Summary
Dopamine (DA) plays many roles in the brain, especially in movement, motivation, and reinforcement of behavior; however, its role in regulating innate immunity is not clear. Here, we show that DA can induce DNA-based extracellular traps in primary, adult, human microglia and BV2 microglia cell line. These DNA-based extracellular traps are formed independent of reactive oxygen species, actin polymerization, and cell death. These traps are functional and capture fluorescein (FITC)-tagged Escherichia coli even when reactive oxygen species production or actin polymerization is inhibited. We show that microglial extracellular traps are present in Glioblastoma multiforme. This is crucial because Glioblastoma multiforme cells are known to secrete DA. Our findings demonstrate that DA plays a significant role in sterile neuro-inflammation by inducing microglia extracellular traps.
Subject areas: Molecular Biology, Cellular Neuroscience, Immunology, Cell Biology
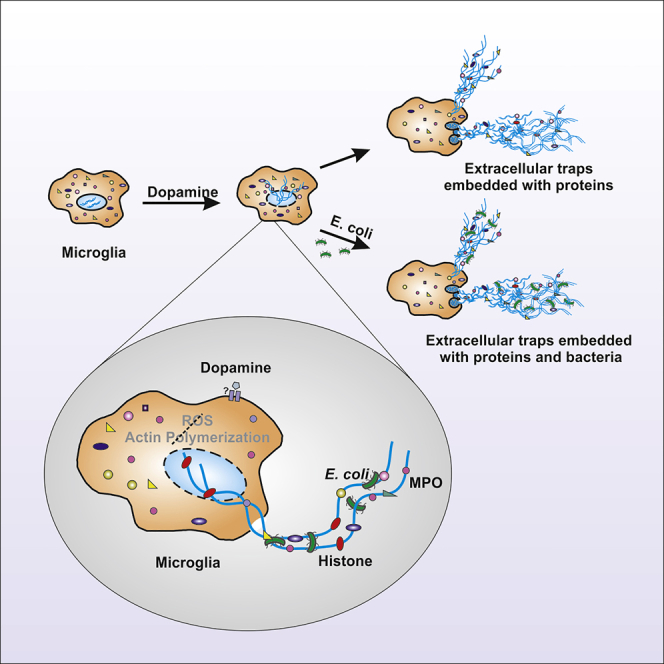
Graphical Abstract
Highlights
-
•
Dopamine induces ETs in BV2 microglia and primary adult human microglia
-
•
Induced traps are independent of ROS, cell death, and actin polymerization
-
•
Microglia ETs are functional and can trap E. coli
-
•
Microglia ETs are also present in Glioblastoma multiforme
Molecular Biology; Cellular Neuroscience; Immunology; Cell Biology
Introduction
In 2004, Zychklinsky et al. discovered that neutrophils can control the spread of pathogens by forming extracellular traps (ETs), also referred to as neutrophil extracellular traps (NETs). ETs are composed of nuclear or mitochondrial DNA (Brinkmann et al., 2004; Lood et al., 2016; Pocock and Kettenmann, 2007). ETs are decorated with multiple antimicrobial proteins like myeloperoxidase (MPO) and neutrophil elastase (NE) (Delgado-Rizo et al., 2017; Dwyer et al., 2014; Papayannopoulos, 2017) and are capable of trapping and killing bacteria (Brinkmann et al., 2004), fungi (Urban et al., 2006), parasites (Abi Abdallah et al., 2012), and viruses (Saitoh et al., 2012) It is now known that innate immune cells such as monocytes (Webster et al., 2010), macrophages (Chow et al., 2010), eosinophils ((Gupta et al., 2010)Yousefi et al., 2008), basophils (Morshed et al., 2014; Schorn et al., 2012), and mast cells (Möllerherm et al., 2016) can also form ETs.
Two mechanisms are proposed for NETs formation (NETosis): suicidal or lytic NETosis and vital or non-lytic NETosis (Fuchs et al., 2007; Papayannopoulos, 2017; Pilsczek et al., 2010; Yipp et al., 2012; Yousefi et al., 2019). In lytic NETosis, decondensed chromatin is released with the permeabilization of nuclear envelop and cell membrane leading to immediate cell death (Fuchs et al., 2010; Papayannopoulos, 2017). In non-lytic NETosis, decondensed chromatin is released in vesicles along with granules containing antimicrobial proteins without compromising the cell membrane and cells continue to function normally (Papayannopoulos, 2017; Yipp et al., 2012). Yousefi et al. first reported that viable neutrophils can produce NETs composed of mitochondrial DNA rather than nuclear DNA (Yousefi et al., 2009). Lytic NETosis is most studied and is reactive oxygen species (ROS) dependent (Fuchs et al., 2010; Papayannopoulos, 2017). The precise mechanism behind NETosis is still not known and is a topic of intense research. Stimuli-like PMA (phorbol 12-myristate 13-acetate), LPSs (lipopolysaccharides), platelets, granulocyte-macrophage colony-stimulating factor, oxidized low-density lipoprotein, and statins induce ETs (Caudrillier et al., 2012; Delgado-Rizo et al., 2017; Wang et al., 2019; Chow et al., 2010). Pathologic conditions such as hyperglycemia impair the ability of neutrophils to form NETs and reduce their antimicrobial properties (Joshi et al., 2013).
ETs play an important role in immune surveillance and pathogen clearance, but their persistence post infection makes them inflammatory and harmful (Kolaczkowska et al., 2015; Hakkim et al., 2010; Papayannopoulos, 2017; Pocock and Kettenmann, 2007; Wright et al., 2016). ETs contribute to tissue damage (Czaikoski et al., 2016; Saffarzadeh et al., 2012; Villanueva et al., 2011), vaso-occlusion (Fuchs et al., 2010; Martinod et al., 2013), sterile inflammation (Warnatsch et al., 2015), cancer (Cedervall et al., 2015; Demers et al., 2012; Guglietta et al., 2016), rheumatoid arthritis (Khandpur et al., 2013), and systemic lupus erythematosus (Garcia-Romo et al., 2011; Yu and Su, 2013). Calcium-phosphate-based mineralo-organic particles, some of which are spontaneously generated in the body, induce NETs that leads to inflammation through high-mobility group protein B1 (Peng et al., 2017). It is critical to understand the regulation of formation and disassembly of ETs for therapeutic interventions.
Innate immune cells form ETs; macrophages forming ETs are actively studied. Human peripheral blood monocytes (Jönsson et al., 2013; Halder et al., 2016), human monocyte-derived macrophages (Wong and Jacobs, 2013), human alveolar macrophages (King et al., 2015), THP-1 monocytic cell line (Shen et al., 2016), murine peritoneal macrophages (Chow et al., 2010), and rat macrophages (Bryukhin and Shopova, 2015) form ETs, referred to as macrophage extracellular traps (Doster et al., 2017). Until recently, ET formation by microglia was not reported (Wang et al., 2019). Microglia are resident myeloid cells of the central nervous system (CNS). They play critical role in clearing debris and maintaining homeostasis in the CNS (Li and Barres, 2018). These roles of microglia are regulated by multiple factors including neurotransmitters (Pocock and Kettenmann, 2007; Fan et al., 2018; Yoshioka et al., 2016). Dopamine (DA) is one of the key neurotransmitters in the brain. It controls a variety of functions including locomotor activities, emotion, and cognition (Beninger, 1983; Ferreri et al., 2019; Ott and Nieder, 2019). DA is also important for immunity within the CNS and in the body (Fan et al., 2018; Arreola et al., 2016). Recently, Caragher et al. reported that glioblastoma (GBM) cells express dopamine receptor 2 (DR2) and can synthesize and secrete DA (Caragher et al., 2019). This affects GBM metabolism and may enhance tumor growth. Activation of DR2 increases spheroid forming capacity of GBM cells (Caragher et al., 2019; Weissenrieder et al., 2019). These findings indicate a possible role of DA in the regulation of GBM.
Based on these observations, we investigated whether DA can induce ETs in BV2 microglia cell line and primary adult human microglia. We explored the basic mechanism of microglia ET formation, functionality of these traps, and their presence in GBM.
Results
Dopamine induces formation of extracellular traps in microglia
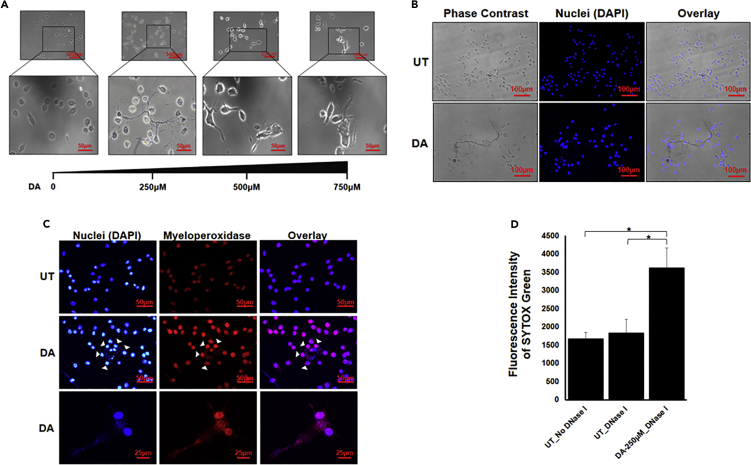
We started with examining whether DA induces ET formation in BV2 microglia cell line. We treated the cells with different concentrations of DA for 24 hours. All the concentrations of DA induced traps like structures in BV2 microglia cell as visualized by phase contrast microscopy (Figure 1A). We used 250μM of DA for further experiments. ETs are composed of decondensed chromatin and hence they are stained by (4′,6-Diamidine-2′-phenylindole dihydrochloride) DAPI. Antimicrobial protein MPO is also embedded on ETs. We stained the DA-induced trap-like structures with DAPI (Figures 1B and 1C) and MPO that confirmed their identity as ETs. We further quantified the presence of ETs in the culture supernatant by digesting them with DNase I which showed that DA was inducing ETs in BV2 microglia cell line (Figure 1D).
Figure 1.
Visualization of DA-induced extracellular traps in BV2 microglia
(A) BV2 microglia were incubated with different concentrations of dopamine (DA) for 24 hr. ETs were clearly visible at all the concentrations of DA. Scale bars, 100μm and 50μm.
(B and C) BV2 microglia were incubated with 250μM of DA for 24 hr. ETs were stained with just DAPI (blue) (B) or DAPI and MPO (C). The images are representative of three experiments. At least 7 frames were imaged per well in a two well chamber slide. Scale bars, 100μm, 50μm, and 25μm.
(D) DNase I was added after 24 hr in DA-treated wells and untreated (UT) wells. Fluorescence was measured in the collected supernatant with the help of SYTOX Green. Graph is representative of 4 experiments. Data are represented as mean ± SEM. ∗p < 0.05 (Student's t-test).
Extracellular traps are being formed independent of cell death
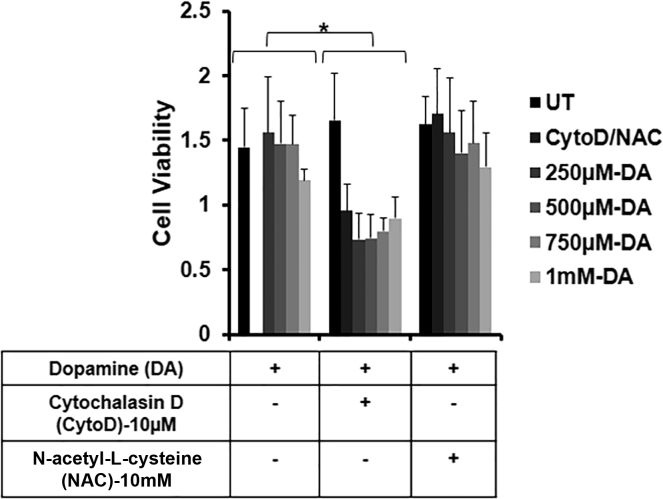
We next performed (Methylthiazolyldiphenyl-tetrazolium bromide) MTT assays to determine whether ETs are induced by lytic ETosis or vital ETosis (Figure 2). DA did not cause significant cell death at any concentration. This confirmed that DA-induced BV2 microglia cells are following vital ETosis. To investigate the mechanism of ET formation in BV2 cells, we have used NAC (N-acetyl-L-cysteine), an antioxidant, and cytochalasin D (CytoD), an actin polymerization inhibitor, along with DA in our experiments. We checked the cytotoxicity of NAC and CytoD along with DA on BV2 microglia cells. NAC and DA did not cause significant cell death while CytoD and DA were toxic to cells.
Figure 2.
Dopamine induces extracellular traps formation independent of cell death
BV2 microglia were seeded in a 96-well microplate and were pretreated with 10μM cytochalasin D (CytoD) for 20 min and 10mM N-acetyl-L-cysteine (NAC) for 3 hr. Pretreatment was followed by dopamine (DA) treatment for 24 hr. Cell viability was observed by taking the absorbance at 570 nm. The graph is representative of 3 experiments. Data are represented as mean ± SEM. ∗p < .05 (Student's t-test).
Microglia form extracellular traps in an ROS-independent manner
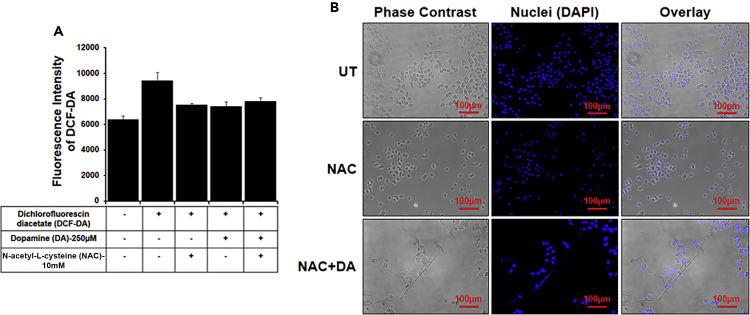
Formation of ETs is ROS dependent and/or independent (Papayannopoulos, 2017). To examine which pathway contributes to ET formation in BV2 microglia, we inhibited ROS production by pretreating the cells with NAC and confirmed the inhibition by dichlorofluorescin diacetate (Figure 3A). We observed that DA itself did not induce ROS; hence, NAC did not have significant effect on the level of ROS. This result suggests that DA induces ETs in an ROS-independent manner. To confirm this finding, we treated ROS inhibited BV2 cells with DA and observed ET formation. Inhibition of ROS did not inhibit the formation of DA-induced ETs as evident from immunofluorescence images (Figure 3B). These results confirmed that DA induces ET formation in BV2 microglia cell via an ROS-independent pathway.
Figure 3.
Dopamine induces extracellular traps independent of ROS
Cells were pretreated with 10mM N-acetyl-L-cysteine (NAC) for 3 hr followed by 250μM dopamine (DA) treatment for 24 hr.
(A) ROS production was checked with the help of dichlorofluorescin diacetate (DCF-DA). The graph is representative of three experiments. Data are represented as mean ± SEM.
(B) ET formation was induced by DA even in the presence of NAC. ETs were stained with DAPI (blue). At least 7 frames were imaged per well. Scale bars, 100μm.
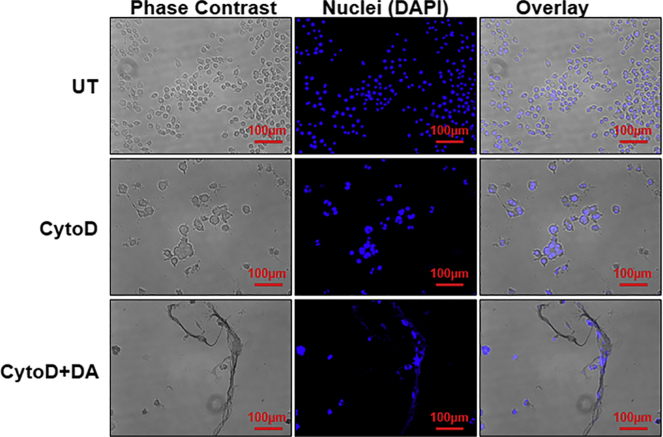
Extracellular trap formation in BV2 microglia cells is independent of actin polymerization
Actin dynamics may play a role in the formation of ETs (Metzler et al., 2014). To investigate whether actin polymerization plays a role in ET formation in microglia, we preincubated BV2 cells with CytoD and then treated them with DA (Figure 4). Immunofluorescence images confirmed that DA induced ET formation independent of actin polymerization, but the viability of cells was significantly reduced—also seen in MTT assays (Figure 2). This indicates that CytoD- and DA-treated BV2 cells may follow lytic ETosis.
Figure 4.
Extracellular trap formation is independent of actin polymerization
Cells were pretreated for 20 min with 10μM cytochalasin D (CytoD) followed by treatment with 250μM dopamine (DA) for 24 hr. Inhibition of actin polymerization had no effect on ET formation. ETs were stained with DAPI (blue). The images are the representative of two experiments. At least 7 frames were imaged per well. Scale bars, 100μm.
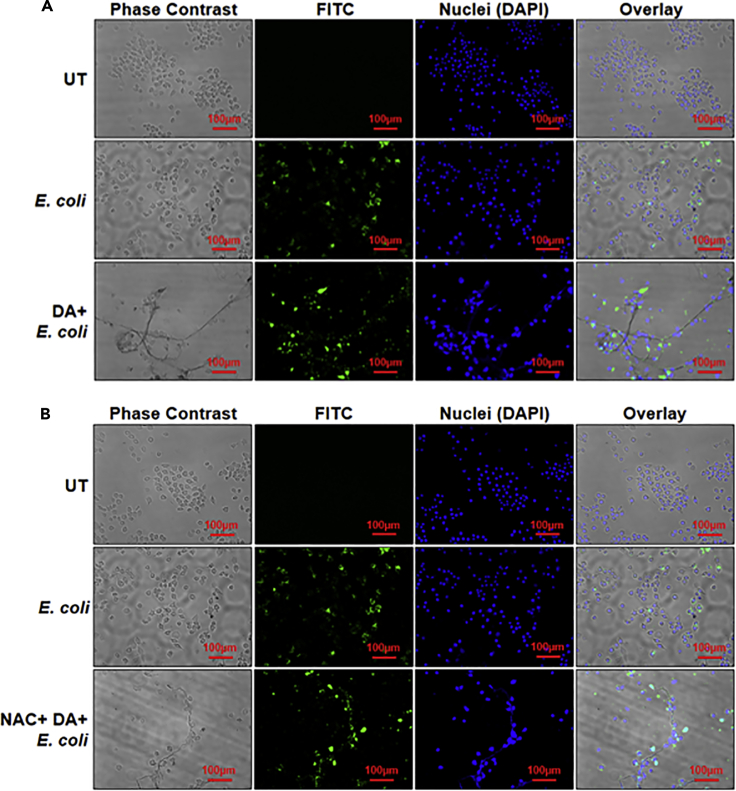
Dopamine-induced extracellular traps are functional
We further investigated whether DA-induced BV2 microglia ETs are functional and can trap bacteria. We incubated (fluorescein) FITC-tagged E. coli with BV2 cells treated with only DA, NAC + DA (Figures 5A and 5B), or CytoD + DA (Figure S1). Immunofluorescence microscopy images confirmed that DA-induced ETs are functional and capture E. coli. NAC had no effect on the functionality of traps; however, CytoD reduced the functionality of traps (Figure S1).
Figure 5.
Dopamine induces formation of functional extracellular traps
(A) BV2 microglia were incubated with dopamine (DA) for 3 hr. FITC-tagged E. coli (green) was added followed by DA incubation, and cells were further incubated for 21 hours. ETs were stained with DAPI (blue). In the overlay image, green dots are overlapping with the blue ETs, suggesting that ETs are trapping E. coli. Images are representative of two experiments. At least 7 frames were imaged per well. Scale bars, 100μm. (B) BV2 microglia were pretreated with N-acetyl-L-cysteine (NAC) and were incubated with dopamine (DA) for 3 hr. FITC-tagged E. coli (green) was added followed by DA incubation, and cells were further incubated for 21 hours. ETs were stained with DAPI (blue). In the overlay image, green dots are overlapping with the blue ETs, suggesting that ETs are trapping E. coli. Images are representative of two experiments. At least 7 frames were imaged per well. Scale bars, 100μm.
Dopamine induces extracellular traps in primary human microglia
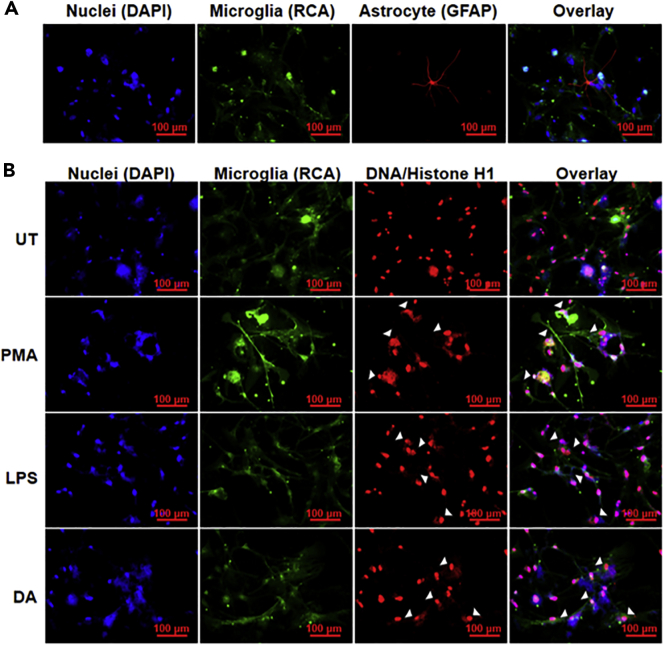
It was clear from our results that DA induces functional ETs in BV2 microglia cell line. We extended our investigation to primary adult human microglia. We isolated primary adult human microglia, as described previously (Agrawal et al., 2020), and characterized them by staining with Ricinus communis agglutinin (RCA) (Figures 6A and S2). To determine whether DA can induce ETs in primary human microglia, we treated the isolated cells with 2.5μM DA for 12 hours (Figure 6). We stained the cells with RCA (green), as a microglial marker, as well as DAPI (blue) and anti-DNA/Histone H1 antibody (red) for ETs (Figure 6B). Immunofluorescence images confirmed that DA induces ETs in primary human microglia.
Figure 6.
Dopamine induces extracellular traps in primary human microglia
(A) Primary human microglia isolated from adult human brain tissues. RCA (green) was used as a microglia marker, and glial fibrillary acidic protein (GFAP) (red) was used as an astrocyte marker. About 80% of the cells isolated were microglia.
(B) Isolated microglia were treated with 2.5μM dopamine (DA) for 12 hr. RCA (green) was used as a microglia marker. ETs (arrow heads) were visualized using DAPI (blue) and DNA/Histone H1 antibodies (red). At least 7 frames were imaged per well of the two well chamber slide. Scale bars, 100μm.
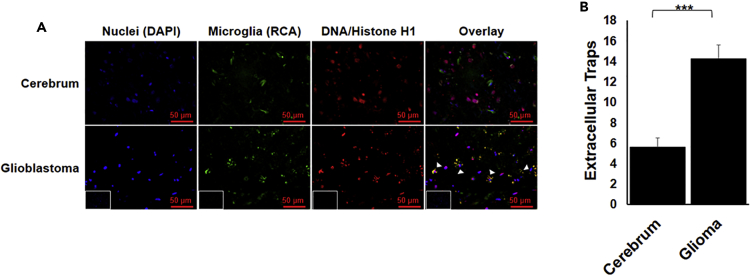
Microglia extracellular traps are present in glioblastoma
Recent studies suggest that GBM cells express DR2 and secrete DA (Caragher et al., 2019; Weissenrieder et al., 2019). These findings encouraged us to investigate whether microglia present in the GBM microenvironment form extracellular traps. We stained GBM tissues with RCA for microglia (green) and H1/DNA antibody for ETs (red). H1/DNA staining was uniformly distributed inside the cells’ nucleus in the human cerebrum tissue, whereas it showed a punctate staining around the cells in case of GBM tissues (Figures 7 and S3). Overlap of H1/DNA and RCA confirmed the formation of ETs by microglia. An algorithm was developed to quantify the formation of ETs. GBM tissues showed significant formation of ETs. There are cells other than microglia that are also showing punctate stain. This may be because of the presence of neutrophils and other cells within GBM tissue (Chen and Hambardzumyan, 2018; Schiffer et al., 2018) that are known to form ETs.
Figure 7.
Presence of microglia extracellular traps in GBM
(A) GBM tissue was stained with DAPI (blue), RCA (green) for microglia, and DNA/Histone H1 (red) for ETs. The formation of ETs by microglia can be visualized by the punctate red staining overlapping with green (arrow heads). Inset represents secondary antibody control. Scale bars, 50μm.
(B) The punctate red staining overlapping with green was quantified. The graph represents average of the quantified value. The result is representative of two experiments. At least 7 frames were imaged per section and quantified. Data are represented as mean ± SEM. ∗∗∗p < .0001 (Student's t-test).
Discussion
ETs play a critical role in health and disease (Daniel et al., 2019; Linders and Madhi, 2020; Papayannopoulos, 2017). We establish that DA can induce formation of ETs in BV2 microglia and primary adult human microglia (Figures 1 and 6). Presence of DA receptors on microglia and cells of adaptive and innate immune system points toward DA’s role in immune regulation (Pocock and Kettenmann, 2007; Mckenna et al., 2002; Sookhai et al., 1999). The ability of DA to induce ETs points toward the role of DA in immune regulation and sterile inflammation. Lymphocytes and dendritic cells can produce DA that can act in an autocrine and/or paracrine manner (Cosentino et al., 2007; Mckenna et al., 2002; Nakano et al., 2009). DA concentration varies in different regions of the brain and can increase up to 1800μM (Ewing et al., 1983; Olefirowicz and Ewing, 1990; Basu et al., 2001; Yan et al., 2015; Goto et al., 2007; Lucas and McMillen, 2002; Matt and Gaskill, 2020). Our results provide a pivotal link between DA and neuro-inflammation.
Cells forming ETs via the suicidal NETosis pathway die immediately while cells following the vital NETosis pathway survive and continue to function normally (Papayannopoulos, 2017; Yipp and Kubes, 2013). We performed MTT assays to investigate the toxicity of DA on BV2 microglia and established that DA is not cytotoxic (Figure 2). This implies that DA-induced ET formation in BV2 microglia does not lead to immediate cell death. Yousefi et al. first showed that neutrophils can survive even after the formation of NETs. Interestingly, the traps formed by viable neutrophils contained mitochondrial DNA instead of nuclear DNA (Yousefi et al., 2009). How long does microglia survive after expelling its DNA and what is the composition of microglia ETs are intriguing areas for future investigations.
Another aspect of the mechanism we observed was that DA-induced ETs follow an ROS-independent pathway. DA can work as an antioxidant (Jodko-Piórecka and Litwinienko, 2015), and our results showed that DA did not induce significant ROS generation in BV2 microglia (Figure 3). We pretreated cells with the ROS scavenger NAC and then with DA to confirm no significant increase in ROS levels. These results proposed that DA-induced ET formation is independent of ROS. We confirmed this by observing the formation of traps even when ROS was inhibited (Figure 3). ROS-independent ET formation is previously reported to follow vital ETosis (Pilsczek et al., 2010). The molecules involved in this pathway are under-explored. ET formation is highly stimulus dependent (Daniel et al., 2019; Papayannopoulos, 2017; Doster et al., 2017). Our result is crucial, as evidence of different stimuli inducing vital ETosis will help in exploring molecular pathways. Actin dynamics plays a crucial role in ET formation (Stojkov et al., 2017; Metzler et al., 2014). But sometimes inhibition of actin polymerization does not affect ET formation (Abi Abdallah et al., 2012; Granger et al., 2017). We observed that DA induces ETs in an actin polymerization independent manner in BV2 cells (Figure 4). It was evident that CytoD does not affect ET formation, but it was cytotoxic as evident from our MTT assay. Inhibiting actin polymerization may induce lytic ETosis in BV2 cells. ETs are hypothesized as an alternate mechanism adopted by immune cells to counter pathogens when their phagocytic capacity is overwhelmed (Branzk et al., 2014). It is possible that, in some cases, inhibiting phagocytosis might push immune cells toward ETs inducing pathways. One of the key functions of ETs is to trap pathogens (Brinkmann et al., 2004; Doster et al., 2017; Papayannopoulos, 2017). DA-induced ETs in BV2 cells successfully trapped FITC-tagged E. coli (Figure 5). Even the ETs formed in presence of NAC or CytoD (Figure S1) trapped E. coli. Thus, inhibition of ROS did not affect functionality of traps. But functionality of traps was compromised in CytoD-treated cells. How CytoD affects ET formation and functionality in microglia needs further investigation. These results established trap formation as an important mechanism of microglial response to counter pathogens.
Encouraged by the results from BV2 microglia, we treated primary human adult microglia with known ET inducers, PMA and LPS (Doster et al., 2017; Papayannopoulos, 2017), and DA; we observed the formation of ETs (Figure 6B). This was exciting and it opened new avenues of microglia ET exploration. PMA and LPS induce ETs in different cells primarily via an ROS-dependent lytic pathway (Fuchs et al., 2007; Khan et al., 2017) while our results in BV2 cells suggest an ROS-independent non-lytic pathway for microglia ET formation. Our results support the heterogeneity of ET formation pathway adopted by cells depending on the stimuli (Petretto et al., 2019; Pieterse et al., 2016; Daniel et al., 2019). ETs are protective and inflammatory (Daniel et al., 2019). They play role in pathogenesis of many diseases including cancer (Daniel et al., 2019; Papayannopoulos, 2017). A recent report by Caragher et al. shows that GBM cells express DR2 and they can synthesize and secrete DA (Caragher et al., 2019). This may increase local concentration of DA within the tumor microenvironment which is yet to be quantified. We found that microglia present in the GBM microenvironment form ETs (Figure 7). Inflammation caused by ETs might be involved in progression of GBM. ETs play a role in metastasis of cancer, and cancer cells in turn can induce ETs (Cools-Lartigue et al., 2013; Tohme et al., 2016; Park et al., 2016). It is necessary to explore if that is true for GBM. Several key questions need future investigations; what is the role of microglia ETs in GBM pathology? Are ETs involved in shaping the microenvironment of GBM or inflammation? Microglia ETs present in the GBM need thorough characterization. It will help us in better elucidating their role in GBM and its microenvironment. ETs play an important role in autoimmune diseases such as lupus and arthritis (Garcia-Romo et al., 2011; Khandpur et al., 2013; Yu and Su, 2013). Our findings that DA can induce ETs point toward a role of DA in other inflammatory diseases in the brain. In diseases like schizophrenia, Tourette syndrome, Parkinson disease, and multiple sclerosis, DA plays a substantial role in the pathophysiology (Arreola et al., 2016; Denys et al., 2013; Brisch et al., 2014). Studying DA-induced microglial ETs may lead to interventions that may help in better management of these disease and GBM.
Limitations of the study
In the present study, we have shown formation of DA-induced microglia extracellular traps composed of DNA. Mitochondrial DNA or/and nuclear DNA contributes to formation of ETs (Lood et al., 2016; Mcilroy et al., 2014). This study does not show whether traps are composed of nuclear DNA or/and mitochondrial DNA. Studying this will help in further elucidating the intermediate signaling molecules involved in extracellular trap formation. We have shown that the traps are functional as they are capable of trapping FITC-tagged E. coli. But the functionality of traps also depends on traps killing bacteria. We have not evaluated whether the traps are capable of killing bacteria which will establish effective functionality of the traps. Protein composition of the traps also varies with cells and stimuli (Papayannopoulos, 2017). As ETs can be inflammatory, extension of this study is needed to characterize microglia ETs to determine their nature.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Dr. Sushmita Jha (sushmitajha@iitj.ac.in).
Material availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and code availability
The published article includes all code generated during this study. Original data have been deposited to Mendley Data: https://doi.org/10.17632/k2r3xsyh2m.1.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
S.J.'s laboratory was established with institutional grants from Indian Institute of Technology Jodhpur (IITJ) and is funded by grants from the Department of Biotechnology (BT/PR12831/MED/30/1489/2015) and the Ministry of Electronics and Information Technology, Government of India (No.4 (16)/2019-ITEA). We thank Dr. Anirban Basu's Lab at National Brain Research Center (NBRC), India, for their kind gift of the BV2 microglia cell line. Modified histone antibodies were a kind gift from Dr. Vijayalakshmi Mahadevan from the Institute of Bioinformatics and Applied Biotechnology. Human brain tissue sections were obtained from the All India Institute of Medical Sciences (AIIMS) Jodhpur and Tata Memorial Hospital, Tata Memorial Center, Mumbai, Maharashtra, India. We thank Dr. Pushpa Potaliya and Dr. Vineet Jain, Department of Anatomy, AIIMS, Jodhpur, for valuable assistance in histological processing of the tissues. We thank Mr. Bharat Pareek, Technical Superintendent, Indian Institute of Technology Jodhpur, for his technical support in our lab.
Author contributions
I.A. designed and performed the experiments (data analysis, standardized primary microglia isolation, immunofluorescence), as well as prepared and edited initial manuscript draft. N.S. performed BV2 microglia cell culture. S.S. did some immunocytochemistry experiments. D.B.C. wrote the code for ET quantification and D.C. and S.A. helped with quantification. D.J. provided brain tissues for microglia isolation. S.G., S.E., and T.G. provided tissue sections for IHC studies. S.J. conceptualized the study, designed experiments, wrote initial draft, and edited and reviewed the manuscript. All authors reviewed the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101968.
Supplemental information
References
- Abi Abdallah D.S., Lin C., Ball C.J., King M.R., Duhamel G.E., Denkers E.Y. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 2012;80:768–777. doi: 10.1128/IAI.05730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal I., Saxena S., Nair P., Jha D., Jha S. Obtaining Human Microglia from Adult Human Brain Tissue. JoVE. 2020;162:e61438. doi: 10.3791/61438. [DOI] [PubMed] [Google Scholar]
- Arreola R., Alvarez-Herrera S., Pérez-Sánchez G., Becerril-Villanueva E., Cruz-Fuentes C., Flores-Gutierrez E.O., Garcés-Alvarez M.E., De La Cruz-Aguilera D.L., Medina-Rivero E., Hurtado-Alvarado G. Immunomodulatory effects mediated by dopamine. J. Immunol. Res. 2016;2016:3160486. doi: 10.1155/2016/3160486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Nagy J.A., Pal S., Vasile E., Eckelhoefer I.A., Susan Bliss V., Manseau E.J., Dasgupta P.S., Dvorak H.F., Mukhopadhyay D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 2001;7:569–574. doi: 10.1038/87895. [DOI] [PubMed] [Google Scholar]
- Beninger R.J. The role of dopamine in locomotor activity and learning. Brain Res. 1983;287:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- Branzk N., Lubojemska A., Hardison S.E., Wang Q., Gutierrez M.G., Brown G.D., Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014;15:1017. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Brisch R., Saniotis A., Wolf R., Bielau H., Bernstein H.-G., Steiner J., Bogerts B., Braun K., Jankowski Z., Kumaratilake J. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front. Psychiatry. 2014;5:47. doi: 10.3389/fpsyt.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryukhin G.V., Shopova A.V. Characteristics of mononuclear extracellular traps in the offspring of female rats with drug-induced hepatitis. Bull. Exp. Biol. Med. 2015;159:435–437. doi: 10.1007/s10517-015-2984-8. [DOI] [PubMed] [Google Scholar]
- Caragher S.P., Shireman J.M., Huang M., Miska J., Atashi F., Baisiwala S., Hong Park C., Saathoff M.R., Warnke L., Xiao T. Activation of dopamine receptor 2 prompts transcriptomic and metabolic plasticity in glioblastoma. J. Neurosci. 2019;39:1982. doi: 10.1523/JNEUROSCI.1589-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudrillier A., Kessenbrock K., Gilliss B.M., Nguyen J.X., Marques M.B., Monestier M., Toy P., Werb Z., Looney M.R. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedervall J., Zhang Y., Huang H., Zhang L., Femel J., Dimberg A., Olsson A.-K. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 2015;75:2653. doi: 10.1158/0008-5472.CAN-14-3299. [DOI] [PubMed] [Google Scholar]
- Chen Z., Hambardzumyan D. Immune microenvironment in glioblastoma subtypes. Front. Immunol. 2018;9:1004. doi: 10.3389/fimmu.2018.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow O.A., Von Köckritz-Blickwede M., Bright A.T., Hensler M.E., Zinkernagel A.S., Cogen A.L., Gallo R.L., Monestier M., Wang Y., Glass C.K., Nizet V. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools-Lartigue J., Spicer J., Mcdonald B., Gowing S., Chow S., Giannias B., Bourdeau F., Kubes P., Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino M., Fietta A.M., Ferrari M., Rasini E., Bombelli R., Carcano E., Saporiti F., Meloni F., Marino F., Lecchini S. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109:632–642. doi: 10.1182/blood-2006-01-028423. [DOI] [PubMed] [Google Scholar]
- Czaikoski P.G., Mota J.M.S.C., Nascimento D.C., Sônego F., Castanheira F.V.E.S., Melo P.H., Scortegagna G.T., Silva R.L., Barroso-Sousa R., Souto F.O. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One. 2016;11:e0148142. doi: 10.1371/journal.pone.0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C., Leppkes M., Muñoz L.E., Schley G., Schett G., Herrmann M. Extracellular DNA traps in inflammation, injury and healing. Nat. Rev. Nephrol. 2019;15:559–575. doi: 10.1038/s41581-019-0163-2. [DOI] [PubMed] [Google Scholar]
- Delgado-Rizo V., Martínez-Guzmán M., Iñiguez-Gutierrez L., García-Orozco A., Alvarado-Navarro A., Fafutis-Morris M. Neutrophil extracellular traps and its implications in inflammation: an overview. Front. Immunol. 2017;8:81. doi: 10.3389/fimmu.2017.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers M., Krause D.S., Schatzberg D., Martinod K., Voorhees J.R., Fuchs T.A., Scadden D.T., Wagner D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. U S A. 2012;109:13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys D., De Vries F., Cath D., Figee M., Vulink N., Veltman D.J., Van Der Doef T.F., Boellaard R., Westenberg H., Van Balkom A. Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder. Eur. Neuropsychopharmacol. 2013;23:1423–1431. doi: 10.1016/j.euroneuro.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Doster R.S., Rogers L.M., Gaddy J.A., Aronoff D.M. Macrophage extracellular traps: a scoping review. J. Innate Immun. 2017;10:3–13. doi: 10.1159/000480373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer M., Shan Q., D’ortona S., Maurer R., Mitchell R., Olesen H., Thiel S., Huebner J., Gadjeva M. Cystic Fibrosis Sputum DNA has NETosis characteristics and NET release is regulated by MIF. J. Innate Immun. 2014;6:765–779. doi: 10.1159/000363242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing A.G., Bigelow J.C., Wightman R.M. Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science. 1983;221:169. doi: 10.1126/science.6857277. [DOI] [PubMed] [Google Scholar]
- Fan Y., Chen Z., Pathak J.L., Carneiro A.M.D., Chung C.Y. Differential regulation of adhesion and phagocytosis of resting and activated microglia by dopamine. Front. Cell. Neurosci. 2018;12:309. doi: 10.3389/fncel.2018.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri L., Mas-Herrero E., Zatorre R.J., Ripollés P., Gomez-Andres A., Alicart H., Olivé G., Marco-Pallarés J., Antonijoan R.M., Valle M. Dopamine modulates the reward experiences elicited by music. Proc. Natl. Acad. Sci. U S A. 2019;116:3793. doi: 10.1073/pnas.1811878116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell. Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., Wrobleski S.K., Wakefield T.W., Hartwig J.H., Wagner D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romo G.S., Caielli S., Vega B., Connolly J., Allantaz F., Xu Z., Punaro M., Baisch J., Guiducci C., Coffman R.L. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Otani S., Grace A.A. The Yin and Yang of dopamine release: a new perspective. Neuropharmacology. 2007;53:583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger V., Faille D., Marani V., Noël B., Gallais Y., Szely N., Flament H., Pallardy M., Chollet-Martin S., De Chaisemartin L. Human blood monocytes are able to form extracellular traps. J. Leukoc. Biol. 2017;102:775–781. doi: 10.1189/jlb.3MA0916-411R. [DOI] [PubMed] [Google Scholar]
- Guglietta S., Chiavelli A., Zagato E., Krieg C., Gandini S., Ravenda P.S., Bazolli B., Lu B., Penna G., Rescigno M. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat. Commun. 2016;7:11037. doi: 10.1038/ncomms11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.K., Joshi M.B., Philippova M., Erne P., Hasler P., Hahn S., Resink T.J. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Hakkim A., Fürnrohr B.G., Amann K., Laube B., Abed U.A., Brinkmann V., Herrmann M., Voll R.E., Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. U S A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder L.D., Abdelfatah M.A., Jo E.A.H., Jacobsen I.D., Westermann M., Beyersdorf N., Lorkowski S., Zipfel P.F., Skerka C. Factor H binds to extracellular DNA traps released from human blood monocytes in response to Candida albicans. Front. Immunol. 2016;7:671. doi: 10.3389/fimmu.2016.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodko-Piórecka K., Litwinienko G. Antioxidant activity of dopamine and L-DOPA in lipid micelles and their cooperation with an analogue of α-tocopherol. Free Radic. Biol. Med. 2015;83:1–11. doi: 10.1016/j.freeradbiomed.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Jönsson B.E., Bylund J., Johansson B.R., Telemo E., Wold A.E. Cord-forming mycobacteria induce DNA meshwork formation by human peripheral blood mononuclear cells. Pathog. Dis. 2013;67:54–66. doi: 10.1111/2049-632X.12007. [DOI] [PubMed] [Google Scholar]
- Joshi M.B., Lad A., Bharath Prasad A.S., Balakrishnan A., Ramachandra L., Satyamoorthy K. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 2013;587:2241–2246. doi: 10.1016/j.febslet.2013.05.053. [DOI] [PubMed] [Google Scholar]
- Khan M.A., Farahvash A., Douda D.N., Licht J.-C., Grasemann H., Sweezey N., Palaniyar N. JNK activation turns on LPS- and gram-negative bacteria-induced NADPH oxidase-dependent suicidal NETosis. Sci. Rep. 2017;7:3409. doi: 10.1038/s41598-017-03257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandpur R., Carmona-Rivera C., Vivekanandan-Giri A., Gizinski A., Yalavarthi S., Knight J.S., Friday S., Li S., Patel R.M., Subramanian V. Nets are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P.T., Sharma R., O’sullivan K., Selemidis S., Lim S., Radhakrishna N., Lo C., Prasad J., Callaghan J., Mclaughlin P. Nontypeable Haemophilus influenzae induces sustained lung oxidative stress and protease expression. PLoS One. 2015;10:e0120371. doi: 10.1371/journal.pone.0120371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E., Jenne C.N., Surewaard B.G.J., Thanabalasuriar A., Lee W.-Y., Sanz M.-J., Mowen K., Opdenakker G., Kubes P. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat. Commun. 2015;6:6673. doi: 10.1038/ncomms7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Barres B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018;18:225–242. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- Linders J., Madhi R. Extracellular Cold-Inducible RNA-Binding Protein Regulates Neutrophil Extracellular Trap Formation and Tissue Damage in Acute Pancreatitis. Laboratory Investigation (Nature publishing group); 2020. [DOI] [PubMed] [Google Scholar]
- Lood C., Blanco L.P., Purmalek M.M., Carmona-Rivera C., De Ravin S.S., Smith C.K., Malech H.L., Ledbetter J.A., Elkon K.B., Kaplan M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016;22:146. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas L.A.C., Mcmillen B.A. Differences in brain area concentrations of dopamine and serotonin in myers' high ethanol preferring (mHEP) and outbred rats. J. Neural Transm. 2002;109:279–292. doi: 10.1007/s007020200023. [DOI] [PubMed] [Google Scholar]
- Martinod K., Demers M., Fuchs T.A., Wong S.L., Brill A., Gallant M., Hu J., Wang Y., Wagner D.D. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc. Natl. Acad. Sci. U S A. 2013;110:8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt S.M., Gaskill P.J. Where is dopamine and how do immune cells see it?: dopamine-mediated immune cell function in health and disease. J. Neuroimmune Pharmacol. 2020;15:114–164. doi: 10.1007/s11481-019-09851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcilroy D.J., Jarnicki A.G., Au G.G., Lott N., Smith D.W., Hansbro P.M., Balogh Z.J. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J. Crit. Care. 2014;29:1133.e1–1133.e5. doi: 10.1016/j.jcrc.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Mckenna F., Mclaughlin P.J., Lewis B.J., Sibbring G.C., Cummerson J.A., Bowen-Jones D., Moots R.J. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J. Neuroimmunol. 2002;132:34–40. doi: 10.1016/s0165-5728(02)00280-1. [DOI] [PubMed] [Google Scholar]
- Metzler Kathleen D., Goosmann C., Lubojemska A., Zychlinsky A., Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8:883–896. doi: 10.1016/j.celrep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möllerherm H., Von Köckritz-Blickwede M., Branitzki-Heinemann K. Antimicrobial activity of mast cells: role and relevance of extracellular DNA traps. Front. Immunol. 2016;7:265. doi: 10.3389/fimmu.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshed M., Hlushchuk R., Simon D., Walls A.F., Obata-Ninomiya K., Karasuyama H., Djonov V., Eggel A., Kaufmann T., Simon H.-U., Yousefi S. NADPH oxidase–independent formation of extracellular DNA traps by basophils. J. Immunol. 2014;192:5314. doi: 10.4049/jimmunol.1303418. [DOI] [PubMed] [Google Scholar]
- Nakano K., Higashi T., Takagi R., Hashimoto K., Tanaka Y., Matsushita S. Dopamine released by dendritic cells polarizes Th2 differentiation. Int. Immunol. 2009;21:645–654. doi: 10.1093/intimm/dxp033. [DOI] [PubMed] [Google Scholar]
- Olefirowicz T.M., Ewing A.G. Dopamine concentration in the cytoplasmic compartment of single neurons determined by capillary electrophoresis. J. Neurosci. Methods. 1990;34:11–15. doi: 10.1016/0165-0270(90)90036-f. [DOI] [PubMed] [Google Scholar]
- Ott T., Nieder A. Dopamine and cognitive control in prefrontal cortex. Trends Cogn. Sci. 2019;23:213–234. doi: 10.1016/j.tics.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2017;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- Park J., Wysocki R.W., Amoozgar Z., Maiorino L., Fein M.R., Jorns J., Schott A.F., Kinugasa-Katayama Y., Lee Y., Won N.H. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. translational Med. 2016;8:361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H.-H., Liu Y.-J., Ojcius D.M., Lee C.-M., Chen R.-H., Huang P.-R., Martel J., Young J.D. Mineral particles stimulate innate immunity through neutrophil extracellular traps containing HMGB1. Scientific Rep. 2017;7:16628. doi: 10.1038/s41598-017-16778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petretto A., Bruschi M., Pratesi F., Croia C., Candiano G., Ghiggeri G., Migliorini P. Neutrophil extracellular traps (NET) induced by different stimuli: a comparative proteomic analysis. PLoS One. 2019;14:e0218946. doi: 10.1371/journal.pone.0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse E., Rother N., Yanginlar C., Hilbrands L.B., Van Der Vlag J. Neutrophils discriminate between lipopolysaccharides of different bacterial sources and selectively release neutrophil extracellular traps. Front. Immunol. 2016;7:484. doi: 10.3389/fimmu.2016.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsczek F.H., Salina D., Poon K.K.H., Fahey C., Yipp B.G., Sibley C.D., Robbins S.M., Green F.H.Y., Surette M.G., Sugai M. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010;185:7413. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- Pocock J.M., Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Saffarzadeh M., Juenemann C., Queisser M.A., Lochnit G., Barreto G., Galuska S.P., Lohmeyer J., Preissner K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., Uehata T., Iwasaki H., Omori H., Yamaoka S. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Schiffer D., Annovazzi L., Casalone C., Corona C., Mellai M. Glioblastoma: microenvironment and niche concept. Cancers. 2018;11:5. doi: 10.3390/cancers11010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorn C., Janko C., Latzko M., Chaurio R., Schett G., Herrmann M. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front. Immunol. 2012;3:277. doi: 10.3389/fimmu.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F., Tang X., Cheng W., Wang Y., Wang C., Shi X., An Y., Zhang Q., Liu M., Liu B., Yu L. Fosfomycin enhances phagocyte-mediated killing of Staphylococcus aureus by extracellular traps and reactive oxygen species. Sci. Rep. 2016;6:19262. doi: 10.1038/srep19262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookhai S., Wang J.H., Mccourt M., O'connell D., Redmond H.P. Dopamine induces neutrophil apoptosis through a dopamine D-1 receptor-independent mechanism. Surgery. 1999;126:314–322. [PubMed] [Google Scholar]
- Stojkov D., Amini P., Oberson K., Sokollik C., Duppenthaler A., Simon H.-U., Yousefi S. ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. J. Cell Biol. 2017;216:4073. doi: 10.1083/jcb.201611168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohme S., Yazdani H.O., Al-Khafaji A.B., Chidi A.P., Loughran P., Mowen K., Wang Y., Simmons R.L., Huang H., Tsung A. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban C.F., Reichard U., Brinkmann V., Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- Villanueva E., Yalavarthi S., Berthier C.C., Hodgin J.B., Khandpur R., Lin A.M., Rubin C.J., Zhao W., Olsen S.H., Klinker M. Netting neutrophils induce endothelial damage, infiltrate tissues and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Wang Y., Shi X., Tang X., Cheng W., Wang X., An Y., Li S., Xu H., Li Y. The TRAPs from microglial vesicles protect against Listeria infection in the CNS. Front. Cell. Neurosci. 2019;13:199. doi: 10.3389/fncel.2019.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnatsch A., Ioannou M., Wang Q., Papayannopoulos V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster S.J., Daigneault M., Bewley M.A., Preston J.A., Marriott H.M., Walmsley S.R., Read R.C., Whyte M.K.B., Dockrell D.H. Distinct cell death programs in monocytes regulate innate responses following challenge with common causes of invasive bacterial disease. J. Immunol. 2010;185:2968–2979. doi: 10.4049/jimmunol.1000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenrieder J.S., Reed J.L., Green M.V., Moldovan G.L., Koubek E.J., Neighbors J.D., Hohl R.J. The dopamine D2 receptor contributes to the spheroid formation behavior of U87 glioblastoma cells. Pharmacology. 2019;105:19–27. doi: 10.1159/000502562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.-W., Jacobs W.R. Mycobacterium tuberculosis exploits human interferon γ to stimulate macrophage extracellular trap formation and necrosis. J. Infect. Dis. 2013;208:109–119. doi: 10.1093/infdis/jit097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T.K., Gibson P.G., Simpson J.L., Mcdonald V.M., Wood L.G., Baines K.J. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology. 2016;21:467–475. doi: 10.1111/resp.12730. [DOI] [PubMed] [Google Scholar]
- Yan Y., Jiang W., Liu L., Wang X., Ding C., Tian Z., Zhou R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- Yipp B.G., Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- Yipp B.G., Petri B., Salina D., Jenne C.N., Scott B.N.V., Zbytnuik L.D., Pittman K., Asaduzzaman M., Wu K., Meijndert H.C. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012;18:1386. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka Y., Sugino Y., Tozawa A., Yamamuro A., Kasai A., Ishimaru Y., Maeda S. Dopamine inhibits lipopolysaccharide-induced nitric oxide production through the formation of dopamine quinone in murine microglia BV-2 cells. J. Pharmacol. Sci. 2016;130:51–59. doi: 10.1016/j.jphs.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Yousefi S., Gold J.A., Andina N., Lee J.J., Kelly A.M., Kozlowski E., Schmid I., Straumann A., Reichenbach J., Gleich G.J., Simon H.-U. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008;14:949. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- Yousefi S., Mihalache C., Kozlowski E., Schmid I., Simon H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- Yousefi S., Stojkov D., Germic N., Simon D., Wang X., Benarafa C., Simon H.-U. Untangling “NETosis” from NETs. Eur. J. Immunol. 2019;49:221–227. doi: 10.1002/eji.201747053. [DOI] [PubMed] [Google Scholar]
- Yu Y., Su K. Neutrophil extracellular traps and systemic lupus erythematosus. J. Clin. Cell. Immunol. 2013;4:139. doi: 10.4172/2155-9899.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all code generated during this study. Original data have been deposited to Mendley Data: https://doi.org/10.17632/k2r3xsyh2m.1.