34) | 41% (15) | |
|
19% (17) | 8% (3) | |
| EF angio* (%) | 36 (+/−12) | 35 (+−13) | 0.92 |
| Laboratories | |||
| Troponin I (ng/ml) admission* | 3.12 (5.62) | 1.32 (2.14) | 0.013 |
| Troponin I (ng/ml) maximum * | 8.35 (16.40) | 5.19 (17.13) | 0.35 |
| CK (U/l) on admission* | 462 (1208) | 173 (177) | 0.03 |
| CK (U/l) maximum* | 974 (2860) | 301 (328) | 0.03 |
| HB (g/dl)* | 12.8 (1.9) | 13.3 (1.8) | 0.20 |
| Leukocytes (103/µl)* | 11.0 (5.3) | 10.7 (6.7) | 0.76 |
| Intrashospital death | 8% (7) | 11% (4) | 0.59 |
*Mean (standard deviation); BMI, body mass index; HR, heart rate; RR, blood pressure; EF, ejection fraction; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; AP, angina pectoris; TTS, tako-tsubo-syndrome.
3.2. Follow-up
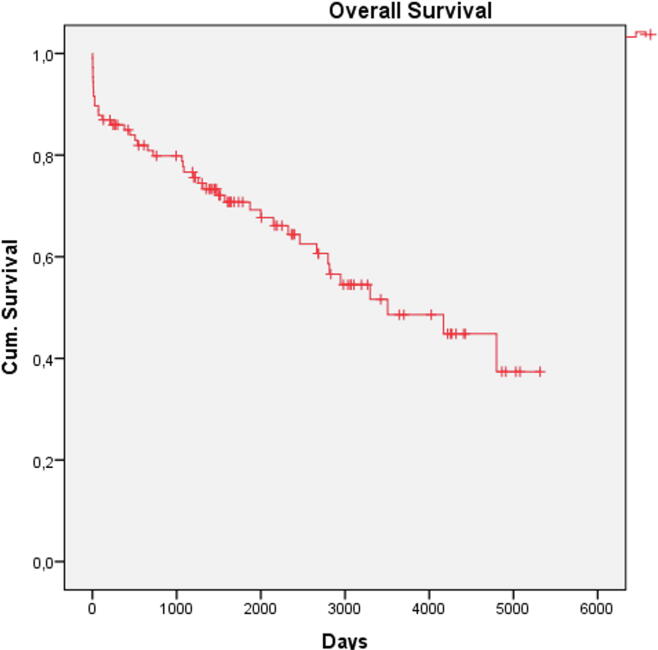
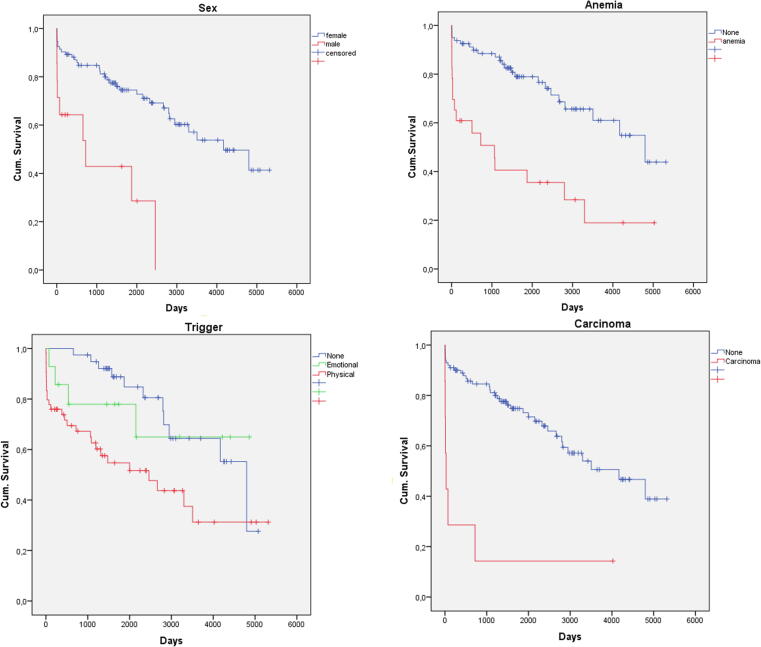
Follow-up data was available for 84% (107/127). Median FU time was 4.4 years (IQR 1.4–7.7 years). Death occurred in 39% (42/107), cardiovascular death in 22% (23/107). Death occurred in 10% (n = 11) intra-hospital, in 10% (n = 11) within 30 days and in 14% within 1 year after primary admission (Fig. 2). All patients who presented after cardiopulmonary resuscitation (CPR) died during follow-up, leading to a significant difference of survival probability depending on the clinical presentation (p = 0.004). Patients who died during follow-up had a trend to higher cardiac enzymes during primary hospitalization (Table 2). Re-TTS occurred in 10% (typical 8%, atypical 13%, p = 0.51) and was not associated with higher mortality (p = 0.40). In univariate analysis, male sex (OR 3.27 (95CI (1.01–11.58) Fig. 3A), patients after CPR (OR 1.24 (95%CI 1.07–1.43), patients with anemia (OR 5.67 (95% CI 2.06–15.58), Fig. 3B), with physical trigger (OR 2.53 (95% CI1.14–5.64); p = 0.018; Table 2, Fig. 3C) and with carcinoma (OR 10.67 (95%CI 1.24–91.25), Fig. 3D) were more likely to die during follow-up. In the logistic multivariate regression analysis including these parameters, only anemia (OR 3.93 (95%CI 1.02–2.08), p = 0.015) showed independent significant influence on long-term mortality.
Fig. 2.
Kaplan-Meier-Analysis: Overall survival of the cohort.
Table 2.
Parameters of patients who were still alive vs. patients, who died during follow-up.
| Alive | Died | P | |
|---|---|---|---|
| N = 65 | N = 42 | ||
| Age* (years) | 68 (11) | 70 (10) | 0.35 |
| BMI* (kg/m2) | 24.5 (4.2) | 25.4 (4.0) | 0.31 |
| Female | 92% (60) | 78.5% (33) | 0.04 |
| Male | 8% (5) | 21% (9) | |
| HR > 90 bpm | 31% (20) | 41% (17) | 0.36 |
| RR systolic < 90 mmHg | 3% (2) | 12% (5) | 0.10 |
| HR > 90 bpm & RR systolic < 90 mmHg | 2% (1) | 7% (3) | 0.17 |
| Laboratory Measurements | |||
| Troponin I (ng/ml) admission* | 2.18 (3.78) | 2.39 (5.16) | 0.81 |
| Troponin I (ng/ml) maximum* | 6.19 (12.90) | 9.94 (23.35) | 0.30 |
| Creatinkinase (U/l) on admission * | 206 (187) | 452(833) | 0.025 |
| Creatin (U/l) maximum * | 421 (745) | 930 (1816) | 0.049 |
| Hemoglobin (g/dl) * | 13.5 (1.4) | 12.4 (2.1) | 0.04 |
|
11% (7) | 38% (16) | <0.001 |
| Leukocytes | 10.3 (5.3) | 12.3 (6.4) | 0.09 |
| Echocardiography | |||
| EF on admission* (%) | 36 (10) | 31 (12) | 0.45 |
| EF prior discharge* (%) | 46 (11) | 41 (9) | 0.06 |
| Laevocardiography | |||
| EF angio (%) | 36 (12) | 34 (13) | 0.21 |
| Typical contraction pattern | 66 (43) | 79 (33) | 0.17 |
| Indication for angiography | 0.004 | ||
|
19% (12) | 21% (9) | |
|
62% (40) | 38% (16) | |
|
11% (7) | 14% (6) | |
|
9% (6) | 7% (3) | |
|
0% (0) | 18% (8) | |
| Identifyable trigger | 57% (37) | 74% (31) | 0.08 |
|
15% (10) | 10% (4) | 0.07 |
|
42% (27) | 64% (27) | |
| Carcinoma | 2% (1) | 14% (6) | 0.009 |
| Re-TTS | 11% (7) | 7% (3) | 0.53 |
*Mean (standard deviation); BMI, body mass index; HR, heart rate; RR, blood pressure; EF, ejection fraction; STEMI, st-segment elevation myocardial infarction; NSTEMI, non-st-segment elevation myocardial infarction; AP, angina pectoris; TTS, tako-tsubo-syndrome.
Fig. 3.
Kaplan-Meier Survival Analysis: Sex (A), Anemia (B), Trigger (C), Carcinoma (D).
71% (76/107) of the patients originally presented with typical and 29% (31/107) with atypical contraction patterns. Neither rehospitalisation (typical 13% vs atypical 7%, p = 0.66) nor death (typical 43% vs. atypical 29%, p = 0.17) re-TTS (typical 8% vs. typical 13% p = 0.39) or cardiovascular death (typical 26% vs. atypical 10%, p = 0.05) differed significantly between patients with typical and atypical TTS. Combining the endpoints CV death and rehospitalisation patients with typical TTS showed a significantly higher risk (typical 39% vs. atypical 17%, p = 0.02; see Table 3).
Table 3.
Long-term outcome of patients with typical and atypical contraction abnormality pattern.
| Typical | Atypical | P | |
|---|---|---|---|
| N = 76 | N = 31 | ||
| Death | 43% (33) | 29% (9) | 0.17 |
| CV Death | 26% (20) | 10% (3) | 0.05 |
| Rehospitalisation (alive) | 13% (10) | 7% (2) | 0.66 |
| CV Death & Rehospitalisation | 39% (30) | 17% (5) | 0.02 |
| Re-TTS | 8% (6) | 13% (4) | 0.39 |
CV, cardiovascular, TTS, takotsubo-syndrome.
The questionnaire on quality of life was completed by 91% of the patients alive at the time of follow-up (59/65; 39 typical, 20 atypical). 44% of the patients still claimed to suffer from dyspnea or angina. 39% reported dyspnea NYHAI, 22% NYHA II and 15% NYHA III. 1 patient claimed to have dyspnea caused by emotional stress. 73% were free of angina, only 1 patient claimed to experience angina during emotional stress. No differences could be seen in any of the symptoms regarding the different contraction patterns. Quality of life was stated to be “good” in 69% (n = 41), “ok” in 27% (n = 16) and “bad” in 3% (n = 2).
14% reported at least one further episode or symptoms of AMI. 20% underwent coronary angiography during the follow-up period, while in only one patient a percutaneous coronary intervention (PCI) was performed. A recurrent episode of TTS was diagnosed in 9% (n = 10) with a median time of 4.5 years (IQR 2.9–5.5) from the first episode. In case of recurrence 80% (n = 8) showed a typical contraction pattern, 20% (n = 2) an atypical contraction pattern. One patient showed typical contraction pattern in the primary and an atypical in the recurrent episode, whereas 1 patient showed atypical contraction pattern in the primary and typical contraction pattern during relapse. Four patients showed an emotional trigger for the repeat episode, 3 patients a physical and in 3 no trigger could be identified. Compared to the primary episode, the kind of trigger changed in 4 cases.
4. Discussion
Our analysis of an all-comer cohort of patients presenting with TTS shows:
-
(1)
Patients with TTS show a high in-hospital and long-term mortality. Anemia was the only independent predictor of long-term mortality.
-
(2)
The majority of patients with TTS present with the typical contraction pattern. Patients with a typical contraction abnormality showed higher levels of cardiac enzymes, but without significant higher intra-hospital or long-term mortality.
-
(3)
Symptoms persist in nearly half of the patients independent on the initial contraction abnormality pattern. Another episode of TTS occurred in nearly every 10th patient within the follow-up period of 4.4 years.
TTS is a severe condition with a high mortality – not only in the acute phase, but also in the long-term. The mortality rate of our cohort even exceeds previously published data [5], [11], [7]. In spite the commonly used term “broken heart syndrome”, a minority of patients in our as well as in other registries with TTS shows an emotional trigger [5]. To the contrary, in our cohort the majority of patients presented with a physical trigger. Physical triggers contain critical illnesses such as neurological disorders, bone fractures or sepsis and are known to be unfavorable for the patients‘ outcome [7], [12]. In the latest data on patients with TTS with a focus on the trigger, a physical trigger and especially carcinoma were associated with worse long-term outcome [7]. Thus, the high percentage of physical triggers of various prognosis (e.g. sepsis, malignoma) might partly explain the high mortality in our cohort. Other reasons for the very high mortality rate in our cohort could be the higher percentage of male patients as well as the relatively low LV-EF on admission. Both are known to be of unfavorable outcome [5], [8], [11], [7]. However, none of these was an independent predictor for death. Instead, the only independent predictor for mortality in our cohort was anemia. Anemia is associated with frequent and severe comorbidities including cancer and therefore higher risk of mortality within the population [13]. Thus, anemia was also shown to be unfavorable for patients presenting with ACS [14]. Our data - as previous studies- again shows analogies in the outcome of patients with TTS and with ACS [5], [7], [15].
TTS is widely associated with the typical wall motion abnormality of apical ballooning- present in ¾ of our cohort. Patients with typical contraction pattern show more frequent ischemia related changes in the ECG. ST segment elevation infarction was previously shown to be more frequent in patients with typical TTS [9]. In our cohort, patients with typical contraction pattern show higher levels of troponin on admission, higher CK on admission and higher peak CK than patients with atypical patterns. This finding is different from previous studies, reporting poorer LV-function, higher BNP levels, but no differences in ischemic enzymes [9]. Whereas previous data revealed discrepant data on mortality in dependence of the contraction pattern [8], [9], our data showed no difference in in-hospital and long-term outcome.
In our cohort the majority reports persisting symptoms. Interestingly, patients with different contraction pattern did not differ in the extent of chronic symptoms. TTS was lately revealed to be more a chronic than an acute disease [16], [17], [18]. In animal models, histological abnormalities persist despite echocardiographic normalization of the LVEF [19]. In fact, even though wall motion abnormalities may resolve, inflammation and myocardial dysfunction seem to be persisting in patients with TTS [17], [18]. The high rate of persisting symptoms in our cohort supports the notion of a chronic disease and is in agreement with data from a smaller cohort [20]. It once again raises the question if microvascular dysfunction might persist after the acute phase and thus, permanent medication could be off use for these patients. Until now data on that showed, that microvascular dysfunction resolves after the acute event [21]. In case of clinical recurrence the pattern of wall motion abnormalities differs in a considerable percentage of the patients [22]. In our cohort, this percentage was 20%. This variability shows, that not only a certain region of the myocardium is vulnerable in patients with TTS. Rather, the tendency for TTS is present within the whole myocardium appearing in acute regional phases. This strengthens the theory of TTS as a chronic condition and also matches to our main finding, that the contraction pattern does not influence long-term prognosis regarding mortality.
The poor long-term prognosis and the categorization as a chronic disease as well as the high percentage of patients with persisting symtpoms show the need for a closer follow up and a specified treatment of the patients. In the past, initial treatment following the diagnosis of TTS was adopted from the treatment for acute heart failure or AMI. However, data on the efficacy of such treatment, including beta-blockers and ACE inhibitors, are scarce – both regarding recovery of LV function and avoiding the recurrence of TTS [5], [16].
4.1. Limitations of the study
Despite the recommendation by the guidelines, we cannot provide MRI results of each patients due to low capacity at our center. Additionally, the study – as any study providing details on outcome of patients with TTS – cannot exclude the influence physical triggers on the outcome of the patients. This trigger – partly severe co-morbidities - might be a high bias. Still, multivariate analysis could not reveal a single morbidity despite anemia with direct influence on mortality.
5. Conclusion
Patients with TTS have poor long-term prognosis. Contraction pattern at presentation had no influence on overall long-term morality despite higher cardiac enzymes. Overall, mortality in patients presenting with TTS approaches 40% within 4 years, nearly half of the surviving patients show persisting symptoms, and recurrence rate is approximately 10%. Thus, TTS needs to be seen as a serious – maybe even- chronic condition andsfuture studies should focus on the potential need for specific long-term therapy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008;155:408–416. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Tamis-Holland J., Jneid H., Reynolds H., Agewall S., Brilakis E., Brown T., Lerman A., Cushman M., Kumbhani D., Aslanian-Engoren C., Bolger A., Beltrame J. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease. Circulation. 2019;139:e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 3.Collet J.-P., Thiele H., Barbato E., Barthelemy O., Bauersachs J., Bhatt D., Dendale P., Dorobantu M., Edvardsen T., Folliguet T., Gale C., Gilard M., Jobs A., Jüni P., Lambrinou E., Lewis B., Mehilli J., Meliga E., Markely B., Mueller C., Roffi M., Rutten F., Sibbing D., Siontis G. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST elevation The Task Force for the management of acute coronary syndromes -segment elevation of. Eur. Heart J. epub ahead. 2020;2020:1–79. doi: 10.1093/eurheartj/ehaa575. [DOI] [Google Scholar]
- 4.Ibanez B., James S., Agewall S., Antunes M., Bucciarelli-Ducci C., Bueno H., Caforio A., Crea F., Goudevenos J., Halvorsen S., Hindricks G., Kastrati A., Lenzen M., Prescott E., Roffi M., Valgimigli M., Varenhorst C., Vranckx P., Widimsky P. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST -segment elevation The Task Force for the management of acute myocardial infarction. Eur. Heart J. 2017;39(2018):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 5.C. Templin, J. Ghadri, J. Diekmann, L. Napp, D. Bataiosu, M. Jaguszewski, V. Cammann, A. Sarcon, V. Geyer, N. CA, B. Seifert, J. Hellermann, M. Schwyzer, K. Eisenhardt, J. Jenewein, J. Franke, H.A. Katus, C. Burgdorf, A. Cuneo, K. Kuck, C. Jacobshagen, G. Hasenfuss, M. Karakas, W. Koenig, G. Opolski, R. Dworakowski, P. Maccarthy, C. Kaiser, S. Osswald, L. Galiuto, P. Erne, J.J. Bax, I. Ford, F. Ruschitzka, A. Prasad, T.F. Lüscher, Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy, N. Engl. J. Med. (2015) 929–938. doi:10.1056/NEJMoa1406761. [DOI] [PubMed]
- 6.Gomez-Limon J.M., Isaza-Arana S., Robledo-Carmona J., Alania-Torres E., Torres-Llergo J., Valle-Racero J.I., Lopez-Pardo F., Martinez-Martinez A., Jimenez-Navarro M., Urbano-M J.A. Oral, clinical and echocardiographic course in tako tsubo cardiomyopathy : Long term follow up from a multicenter study. Int. J. Cardiol. 2017;228:97–102. doi: 10.1016/j.ijcard.2016.11.256. [DOI] [PubMed] [Google Scholar]
- 7.Ghadri J., Kato K., Cammann V., Gili S., Jurisic S., Di Vece D., Candreva A., Ding K.J., Micek J., Szawan K.A., Bacchi B., Bianchi R., Levinson R.A., Wischnewsky M., Seifert B., Schlossbauer S.A., Citro R., Bossone E., Münzel T., Knorr M., Heiner S., Ascenzo F.D., Franke J., Galiuto L., Crea F., Dichtl W., Empen K., Felix S.B., Delmas C., Lairez O., El-battrawy I., Akin I., Borggrefe M., Horowitz J., Kozel M., Tousek P., Widimský P., Gilyarova E., Shilova A., Gilyarov M., Winchester D.E., Ukena C., Bax J.J. Long-term prognosis of patients with takotsubo syndrome. JACC. 2018;72:874–882. doi: 10.1016/j.jacc.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Stiermaier T., Möller C., Graf T., Eitel C. Prognostic usefulness of the ballooning pattern in patients with takotsubo cardiomyopathy. Am. J. Cardiol. 2016;118:1737–1741. doi: 10.1016/j.amjcard.2016.08.055. [DOI] [PubMed] [Google Scholar]
- 9.Ghadri J., Cammann V., Napp C., Jurisic S., Diekmann J., Bataiosu D.R., Seifert B., Jaguszewski M., Sarcon A., Lüscher T.F., Templin C., Takotsubo I., Registry I. Differences in the clinical profile and outcomes of typical and atypical takotsubo syndrome data from the international takotsubo registry. JAMA Cardiol. 2016;1:335–340. doi: 10.1001/jamacardio.2016.0225. [DOI] [PubMed] [Google Scholar]
- 10.Di Vece D., Citro R., Al E. Outcomes Associated With Cardiogenic Shock in Takotsubo Syndrome. Circulation. 2019;139:413–415. doi: 10.1161/CIRCULATIONAHA.118.036164. [DOI] [PubMed] [Google Scholar]
- 11.Stiermaier T., Moeller C., Oehler K., Desch S., Graf T., Eitel C., Vonthein R., Schuler G., Thiele H., Eitel I. Long-term excess mortality in takotsubo cardiomyopathy : predictors, causes and clinical consequences. Eur. Hear. J. Hear. Fail. 2015:650–656. doi: 10.1002/ejhf.494. [DOI] [PubMed] [Google Scholar]
- 12.Redfors B., Vedad R., Angerås O., Råmunddal T., Petursson P., Haraldsson I., Ali A., Dworeck C., Odenstedt J., Ioaness D., Libungan B., Shao Y., Albertsson P., Stone G.W., Omerovic E. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction — A report from the SWEDEHEART 1 registry. Int. J. Cardiol. 2015;185:282–289. doi: 10.1016/j.ijcard.2015.03.162. [DOI] [PubMed] [Google Scholar]
- 13.Moghaddam N., Wong G., Cairns J., Goodman S., Perry-Arnesen M., Tocher W., Mackay M., Singer J., Lee T., Rao S., Fordyce C. Association of anemia with outcomes among ST-segement-elevation myocardial infarction patients receiving primary percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.118.007175. [DOI] [PubMed] [Google Scholar]
- 14.Sabatine M.S., Morrow D.A., Giugliano R.P., Burton P.B.J., Murphy S.A., Mccabe C.H., Gibson C.M., Braunwald E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–2049. doi: 10.1161/01.CIR.0000162477.70955.5F. [DOI] [PubMed] [Google Scholar]
- 15.Böhm M., Cammann V.L., Ghadri J.R., Ukena C., Gili S., Di Vece D., Kato K., Ding K.J., Szawan K.A., Micek J., Jurisic S., Ascenzo F.D., Frangieh A.H., Rechsteiner D., Seifert B., Ruschitzka F., Lüscher T., Templin C. Interaction of systolic blood pressure and resting heart rate with clinical outcomes in takotsubo syndrome : insights from the International Takotsubo Registry. Eur. J. Heart Fail. 2018;20:1021–1030. doi: 10.1002/ejhf.1162. [DOI] [PubMed] [Google Scholar]
- 16.Singh K., Carson K., Usmani Z., Sawhney G., Shah R., Horowitz J. Systematic review and meta-analysis of incidence and correlates of recurrence of takotsubo cardiomyopathy ☆. Int. J. Cardiol. 2014;174:696–701. doi: 10.1016/j.ijcard.2014.04.221. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen T.H., Neil C.J., Sverdlov A.L., Mahadavan G., Chirkov Y.Y., Kucia A.M., Stansborough J., Beltrame J.F., Selvanayagam J.B., Zeitz C.J., Struthers A.D., Frenneaux M.P., Horowitz J.D. N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am. J. Cardiol. 2011;108:1316–1321. doi: 10.1016/j.amjcard.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 18.Neil C.J., Nguyen T.H., Singh K., Raman B., Stansborough J., Dawson D., Frenneaux M.P., Horowitz J.D., Hons B. Relation of delayed recovery of myocardial function after takotsubo cardiomyopathy to subsequent quality of life. Am. J. Cardiol. 2015;115:1085–1089. doi: 10.1016/j.amjcard.2015.01.541. [DOI] [PubMed] [Google Scholar]
- 19.Sachdeva J., Dai W., Kloner R.A. Functional and histological assessment of an experimental model. J. Am. Hear. Assoc. 2014;3 doi: 10.1161/JAHA.114.000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parodi G., Bellandi B., Del Pace S., Cardiomyopathy T. Natural history of Tako-Tsubo. Chest. 2011;139:887–892. doi: 10.1378/chest.10-1041. [DOI] [PubMed] [Google Scholar]
- 21.Pelliccia F., Kaski J., Crea F., Camici P. Pathophysiology of Takotsubo syndrome. Circulatation. 2017:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 22.Kato K., Di Vece D., Camman V., Micek J., Szawan K., Bacchi B., Lüscher T., Ruschitzka F., Ghadi J., Templin C. Takotsubo recurrence. J. Am. Coll. Cardiol. 2019;73:981–986. doi: 10.1016/j.jacc.2018.12.033. [DOI] [PubMed] [Google Scholar]