Summary
In adult males, spermatogonia maintain lifelong spermatozoa production for oocyte fertilization. To understand spermatogonial metabolism we compared gene profiles in rat spermatogonia to publicly available mouse, monkey, and human spermatogonial gene profiles. Interestingly, rat spermatogonia expressed metabolic control factors Foxa1, Foxa2, and Foxa3. Germline Foxa2 was enriched in Gfra1Hi and Gfra1Low undifferentiated A-single spermatogonia. Foxa2-bound loci in spermatogonial chromatin were overrepresented by conserved stemness genes (Dusp6, Gfra1, Etv5, Rest, Nanos2, Foxp1) that intersect bioinformatically with conserved glutathione/pentose phosphate metabolism genes (Tkt, Gss, Gclc, Gclm, Gpx1, Gpx4, Fth), marking elevated spermatogonial GSH:GSSG. Cystine-uptake and intracellular conversion to cysteine typically couple glutathione biosynthesis to pentose phosphate metabolism. Rat spermatogonia, curiously, displayed poor germline stem cell viability in cystine-containing media, and, like primate spermatogonia, exhibited reduced transsulfuration pathway markers. Exogenous cysteine, cysteine-like mercaptans, somatic testis cells, and ferroptosis inhibitors counteracted the cysteine-starvation-induced spermatogonial death and stimulated spermatogonial growth factor activity in vitro.
Subject Areas: Biological Sciences, Developmental Biology, Systems Biology
Graphical Abstract

Highlights
-
•
Foxa2-bound loci are enriched with spermatogonial stemness genes in the rat germline
-
•
Spermatogonial stemness genes couple to glutathione/pentose phosphate pathways
-
•
Mammalian spermatogonia are deficient in transsulfuration pathway gene products
-
•
Cysteine-like factors counteract spermatogonial ferroptosis in soma-depleted cultures
Biological Sciences; Developmental Biology; Systems Biology
Introduction
Spermatogonial stem cells maintain spermatozoa production throughout male reproductive life by the testis-specific process of spermatogenesis (Clermont, 1972). Spermatogonial stem cells maintain spermatogenesis by their abilities to self-renew or differentiate into progenitor spermatogonia that are primed for development into spermatozoa (de Rooij, 2017). Donor spermatogonial stem cells isolated from rodent testes effectively regenerate spermatogenesis and restore fertility to male-sterile recipients (Brinster and Avarbock, 1994; Brinster and Zimmermann, 1994). Remarkably, stem spermatogonia retain their ability to clonally regenerate spermatogenesis in vivo following long-term culture in vitro (Nagano et al., 1998). Spermatogonial culture systems, therefore, hold largely untapped potential for studying genetically encoded mechanisms that govern spermatogenesis and to provide sources of haploid gametes that would advance a broad spectrum of reproduction applications (Kubota and Brinster, 2006).
Knowledge gaps persist on metabolic states that support distinct premeiotic, meiotic, and post-meiotic steps in spermatozoan development (Rato et al., 2012). Sperm-specific metabolic enzymes essential for hypermotility have been systematically investigated using knockout mice and found to drive ATP production via lower glycolytic reactions (Danshina et al., 2010; Miki et al., 2004; Odet et al., 2008). Spermatozoan glycolytic flux further depended on Gapdhs and Pgk2 fueling a glucose-sensing Ldhc4 complex (Odet et al., 2011, 2013). Spermatogonial and spermatocyte metabolism has yet to be investigated as rigorously as spermatozoan metabolism. Recent reports on spermatogonia demonstrated Myc- and JNK-mediated glycolysis increase the frequency of stem spermatogonia self-renewal divisions (Kanatsu-Shinohara et al., 2016, 2019). Beneficial effects of glycolysis on spermatogonial stem cell maintenance over time in sub-culture (Helsel et al., 2017a) may signify existence of premeiotic metabolic processes that promote the longer-term preservation and stability of replicating heritable DNA (Christodoulou et al., 2019; Milanese et al., 2019).
Rodent spermatogonial stem cell cultures provide soma-free platforms for dissecting biological processes in the germline that support spermatogenesis (Hamra, 2017). Undifferentiated spermatogonial lines endowed with germline stem cells are derived from rodent testes using culture media containing glial-cell-line-derived neurotrophic factor (Gdnf) and fibroblast growth factor 2 (Fgf2) (Kanatsu-Shinohara et al., 2003). Here, we exploited a rat culture system to annotate metabolic gene networks enriched in rat, mouse, monkey, and human spermatogonia. We found conserved (e.g. Glutathione Biosynthetic Enzymes) and species-dependent (e.g. Forkhead Transcription Factors) gene signatures enriched in rat undifferentiated spermatogonia.
Conserved spermatogonial gene profiles reported here are overrepresented by glutathione, NADPH, and pentose phosphate pathway gene sets that marked a 3.4-fold higher GSH:GSSG ratio in undifferentiated versus differentiating rat spermatogonia. We further report a dynamic metabolic shift during rat spermatogonial differentiation in vitro marked by reduced levels of the non-oxidative pentose phosphate pathway enzyme Transketolase (Tkt) and the accumulation of upper glycolytic/non-oxidative pentose phosphate pathway metabolites. Enrichment for glutathione and pentose phosphate pathway molecular markers across rat, mouse, monkey, and human premeiotic male germlines led us to identify an anti-ferroptotic pro-cysteine-like factor uptake pathway that stimulated clonal development of spermatogenic cells on laminin in synergy with the spermatogonial growth factor, Gdnf.
Results
Gene Profiling in Rat Spermatogonia
To annotate rat spermatogonial gene expression profiles, we sequenced transcriptomes in rat spermatogonial lines before and after inducing their premeiotic differentiation in culture on laminin using spermatogonial differentiation medium (SD Medium) (Figure 1A, Table S1). Prior to inducing premeiotic differentiation with SD Medium, rat undifferentiated spermatogonial lines were derived and maintained on mouse embryonic fibroblasts (MEFs) using a culture medium containing GDNF and FGF2 (SG Medium) (Figures 1A and S1A). Rat undifferentiated spermatogonia (USg) cultured in SG Medium are enriched with spermatogonial stem cells (Wu et al., 2009). Unlike SG Medium, SD Medium contains all-trans retinoic acid and Neuregulin-1 and/or Kit ligand as essential growth factors for differentiating spermatogonia (DSg) in vitro (Chapman et al., 2015a; Hamra et al., 2007). Transcriptomes from rat spermatocyte cultures (Spc) (Hamra et al., 2002) were also sequenced to validate markers for spermatogonia entering meiosis in vitro (Table S1).
Figure 1.
RNA Profiling in Rat Stem and Differentiating Spermatogonia
(A) Soma-free in vitro culture system to study molecular and cellular mechanisms in rat undifferentiated spermatogonia (USg) derived and maintained in SG medium prior to inducing development into differentiating spermatogonia (DSg) by culture in SD Medium.
(B) Heatmap of 2-way hierarchal clustering using Z score for normalized values (Kendall's Tau distance measurement method). Differentially expressed gene clusters (C1, C2, C3, C4) in rat USg, DSg, and spermatocytes (Spc). Clusters C1 and C2 are enriched selectively in rat USg, whereas genes in Cluster C3 and C4 are selectively enriched in DSg.
(C) Intersection analyses on rat USg-enriched (n = 1279) versus rat DSg-enriched (n = 692) transcripts (p < 0.02, >2FC) with rat Spc-enriched (n = 2430) transcripts (p < 0.02, >2FC versus DSg).
(D) Rat spermatogenic cell gene profiles for transcripts selectively enriched in rat USg (left: p < 5e-10, n = 99) and gene profiles selectively enriched in rat DSg (right; p < 0.0006, n = 100). Transcripts per million kb (TPM).
(E) Intersection analyses on rat USg-enriched (n = 1279) and DSg-enriched (n = 692) transcripts (p < 0.02, >2FC) with conserved rat and mouse Spc-enriched transcripts (n = 841).
(F) Gene profiles for transcripts enriched in rat DSg and rat Spc (n = 129) (left) compared with transcripts enriched in rat DSg and the rat and mouse Spc marker transcripts (n = 47) (right).
(G) WebGenStalt.com Gene Ontology (GO) gene set enrichment ratios in rat USg- and DSg-enriched transcripts (p < 0.02, >2.8FC) and in rat Spc-enriched transcripts (p < 0.02, >4FC versus DSg). Shown: rat USg and DSg Wikipathway analyses, rat genome; rat Spc Reactome pathway analysis, human genome.
In silico analyses on the generated transcriptomes (USg versus DSg) revealed 4 major clusters (C1, C2, C3, C4) of differentially expressed genes (DEGs) (120 h) (Figure 1B). Genes modeling C1 were enriched in USg and progressively decreased in abundance from DSg to Spc (n = 918) (Figures 1C and 1D, and Table S1). Genes modeling C2 were transiently downregulated in DSg but upregulated with further differentiation (n = 361) (e.g. Id1, Id2, Id3, Id4, Ctsh, Apoe, Cited2, Ier3, Phlda1, Rnc3, Penk) (Figures 1C, 1D, and S1B,and Table S1). In total, we identified 1,279 transcripts enriched in USg (p < 0.02, >2-fold) relative to DSg (Table S1). As notable examples, rat spermatogonial Upk1b, Sgk1, Ctsh, Meox1, Foxa2, Hmha1, Gfra1, Fgfr3, Snap91, Ret, Dusp4, and Cited2 were 16.4 ± 1.9-fold (±SD) more abundant after culturing 120 h in SG Medium versus SD Medium (n = 12 transcripts, average TPM>200 in SD Medium, p < 5e-10, Table 1).
Table 1.
Rat Spermatogonial Gene Expression during Culture in SG versus SD MEDIUM
| RNAs Enriched in Rat USg versus DSg |
RNAs Enriched in Rat DSg versus USg |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein Coding Gene | Mean TPM |
Species | Protein Coding Gene | Mean TPM |
Species | ||||
| USg | DSg | Spc | USg | DSg | Spc | ||||
| Upk1b | 202 | 4 | 3 | R | Cyp26a1 | 1 | 131 | 2 | R |
| Sgk1 | 184 | 9 | 65 | R | Kcnd2 | 1 | 29 | 4 | R |
| Ctsh | 704 | 45 | 127 | R M | Nphs1 | 1 | 23 | 2 | R |
| Meox1 | 108 | 5 | 1 | R | Mpped2 | 5 | 87 | 11 | R |
| Foxa2 | 107 | 4 | 4 | R Ma | Kit | 39 | 363 | 36 | R M Hc |
| Hmha1 | 169 | 9 | 7 | R M | Polq | 3 | 25 | 25 | R |
| Hoxc4 | 83 | 6 | 3 | R | Ctgf | 3 | 25 | 46 | R |
| Apoe | 572 | 24 | 840 | R M | Kirrel2 | 1 | 19 | 1 | R |
| Irx2 | 59 | 3 | 4 | R H | Erbb3 | 6 | 47 | 5 | R H |
| Gfra2 | 83 | 7 | 2 | R M | Lonrf3 | 38 | 257 | 43 | R M |
| Lhx1 | 72 | 2 | 2 | R M | Elovl2 | 2 | 15 | 37 | R |
| Spry4 | 130 | 16 | 40 | R M | Foxn4 | 3 | 26 | 2 | R M |
| Fgfr3 | 225 | 29 | 5 | R H | Baz1a | 21 | 132 | 293 | R M H |
| Tcl1a | 183 | 18 | <1 | R Hb | Mob1b | 21 | 134 | 69 | R |
| Adgrg1 | 91 | 9 | 66 | R M | Nrcam | 1 | 8 | 6 | R M |
| Lbp | 104 | 9 | 3 | R | Slc35c1 | 3 | 25 | 25 | R |
| Noto | 148 | 5 | 2 | R | Cyp26b1 | 5 | 36 | 8 | R |
| Cited2 | 119 | 14 | 416 | R M H | Rarb | 2 | 33 | 2 | R |
| Frzb | 58 | 2 | 1 | R M | Ngfr | 1 | 11 | 10 | R |
| Cldn4 | 117 | 10 | 5 | R | Igfbp2 | 77 | 582 | 58 | R M Hc |
| Snap91 | 118 | 15 | 3 | R M | Trove2 | 7 | 40 | 32 | R H |
| Dusp4 | 156 | 12 | 18 | R M H | Fmo2 | <1 | 7 | 5 | R |
| Ier3 | 138 | 19 | 939 | R M | Nr0b1 | <1 | 6 | 3 | R |
| Tnfsf12 | 81 | 5 | 12 | R M | Ankrd34b | 6 | 35 | 3 | R |
| Gfra1 | 102 | 15 | 2 | R M H | Dynlt3 | 3 | 22 | 27 | R |
| Phlda1 | 64 | 9 | 92 | R M | Dclre1c | 7 | 42 | 13 | R |
| Mest | 150 | 22 | 5 | R Hb | Ero1a | 31 | 163 | 47 | R |
| Enc1 | 227 | 38 | 36 | R | Tfcp2l1 | 2 | 11 | 10 | R |
| Rcn3 | 107 | 10 | 91 | R M | Kiaa0513 | 4 | 22 | 14 | R |
| Rnf125 | 63 | 7 | 12 | R | Tc2n | 6 | 42 | 36 | R M H |
| Igsf21 | 127 | 11 | 1 | R M | Stra6 | 1 | 10 | 4 | R |
| Ret | 212 | 39 | 7 | R M | Cpne7 | 2 | 18 | 2 | R, Hc |
| Gpx2 | 108 | 8 | 7 | R | Mmd2 | 1 | 7 | 24 | R |
| T | 36 | 3 | 3 | R M | Tbx3 | 15 | 86 | 22 | R |
| Fblim1 | 77 | 10 | 14 | R M | Zfp763 | 2 | 11 | 3 | R M |
| Egr3 | 85 | 4 | 42 | R M | Hist1h1d | 357 | 1726 | 1227 | R |
| Penk | 441 | 14 | 1094 | R M | Tgfbr1 | 12 | 65 | 50 | R |
| Tfap2c | 83 | 7 | 1 | R | Ncoa1 | 14 | 62 | 51 | R M |
Transcripts per Million Kb (Mean TPM values, Table S1) from rat undifferentiated spermatogonia (USg), differentiating spermatogonia (DSg), and spermatocyte (Spc) cultures (n = 2 rats/cell population). Left: R = Rat, enriched >7-fold in USg versus DSg, P < 5e-10; M = Mouse, enriched >2-fold in GFP-ID4 Bright versus GFP-ID4 Dim spermatogonia, p < 0.02.
aFPKM <1 in Mouse GFP-ID4 Bright spermatogonia (Helsel et al., 2017b); H=Human, enriched in spermatogonial transition gene clusters 1 > 2 upb or 1 > 2 down (Guo et al., 2018). Right: R = Rat, enriched >3-fold in DSg versus USg, P < 5 × 10−5; M = Mouse, enriched >2-fold in GFP-ID4 Dim versus GFP-ID4 Bright spermatogonia, p < 0.02; H=Human, enriched in spermatogonial transition gene clusters 2 > 3 up or 2 > 3 downc.
In contrast to USg gene profiles, we identified a total of 692 C3-like (n = 563) plus C4-like (n = 129) transcripts enriched in DSg compared with USg that increased in relative abundance during culture in SD Medium (p < 0.02, >2-fold) (Figures 1C and 1D, and Table S1). Spermatogonial Cyp26a1, Mpped2, Kit, Erbb3, Lonrf3, Baz1a, Mob1b, Igfbp2, Ero1a, Tbx3, Hist1h1d, and Tgfbr1 were 6.5 ± 2.4-fold more abundant on average after culturing 120 h in SD Medium versus SG Medium (n = 12 transcripts, average TPM>45 in SD Medium, p < 5 × 10−5, Table 1). Changes in transcript abundance during culture in SD Medium were verified by qtPCR using Zbtb16, Gfra1, Foxa2, and Snap91 as markers enriched in USg and Stra8, Kit, and Erbb3 as markers enriched in DSg (Figure S1C).
Genes clustering such as C3 and C4 in DSg overlapped by 45% with genes downregulated in Stra8-deficient mouse preleptotene spermatocytes (Kojima et al., 2019) (n = 313 of 692; Figure S1D). More stringent filtering for transcripts marking the onset of meiosis in DSg cultures defined a gene set containing 841 conserved pachytene spermatocyte transcripts enriched in both rat and mouse Spc (Ball et al., 2016) (Table S1). The 841 conserved rat and mouse Spc transcripts uniquely overlapped with 6.1% (n = 78 of 1,279) and 6.7% (n = 47 of 692) of transcripts most enriched in USg and DSg, respectively (Figures 1E and 1F, and Table S1). The latter gene set (n = 47), marking meiotic entry by rat spermatogonia on laminin in SD Medium, included Baz1a, Suco, Itgb3bp, Sephs2, Ngly1, Myo19, Gstt3, Nr2c1, Phf7, and Zfp949 (Figures 1E and 1F, and Table S1) and overlapped by 70% (n = 33 of 47) with Stra8-regulated genes in mouse preleptotene spermatocytes (Kojima et al., 2019) (Figures S1D and S1E), as reflected in the human testis transcriptome atlas (Guo et al., 2018).
Rat Undifferentiated Spermatogonia Are Enriched in Glutathione, NADPH and Pentose Phosphate Metabolism Transcripts
Gene ontology (GO) overrepresentation analyses were used to identify overlap between transcripts enriched in rat USg or DSg (p < 0.02, >2.8FC) (Table S1) and Wikipathway and Reactome pathway database gene sets (Table S2). Notably, transcripts enriched in rat USg were overrepresented by Pentose Phosphate Pathway (WP282, p < 0.05), Glutathione Metabolism (WP469, p < 0.025), and Relationship between Glutathione and NADPH (WP2562, p < 0.003) Wikipathway gene sets (Figure 1G and Table S2). In contrast to USg, transcripts enriched in rat DSg were overrepresented by Nuclear Receptors (WP217, p < 0.0003), Retinol Metabolism (WP1297, p < 0.0005), and Nuclear Receptors in Lipid Metabolism and Toxicity (WP139, p < 0.001) gene sets (Figure 1G and Table S2). USg undergoing spermatogenic differentiation in SD Medium containing RA (Figures 1F, S1D, and S1E) is consistent with RA-stimulated spermatogonial differentiation in rat testes (van Pelt and de Rooij, 1991).
The Rat Germline Is Endowed with Foxa-Family Proteins
Among genes that have yet to be rigorously studied in rat USg, Foxa2 stood out as a gene effectively shut down by SD Medium (Table 1, Figure S1C) and displayed a gene signature that co-purified with spermatogonial stem cell activity in testis cell cultures (Hamra et al., 2004). Three Foxa-family genes encode for Forkhead Box DNA-binding domain transcription factors (Foxa1, Foxa2, Foxa3) (Lam et al., 2013) (Figure 2A) that regulate chromatin activity via their co-activation and linker histone domains and that are known for regulating somatic cell metabolic enzyme gene expression (Bochkis et al., 2008; Rausa et al., 2000; Wang et al., 2011; Wolfrum et al., 2004). Foxa-family genes are also critical for stem and progenitor cell fate determination in mesoderm, ectoderm, and endoderm (Monaghan et al., 1993; Weinstein et al., 1994).
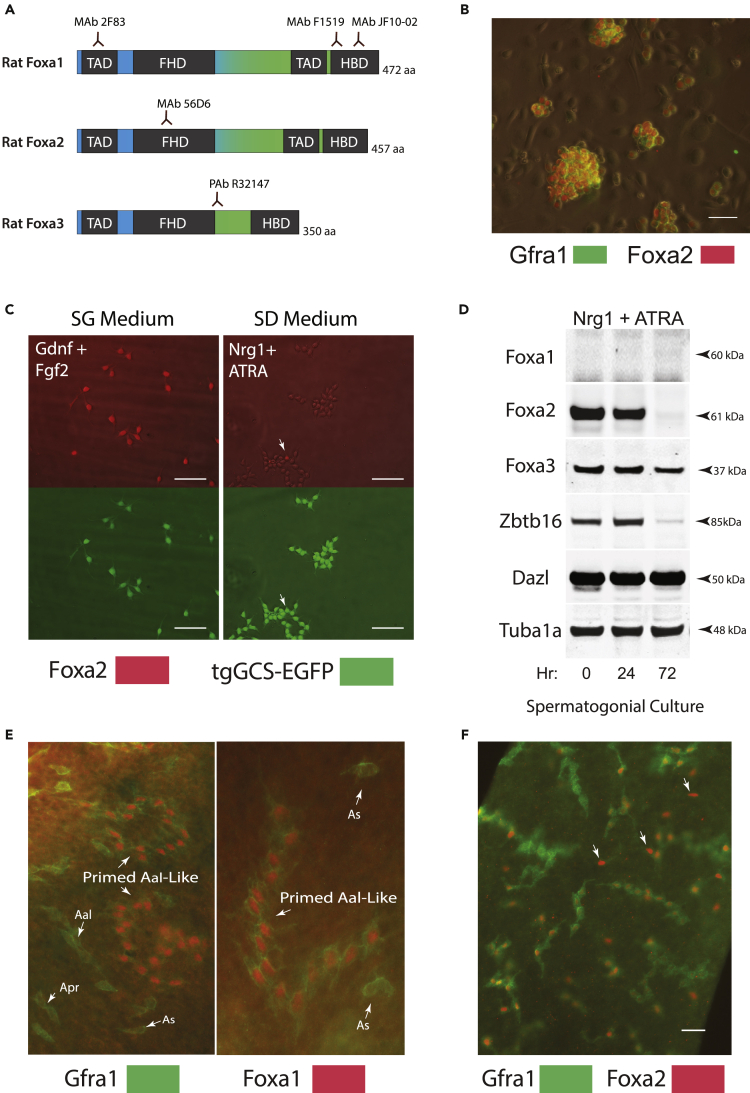
Figure 2.
Rat Spermatogonial Stem Cell Lines Express Foxa2 and Foxa3
(A) Foxa family proteins encoded by rat Foxa1, Foxa2, and Foxa3. TAD, transcriptional co-activation domain. FAD, Forkhead box domain binds to DNA. Carboxy terminal winged helix histone binding domains (HBD) are related to linker histones. Monoclonal antibodies (MAb) raised to distinct Foxa1, Foxa2, and Foxa3 domains.
(B) Gfra1 and Foxa2 co-labeling in Brown Norway rat (BN) spermatogonial cultures on MEFs at passage 14. Scale: 40 μm.
(C) Foxa2 is abundant in tgGCS-EGFP+ Sprague-Dawley rat spermatogonial stem cells (passage 12) after culturing in SG Medium (Gdnf + Fgf2) and is effectively downregulated in rat spermatogonia after culturing in SD Medium (ATRA + Nrg1) on laminin for 120 h. Top panels, Foxa2 MAb D56D6 (Red). Bottom panels, tgGCS-EGFP germline marker (Green). Arrow points to single germ cell that retained higher nuclear Foxa2 after culture in SD Medium. Scale: 60 μm
(D) Western blot on Foxa-family proteins in spermatogonial stem cell lines cultured on laminin at respective times after moving from SG (0 h) to SD Medium (24 h, 72 h). Dazl, germ cell marker loading control. Zbtb16 and Dazl combination marks rat type A spermatogonia. Tuba1a, loading control.
(E) Co-labeling for Foxa1 and Gfra1 in adult rat (d120) seminiferous tubule whole mount. Foxa1 was detected selectively in longer chains of Gfra1+ Aal-like spermatogonia typically consisting of 8–32 labeled nuclei (Huckins, 1971c).
(F) Co-labeling for Foxa2 and Gfra1 in a rat seminiferous tubule whole mount (D120). Arrows point to Foxa2+ As spermatogonia with relatively low Gfra1 labeling compared with longer Gfra1+ Aal spermatogonia. Scale: 40 μm
Also see Figure S2.
To investigate Foxa-family genes in the rat germline in more detail, we conducted protein detection studies using antibodies as depicted in Figure 2A. Foxa2 labeling using monoclonal antibody (MAb) D56D6 was detected in nuclei of Gfra1+ rat spermatogonial lines maintained in SG Medium on MEFs (Figures 2B and S2A) or laminin (Figures 2C and S2B). Although Foxa2 was abundant in type A spermatogonia maintained in SG Medium, in SD Medium, spermatogonial Foxa2 decreased in relative abundance over a 120-h culture period on either laminin or MEFs (Figures 2C and S2B). Immunoblot analysis revealed Foxa2 was downregulated by 72 h after culture in SD Medium, most like the type A spermatogonial marker Zbtb16 (Figure 2D). Like Foxa2, Foxa3 was detected by western blot in rat USg (Figure 2D). In contrast to Foxa2, spermatogonial Foxa3 did not decrease appreciably after culturing for 72 h in SD Medium (Figure 2D), and Foxa1 was not clearly detected over the 72-h culture period (Figure 2D). The germline marker, DAZL, was detected at similar levels at each time point and combined with Zbtb16, demonstrated the SD-Medium-stimulated downregulation of Foxa2 in type A spermatogonia (Figure 2D).
Foxa2 and Foxa1 selectively mark rat As and primed Aal spermatogonia subtypes
In adult rats, spermatogonial stem cells reside within a population of “A-isolated” (Ais) spermatogonia (Huckins, 1971b, 1971c, 1971d). More commonly, Ais spermatogonia are referred to as “A-single” (As) spermatogonia across species (Lok et al., 1983; Oakberg, 1971). As spermatogonia divide to renew germline stem cells but can also produce syncytia of spermatozoon progenitors termed A-paired (Apr) and A-aligned (Aal) spermatogonia (Huckins, 1971c). To determine if Foxa-family proteins were expressed in As, Apr, and/or Aal spermatogonial subpopulations, immunolocalization studies were conducted in rat seminiferous tubule whole mounts. An antibody raised the carboxy terminal domain of human Foxa1 (MAb 1519; Figure 2A) selectively labeled 8, 16, and 32-cell Gfra1+, Rarg+, Foxa2− spermatogonial syncytia in rats (Figures 2E and S3A–S3C). Foxa1 and Foxa2 were clearly detected in distinct populations of Gfra1+ spermatogonia (Figure S3A). Only 0.2% of total As and Apr and Aal-like spermatogonia scored were both Foxa1+ and Foxa2+, as represented by 2 double-positive Apr-like spermatogonial clones identified in 1 of 174 tubular subsegments (0.5 mm/subsegment, ~9 cm total tubule length scored, 1,997 total immunopositive nuclei scored) or 1 of 38 tubular subsegments containing longer Foxa1+, Foxa2− syncytia among distinct Foxa2+, Foxa1− As, and Apr spermatogonia (Figures S3A and S3B).
In contrast to Foxa1 MAb 1519's labeling profile in Gfra1+ Aal USg (Figures 2E, S3A, and S3B), in adult rats the Foxa2 Mab D56D6 (Figure 2A) selectively labeled Gfra1+ and Zbtb16+ As and Apr spermatogonia (Figures 2F, S3A, S3B, S4A, and S4B) and displayed progressively less intense labeling in cohorts of longer Zbtb16+ and Gfra1+ Aal spermatogonia (Figures 2F, S4A, and S4B). Distinct Foxa3+ testicular cells were not observed in rat seminiferous tubule whole mounts or in rat USg cultures by immunofluorescence labeling, potentially due to the R32147 Foxa3 antibody being better suited for western blot assays (Figure 2A).
Interestingly, two additional MAbs generated to respective amino-terminal (2F83) and carboxy-terminal (JF10-02) domains of human FOXA1, respectively, displayed a labeling profile in seminiferous tubule whole mounts that overlapped with the combined labeling profiles obtained using Foxa1 MAb F1519 plus Foxa2 MAb F56D6 by clearly labeling all As, Apr, and Aal-like Gfra1+ spermatogonia (Figure S5A). In contrast, Foxa1 Mabs JF10-02 and 2F83 and Foxa2 MAb D56D6 did not label spermatogonia in adult mouse seminiferous tubules (Figure S5B). All three MAbs (JF10-02, 2F83, D56D6) selectively labeled rat hepatocytes; however, only Foxa1 Mab JF10-02 and Foxa2 MAb D56D6 labeled mouse hepatocytes (Figure S5C). Co-labeling with Foxa2 MAb 56D6 and Foxa1 MAb JF10-02 again revealed that the longer Aal-4 to Aal-32 Foxa1+ spermatogonial syncytia in rat testes were Foxa2− (arrows; Figure S5A). Thus, Foxa1 MAbs 2F83 and JF10-02 either recognized a distinct form(s) of rat Foxa1 in As and Apr USg than in Aal USg or, quite possibly, the Foxa1 MAbs 2F83 and JF10-02 cross-reacted with Foxa2 and/or other Foxa2-like transcription factors in the rat germline (Table S1).
Next, we investigated Foxa2 localization in rat testes during early postnatal development. Rat spermatogonial stem cells develop from prospermatogonia that migrate from the center of seminiferous cords and colonize the basal compartment of the seminiferous epithelium on postnatal d4-7 (Hilscher et al., 1974). Foxa2+ or GFRa1+ prospermatogonia (Dazl+) were not detected by postnatal d2 to d4 (Figure 3A). Between postnatal d4 and d16, however, Foxa2+ germ cells progressively increased within the Gfra1+ population (Figure 3A). Therefore, Foxa2 was induced in the rat germline concomitantly with a Gfra1+ sub-population of undifferentiated spermatogonia (Figures 3A, S6A, and S6B) shortly after T2 prospermatogonia start their migration to colonize the basal lamina in developing seminiferous tubules (Hilscher et al., 1974).
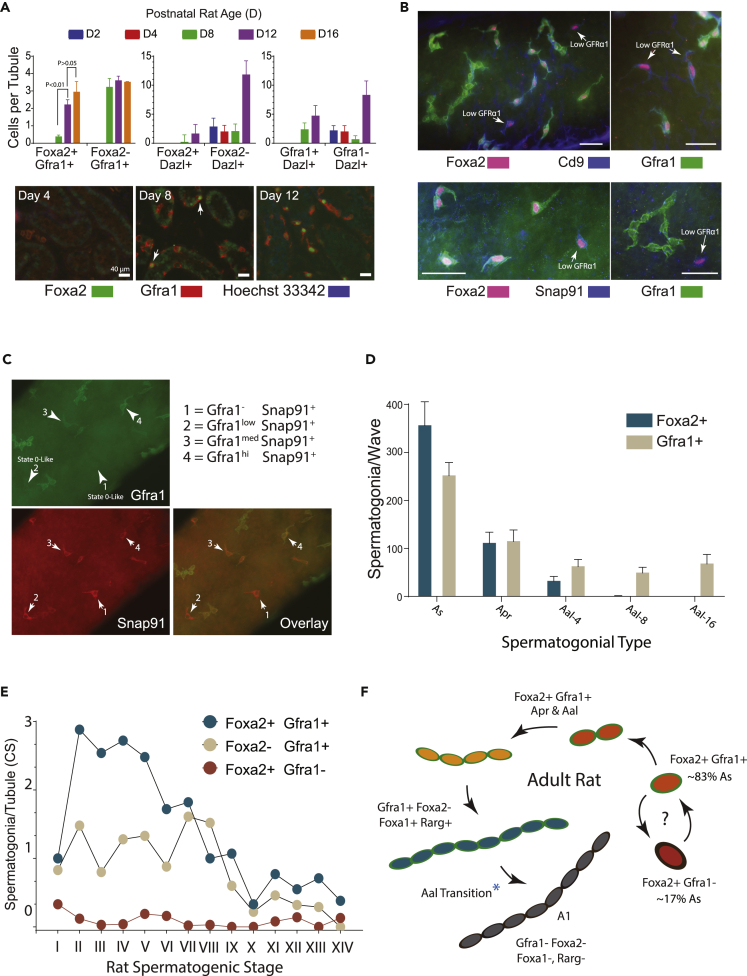
Figure 3.
Foxa1 and Foxa2 Selectively Detect Spermatogonial Types in Rat Testes
(A) Top: relative abundance of Foxa2+ and/or Gfra1+ spermatogonia/seminiferous tubule cross-section in rats at postnatal days 2, 4, 8, 12, and 16 (mean, ±S.E.M, n = 3 rats, 50–60 tubules scored/rat; p values, one-way Anova). Bottom: immunolabeling in rat seminiferous cords/tubules scored in “top” panel. Foxa2+, Gfra1+ testis cells were first detected d8 (arrows). Scale: 30μm.
(B) Foxa2+ As spermatogonial subtypes identified in rat. (Top) Co-labeling for Foxa2, Gfra1, and Cd9 revealed As spermatogonia with relatively high and low Gfra1 labeling intensities. Scale: 30μm. (Bottom) Co-labeling for Foxa2, Gfra1, and Snap91 revealed As spermatogonia with relatively high and low GFRa1 labeling intensities. Note: typical perinuclear cytoplasmic Snap91 localization. Scale: 30μm.
(C) Gradient of Gfra1 labeling in rat Snap91+ As spermatogonia in tubule whole mount.
(D) Relative numbers of rat Gfra1+ and Foxa2+ As, Apr, Aal spermatogonia/spermatogenic wave (mean counts ±SEM, 25–50, 0.5 to 2.0 mm tubule fragments/rat, n = 3 rats). Average spermatogenic wavelength in adult Sprague-Dawley rats = 2.6 cm (Perey et al., 1961).
(E) Relative numbers of Foxa2+ and/or Gfra1+ spermatogonia/seminiferous tubule cross-section (CS) per spermatogenic stage (mean counts, 110–120 tubule CS/rat, n = 3 rats).
(F) Summary of Foxa1 and Foxa2 antibody labeling profiles during steps in the development of Sprague-Dawley rat type As spermatogonia into syncytia of differentiating type A1 spermatogonia drawn from observations made in seminiferous tubule whole mounts and sections in the current study, and founded on the rat USg models proposed by Huckins (Huckins, 1971b, 1971c). Cytoplasmic Gfra1+ (Green) marks As, Apr, and Aal USg. Foxa2 nuclear labeling (red nucleus) is most intense in As and Apr USg (Gfra1+, Cd9+, Snap91+ or Gfra1−, Cd9+, Snap91+) and then declines in intensity in Gfra1+ Aal spermatogonia (orange nucleus). Foxa1 is selectively detected in 8-, 16-, and 32-cell Gfra1+, Rarg+, and Foxa2− spermatogonial syncytia (blue nucleus) prior to differentiating into Foxa1−, Foxa2−, and Gfra1− type A1 spermatogonia (gray nucleus). ∗In mice Rarg+ Aal USg are primed for differentiation into type A1 spermatogonia. The developmental hierarchy of Gfra1+ and Gfra1− undifferentiated type As spermatogonia remains to be defined (?).
Also see Figures S3-S6.
In adult rats, Foxa2 was abundant in undifferentiated type As and Apr spermatogonia as demonstrated by co-labeling with antibodies to Zbtb16, Gfra1, Cd9, and Snap91 (Figures 3B, S4A, and S4B). Populations of Cd9+/Foxa2+, Snap91+/Foxa2+, and Gfra1+/Foxa2+ undifferentiated As spermatogonia were identified by co-labeling with respective cytoplasmic and cell surface markers (Cd9, Snap91, Gfra1). Based on graded Gfra1 labeling within the undifferentiated As population (Figures 3B, 3C, and S6C), categories of Foxa2+ spermatogonia were scored that exhibited relatively high, to low, to background levels of Gfra1 labeling (Figure 3D). Approximately 17% Foxa2+ As spermatogonia demonstrated Gfra1 labeling at background levels (i.e. Gfra1−) (Figures 3D and S6C). Longer Gfra1+/Foxa2− syncytia containing 8 to 32 spermatogonia were commonly observed throughout rat seminiferous tubules and presumably developed from As spermatogonia one or more seminiferous epithelial cycles earlier (12.9 days/rat cycle) (Leblond and Clermont, 1952) (Figures 3E and S4B). Nuclear Foxa2 was also enriched in the stage VIII-IX-dependent Erbb3+, Snap91+, Zbtb16+, Sall4+, Gfra1+ undifferentiated As spermatogonia population (Figures S6D and S6E) (Abid et al., 2014).
Analyses on seminiferous tubule cross-sections in adult rats similarly identified populations of Foxa2+/Gfra1−, Foxa2+/Gfra1+, and Foxa2−/Gfra1+ spermatogonia in each respective stage I-XIV comprising a rat spermatogenic wave (Figure 3E). Based on an “As spermatogonium-specific” labeling profile in seminiferous tubule whole mounts, Foxa2+/Gfra1− As spermatogonia were detected during all stages of the seminiferous epithelial cycle and most commonly during epithelial stages XIV-II, V-IV, and XI-XII (Figure 3E). Foxa-family labeling profiles in rat seminiferous tubules build a model in which undifferentiated Gfra1− and Gfra1+ As spermatogonial sub-types ultimately develop into syncytia of Foxa1+, Foxa2−, Rarg+, Gfra1+ type Aal spermatogonia that, based on studies in mice (Gely-Pernot et al., 2012; Ikami et al., 2015), are developmentally primed for transformation into Gfra1− type A1 differentiating spermatogonia (Figure 3F).
Spermatogonial Foxa Gene Family Profiles in Rat, Mouse, and Human
In preliminary analyses, Foxa1+ and Foxa2+ mouse spermatogonia were not identified by antibody labeling in seminiferous tubule whole mounts (Figure S5B). In additional experiments aimed at determining if Foxa-family expression profiles where conserved across species, we compared our rat spermatogonial line gene lists (Table S1) with published mouse and human spermatogonial gene lists (Table S3). Although Foxa-family transcripts were detected at relatively low levels in mouse spermatogonia (i.e. <1.0 FPKM) (Helsel et al., 2017b), Foxa2 was in fact significantly (p = 0.01) more abundant (~3-fold) in USg (Id4-GFPBright = 0.78 FPKM) relative to DSg (Id4-GFPDim = 0.27 FPKM) (Helsel et al., 2017b).
Similar to mouse, gene expression profiles generated by scRNA sequencing revealed that Foxa-family genes were also not selectively enriched in distinct monkey and human spermatogenic cell populations (Guo et al., 2018; Shami et al., 2020) when compared with rat spermatogonia (Tables S1 and S3). Clear immunolabeling profiles in human testis sections were not observed for FOXA1 and FOXA2; however, FOXA3 demonstrated moderate labeling in human spermatogonia, spermatocytes, and Leydig cells [Human Protein Atlas; (Pontén et al., 2008)]. Thus, authenticating species-dependent Foxa-family expression and potential genetic effects in the male germline will require more detailed analyses. However, when compared with mouse, human, and monkey spermatogonia (Guo et al., 2018; Helsel et al., 2017b; Shami et al., 2020), rat spermatogonia appeared to be selectively endowed with Foxa-family transcription factors and most conspicuously, Foxa1 and Foxa2 (Figures 2 and 3, and Tables S1 and S3).
Spermatogonial Gene Profiles Conserved in Rat, Mouse, and Human
Phenotypic novelty in the rat germline, marked by higher relative levels of Foxa-family gene products than observed in mouse, monkey, and human male germlines (Guo et al., 2018; Shami et al., 2020) (Tables 1 and S1), prompted questions regarding the make-up of conserved gene systems in spermatogonia functionally linked to Foxa2. To identify candidate conserved spermatogonial gene systems regulated by Foxa2, we conducted gene set enrichment analyses (WebGestalt.org) comparing our annotated rat spermatogonial line gene lists (Table S1) across differentially expressed human spermatogenic cell states reported by (Guo et al., 2018). Accordingly, rat USg shared the greatest percentage of enriched genes with human spermatogonial gene sets “1->2 down” and “2->3 down” (Figure 4A) that decreased in relative abundance during human spermatogonial differentiation (Guo et al., 2018) and that best modeled gene profiles enriched in rat undifferentiated spermatogonia prior to loss of germline stem cell activity during differentiation in culture (Hamra et al., 2004).
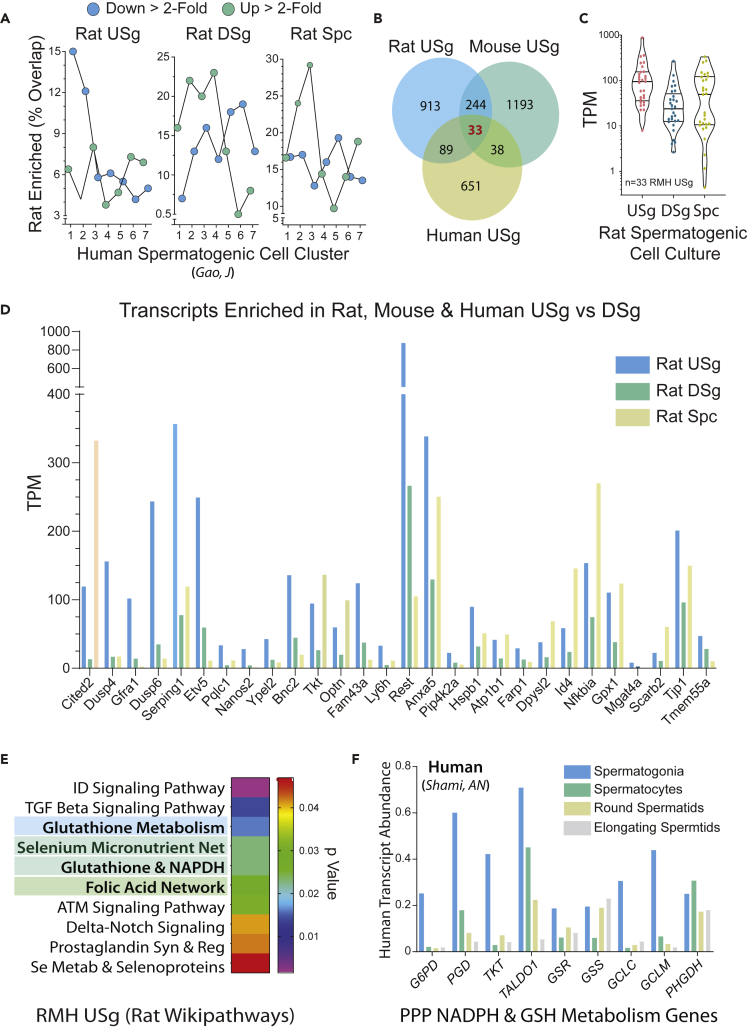
Figure 4.
Rat, Mouse, and Human Spermatogonial Stemness Genes
(A) Overlap shared by rat USg-enriched, DSg-enriched, and Spc-enriched transcripts and human spermatogenic-cell-enriched gene clusters 1–7 reported in the Testis Transcriptional Cell Atlas (Guo et al., 2018).
(B) Intersectional analyses on gene sets enriched in rat, mouse, and human USg. Rat USg-enriched transcripts (n = 1279, p < 0.02, >2FC versus DSg); mouse USg-enriched transcripts (n = 1508, p < 0.02, >2FC GFP-ID4Bright versus GFP-ID4Dim); human USg-enriched transcripts (n = 811, gene sets: 1->2 Down and 2->3 Down) (Guo et al., 2018).
(C) Violin plot of conserved USg-enriched transcripts across rat USg, DSg, and Spc cultures reveals two distinct clusters.
(D) Expression profiles for transcripts composing the conserved USg-enriched gene set in rat USg, DSg, and Spc (n = 33 from panel “B”).
(E) GO Wikipathway gene set enrichment analysis of USg-enriched transcripts conserved across rat, mouse, human (n = 33), plus USg-enriched transcripts conserved across rat and human (n = 89), plus USg-enriched transcripts conserved across mouse and human (n = 38) (see Venn diagram in panel “B”).
(F) Relative abundance of human spermatogenic cell transcripts encoding pentose phosphate, NADPH, and glutathione metabolism enzymes. Ordinate represents relative transcript centroid values reported by (Shami et al., 2020).
In contrast to rat USg, gene sets in DSg and Spc displayed the greatest overlap with human gene sets 1->2 up, 2->3 up, 3->4 up, and 4->5 up (Figure 4A). Spermatogenic cell gene sets 2->3 up, 3->4 up, and 4->5 up are predicted to be enriched with transcripts that increase in relative abundance during human spermatogenic cell differentiation (Guo et al., 2018). Transcripts identified as being enriched in both rat USg (≥2-fold versus DSg, p ≤ 0.02) (Table S1) and human spermatogonial gene sets 1->2 down and 2->3 down (Guo et al., 2018) provided a list of 122 conserved USg transcripts (Table S3) for comparative analyses with transcripts enriched in mouse USg (≥2 fold-enriched Id4-GFPBright versus Id4-GFPDim, p ≤ 0.02) (Helsel et al., 2017b).
Filtering for conserved gene profiles across rat, mouse, and human USg by intersection analyses detected 33 commonly enriched genes (Figures 4B and Table S3) that included established spermatogonial stemness markers (e.g. Nanos2, Id4, Gfra1, Etv5). Transcripts encoding Dusp4, Cited2, and Gfra1 were among the most significantly enriched transcripts in rat USg (p < 5 × 10−11, Table 1) that were also enriched in mouse and human USg (Table S3). Notably, ~60% of the commonly enriched USg transcripts (n = 20 of 33) were more abundant in rat Spc versus rat DSg (Figure 4C), including Cited2, Pqlc1, Serping1, Tkt, Optn, Axna5, Hspb1, Atp1b1, Dpysl2, Id4, Nfkbia, Gpx1, Scarb2, and Tjp (Figure 4D). Conversely, ~40% of commonly enriched transcripts (13 of 33) were more abundant in rat DSg versus rat Spc (Figure 4C), including Dusp4, Gfra1, Dusp6, Etv5, Nanos2, Ypel2, Bnc2, Fam43a, Rest, Pip4k2a, Farp1, Mgat4a, and Tmem55a (Figure 4D). Thus, transcripts enriched across rat, mouse, and human USg formed two distinct gene clusters (Figure 4C) derived from rat gene clusters C1 and C2 (Table S1).
Ontology Gene Sets Conserved across Rat, Mouse, Monkey and Human Spermatogonia
To identify conserved gene systems in spermatogonia, we conducted Gene Ontology (GO) overrepresentation analyses (WebGestalt.org) by comparing transcripts enriched in rat, mouse, and human USg and DSg with gene sets in Wikipathway and Reactome pathway databases (Figure 4E and Table S2). Transcripts enriched in rat, mouse, and human USg (n = 33), together with transcripts enriched in rat and human USg (n = 89) and those enriched in mouse and human USg (n = 38) were pooled to generate a master conserved USg gene set (n = 160; Figure 4B) that was used for GO analyses.
Focusing on the protein-coding genes with annotated gene symbols across species (n = 135 of 160; Table S2), transcripts enriched across rat, mouse, and human USg were overrepresented by Glutathione Metabolism (WP469: Gpx1, Gpx4; p = 1.6 × 10−2), Selenium Micronutrient Network (WP1310:Gpx1, Gpx4; p = 2.3 × 10−2), and Relationship between Glutathione and NADPH (WP2562:Tkt, Fth1, Gpx4; p = 2.3 × 10−2) gene sets (Figure 4E and Table S2). Conserved USg transcripts were also overrepresented by Id Signaling (WP397:Id4, Id1, Id2, Tcf3; p = 1.5 × 10−3), RAF-Independent MAPK1/3 Activation (R-MMU-112409, p = 8.3 × 10−6), Negative Regulation of MAPK Pathway (R-MMU-112409, p = 7.2 × 10−5), and MAPK Family Signaling Cascade (R-MMU-5683057, p = 1.6 × 10−4) gene sets. The Mapk signaling gene sets were represented by Gfra1, Fgfr3, Dusp4, Dusp5, Dusp6, Hspb1, Uba52, and Jak1 (Table S2).
By a similar approach, a set of 133 protein-coding transcripts enriched in rat, mouse, and human DSg (Table S2) were found to be overrepresented by Nuclear Receptors (WP217, p = 2.7 × 10−4), Retinol Metabolism (WP1297, p = 4.2 × 10−4), Complement and Coagulation Cascades (WP547, p = 3.3 × 10−3), Adipogenesis (WP155, p = 4.2 × 10−3), and Nuclear Receptors in Lipid Metabolism and Toxicity (WP139, p = 7.1 × 10−3) gene sets (Table S2), signifying SD Medium both stimulated and supported premeiotic spermatogenic differentiation (Figure 1F). Conserved Spc transcripts were enriched with gene sets that regulate spermatogenic cell metabolism and development, including Hexose Metabolism in Proximal Tubules (WP3916, p < 2 × 10−6), Cilium Assembly (R-MMU-5617833, p < 5 × 10−16), and Intraflagellar Transport (R-MMU-5620924, p < 5 × 10−16) (Table S2).
Enrichment for spermatogonial glutathione metabolism and pentose phosphate pathway gene sets across rat, mouse, and human USg (Figure 4E) are further supported by additional comparative analyses that we conducted using the recently reported primate single cell transcript centroid values in spermatogonia, spermatocytes, round spermatids, and elongating spermatids (Shami et al., 2020) (Figures 4F and S7). Like rat USg (Figure 1G and Table S2), human and monkey spermatogonia were selectively enriched with transcripts encoding non-oxidative (TKT, TALD O 1) and oxidative (G6PD, PGD) pentose phosphate pathway enzymes, glutathione biosynthesis pathway enzymes (GSS, GCLC, GCLM), and glutathione-dependent redox pathway enzymes (GSR, GPX1, GPX4, SOD1) when compared with spermatocytes (Figures 4F and S7).
Foxa2-bound loci are enriched with spermatogonial stemness genes in the rat germline
Due to the robust regenerative potential of rat spermatogonial stem cells (Hamra et al., 2002; Ogawa et al., 1999; Orwig et al., 2002; Wu et al., 2009), we hypothesized Foxa2 functioned as a genetic modifier in the rat germline by positively interacting with gene systems that promote a rat spermatogonial stem cell state. To identify candidate conserved gene systems in spermatogonia that interacted with Foxa2, we annotated Foxa2-bound genetic elements in rat USg chromatin (Chip-Seq; SG Medium) <1Mb from respective gene transcriptional start sites (peak score range 20-743) (Table S4). Distinct Foxa2-binding loci (n = 550; Table S4) were compared with transcripts enriched in rat USg (Table S1) and identified 106 genes in USg (n = 90) and DSg (n = 16) that are potentially regulated by binding Foxa2 (Figure 5A; Table S4). Approximately 45% of target genomic sequences harbored the canonical Foxa1/Foxa2 binding motif (TGTTTAC) (Figure S8A).
Figure 5.
Foxa2-Binding Intersects with Spermatogonial Metabolism Gene Network
(A) Intersectional analyses on gene sets enriched in rat USg and DSg and Foxa2-binding loci identified by ChipSeq in rat USg.
(B) Right: intersectional analyses on conserved gene sets enriched in rat, mouse, and human USg and DSg and Foxa2-binding loci identified by ChipSeq in rat USg. Left: RMH = 7 genes enriched in rat, mouse, and human USg that interact with Foxa2 in rat. RH = 6 genes enriched in rat and human that interact with Foxa2 in rat.
(C) GO Reactome gene set enrichment analysis of Foxa2-binding loci in rat USg that intersect with USg-enriched transcripts conserved across rat, mouse, human (n = 33) + USg-enriched transcripts conserved across rat and human (n = 89).
(D) Antibody labeling profiles in human testis sections for spermatogonial proteins encoded by conserved USg-enriched, Foxa2-binding genes. Images modified from the Human Protein Atlas database (Pontén et al., 2008).
(E) Transcript profiles in rat USg, DSg, and Spc for genes linked to Glycolytic, Pentose Phosphate (PPP), and NADPH metabolic pathways that were selected by GO enrichment analyses.
(F) Transcript profiles in rat USg, DSg, and Spc for genes linked to glutathione redox pathways selected by GO enrichment analyses.
(G) Transcript profiles in rat USg, DSg, and Spc for genes linked to superoxide anion redox pathways selected by GO enrichment analyses.
(H) Labeling profiles in human testis sections for proteins marking conserved metabolic pathways enriched in rat, mouse, and human USg. Images modified from the Human Protein Atlas database (Pontén et al., 2008).
Rat USg-enriched transcripts inclusive to the Foxa2-binding gene set were compared with transcripts enriched across rat, mouse, and human USg and DSg (n = 33) (Figure 4B and Table S3) and identified a conserved spermatogonial Foxa2-binding gene signature (Dusp6, Gfra1, Etv5, Rest, Tkt, Nanos2, Farp1; n = 7 of 33) (RMH, Figure 5B and Table S4). In contrast, spermatogonial Foxa2-binding genes were not found to be inclusive to conserved DSg genes (n = 0 of 11) (Figure 5B). Comparisons using the conserved USg gene set (n = 33), plus transcripts enriched exclusively in rat and human USg (n = 89, Figure 4B), identified seven additional Foxa2-binding loci: Igf2bp2, Piwil4, Cdk17, Zfand3, Cdca7l, and Foxp1 (RH, Figure 5B). Transcripts enriched exclusively in mouse and human USg (n = 38; Figure 4B) lacked genes that overlapped with Foxa2-binding loci identified in rat USg. Fifteen Foxa2-binding elements mapped to 13 distinct genes within or near genes encoding the 33 conserved USg stemness genes (Figure S8B).
Foxa2-binding loci in the USg stemness gene set were annotated as intergenic (Gfra1, Piwil4, Cdk17, Zfand3, Cdca7l, Foxp1), promoter (Dusp6) or intronic (Etv5, Rest, Tkt, Igf2bp2) regions (Figure S8B). Notably, Dusp6 contained tandem Foxa2-binding loci within its promoter region (Promoter: −185 & −717 bp) (Figure S8B). Etv5 also contained tandem Foxa2-binding loci with each binding motif localized to intron 21 of 24 (−78691 & −78,996 bp) (Figure S8B).
The Foxa2-binding locus linked to Foxp1 stood out because Foxp1 was abundant in USg (Figure S8C), and ~20% of Foxa2 ChipSeq-enriched target sequences contained consensus Foxp1 binding motifs (Figure S8A). Rat Forkhead Box-family gene transcript profiles revealed Foxa2, Foxa3, Foxo1, Foxp1, Foxm1, and Foxj3 were all relatively abundant in rat USg (Figure S8D). Like Foxo1 across mammals (Goertz et al., 2011) and Foxa2 in rats (Figures 3D–3F), Foxp1 was selectively expressed by Gfra1+ type A spermatogonia in adult rats (Figure S8E) and in type A-like spermatogonia in adult humans (Figure 5D). Based on mean TPM values > 30, rat USg hold the potential to abundantly express at least eight related Fox-family transcription factors (Figure S8D) that conceivably can compete for variations of the classical Foxa2 consensus DNA binding motif in the germline (Figure S8F).
Foxa2-Binding Spermatogonial Stemness Gene Ontology and Expression
To identify conserved pathways in spermatogonia that are functionally modified by Foxa2 enrichment, GO enrichment analyses were independently conducted on rat Foxa2-binding loci (n = 550; Table S4). Foxa2-binding loci were most significantly overrepresented by Deadenylation of mRNA (R-MMU-429947: p = 3.15 × 10−3), Mapk Family Signaling Cascades (R-MMU-5683057: p = 3.17 × 10−3), and Metabolism of Carbohydrates (R-MMU-71387: p = 4.89 × 10−3) gene sets (Figure 5C; Table S4).
The Deadenylation of mRNA gene set linked to Nanos2, due to inclusion of Cnot6l, Cnot11, and Cnot8 (Table S2) and reported Nanos2 interactions with Cnot-family RNA decay factors that regulate germ cell RNA de-adenylation (Suzuki et al., 2010). Mapk Family Signaling Cascades included the USg-enriched growth factor receptor genes Gfra1 (RMH) and Fgfr1 (RH), as well as the RMH USg-enriched Dusp6 (Table S2). Notably, the mouse Metabolism of Carbohydrates gene set contained the RMH USg-enriched Tkt (Reactome Pathway, p < 0.005) (Figure 5C), again linking Foxa2-binding to glutathione, NADPH, and pentose phosphate metabolic GO processes in USg (Figures 1G, and 4G).
In human testis (Human Protein Atlas), proteins encoded by the conserved USg gene set obtained by filtering on Foxa2-binding genes (Figure 5B) were selectively detected in spermatogonia (Figure 5D), consistent with transcript profiles in USg (Figures 1 and 4). Enrichment for proteins encoded by Foxa2-interacting gene sets in human testes (Figure 5D) prompted additional analyses on spermatogonial metabolism to determine relative abundance of conserved glutathione, NADPH, and pentose phosphate metabolic GO gene-set-encoded (Figures 1G, 4G, and 5C) RNAs and proteins in human spermatogonia.
Glycolytic, Pentose Phosphate, Glutathione Mtabolism in Rat Spermatogonia
Spermatogonial glycolytic pathway gene sets that coupled to glutathione metabolism across rat, mouse, monkey, and human (Figures 4E, 4F, and S7) were evaluated in higher resolution developmentally using rat USg, DSg, and Spc. Like monkey and human spermatogonia (Figures 4F and S7), rat USg were enriched with glutathione, NADPH, and pentose phosphate metabolic enzyme gene sets (Figures 5E–5G) that connected to upper glycolytic (Hk2, G6pd, Gpi, Pfkm), oxidative (Nfe2l3, G6pd, Pgd, Rpia, Rpe), and non-oxidative (Taldo, Tkt) pentose phosphate pathway and serine/glycine biosynthetic enzyme (Pgam1, Phgdh, Psat1, Shmt) transcripts (Figure 5E and Table S1). Transcripts encoding lower glycolytic enzymes Eno1, Pkm, Pkll, Ldha, Ldhb, Ldhc, and Ldhd were less abundant in rat USg, whereas pyruvate and NADPH generating Me1/Mdh1 and Me2/Mdh2 genes, along with the pyruvate oxidizing Pdha2 gene, were abundant in USg (Figure 5E and Table S1).
In agreement with GO analyses, rat USg were enriched with transcripts encoding glutathione reductase (Gsr) and glutathione peroxidases Gpx1, Gpx2, and Gpx4 (Figure 5F). Rat USg also expressed relatively high levels of Sod1, encoding Superoxide dismutase (Mean TPM >1500) (Figure 5G). Cytotoxic superoxide anions generated by oxidative reactions are reduced to hydrogen peroxide by Sod1, and then Gpx-family enzymes rapidly neutralize H2O2 via redox reactions driven by reduced glutathione (Wang et al., 2011). In human testes, enzymes encoded by pentose phosphate, glutathione, and NADPH metabolism genes (Figures 4, 5, and S7) were selectively detected in spermatogonia (Figure 5H; Human Protein Atlas).
Consistent with USg being enriched with gene sets linked to glutathione metabolic processes (Figure 1G) and mirroring gene sets in primate spermatogonia (Figures 4F and S7), rat USg were endowed with transcripts encoding the essential glutathione biosynthetic enzymes, glutamate-cysteine ligase catalytic (Gclc)/modifier (Gclm) subunits, and glutathione synthetase (Gss) (Figure 5F). Glutamate-cysteine ligase catalyzes the first step in glutathione production by generating the dipeptide γ-glutamyl-cysteine (γ-Glu-Cys) from cysteine and glutamate (Koppula et al., 2018). Glutathione synthetase then catalyzes condensation of glycine and γ-glutamyl-cysteine to form the tripeptide, L-glutathione (Koppula et al., 2018). In human testes, GCLC (Atlas HPA036360), GCLM (Atlas CAB009568), and GSS (Atlas HPA054508) also appeared relatively abundant in spermatogonia (Figure 5H; Human Protein Atlas).
Western blot analyses demonstrated that Tkt was abundant in USg cultured in SG Medium but was rapidly downregulated during spermatogonial differentiation in SD Medium (Figure 6A). Transketolase's western blot profile during spermatogonial differentiation in SD Medium resembled the downregulation of Foxa2 and Zbtb16 and was distinct from upregulated Erbb3 (Figure 6A). A 3-fold decrease in Tkt levels in DSg versus USg was associated with a 3.4-fold decrease in GSH:GSSG (Figure 6B and Table S5), a significantly increased glutamate:glutamine (Figure 6B and Table S5), a 4-fold increase in Rpe transcripts (Figure 6C and Table S1), and a 3.4-fold increase in the accumulation of upper glycolytic and pentose phosphate pathway metabolite markers (Figure 6D and Table S5). Thus, USg gene sets (Figures 1 and 4) intersected bioinformatically with conserved glutathione/pentose phosphate metabolism gene sets (Figures 1G, 4E, 4F, 5E, and S7) that marked an elevated glutathione redox state in rat USg (Figure 6B).
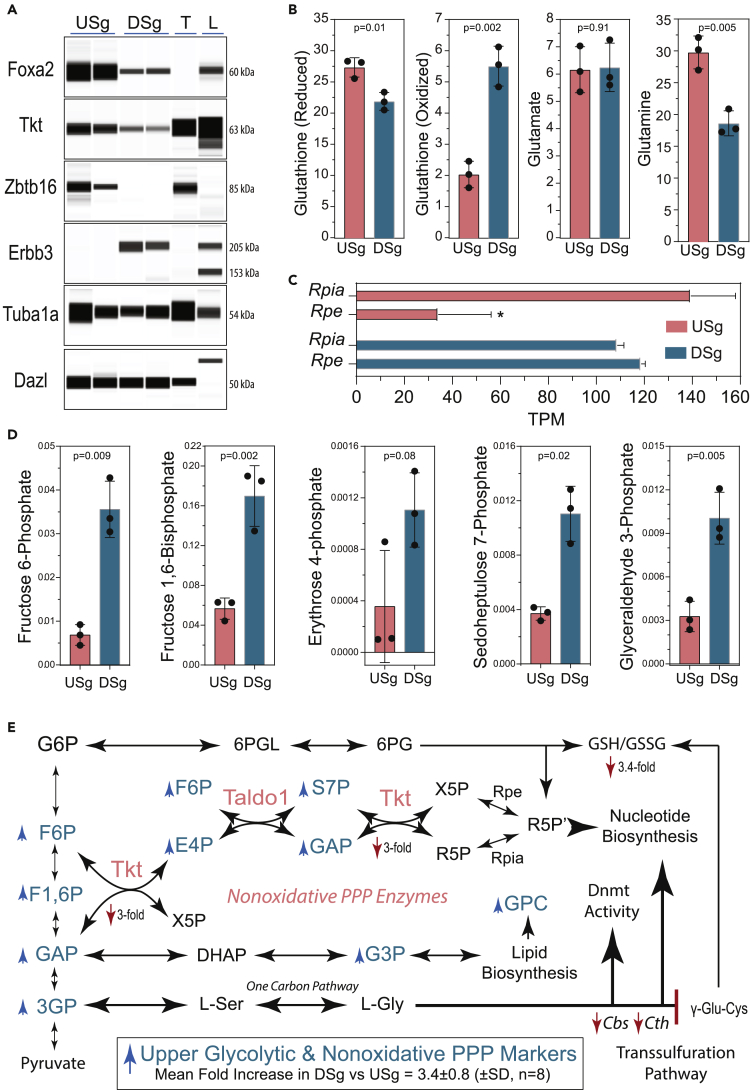
Figure 6.
Accumulation of Upper Glycolytic and Pentose Phosphate Pathway Metabolites during Spermatogonial Differentiation in Culture
(A) Western blot analysis of transketolase in cultures of rat Foxa2+ USg before and after differentiation (DSg) on laminin in SD Medium for 120 h (n = 2 rat spermatogonial lines). T, adult rat testis; L, adult rat liver. Dazl, premeiotic and meiotic pan-germ cell marker; Zbtb16, type A spermatogonia marker; Erbb3, SD Medium-inducible spermatogonial marker; Tuba1a, soma and germ cell marker.
(B) Targeted LC-MS-based metabolite profiling measurements (Table S5) on relative levels of reduced (GSH) and oxidized (GSSG) L-glutathione in USg versus DSg in cultures prepared as described in panel (A) Relative levels of L-Glutamine and L-Glutamate in USg and DSg are shown for comparison (n = 3 replicate cultures/condition).
(C) Abundance of Rpe and Rpia transcripts in rat USg versus DSg (TPM; p < 0.003).
(D) Accumulation of upper glycolytic/pentose phosphate pathway metabolites in USg versus DSg cultures prepared as described in panel A.
(E) Summary of upper glycolytic/pentose phosphate pathway metabolite profiles in USg versus DSg (Table S5), prepared as described in panel A, but processed for targeted LC-MS-based metabolite profiling measurements (Also see Table S5).
Spermatogonia Depend on Extracellularly Supplied Cysteine to Drive Anabolism
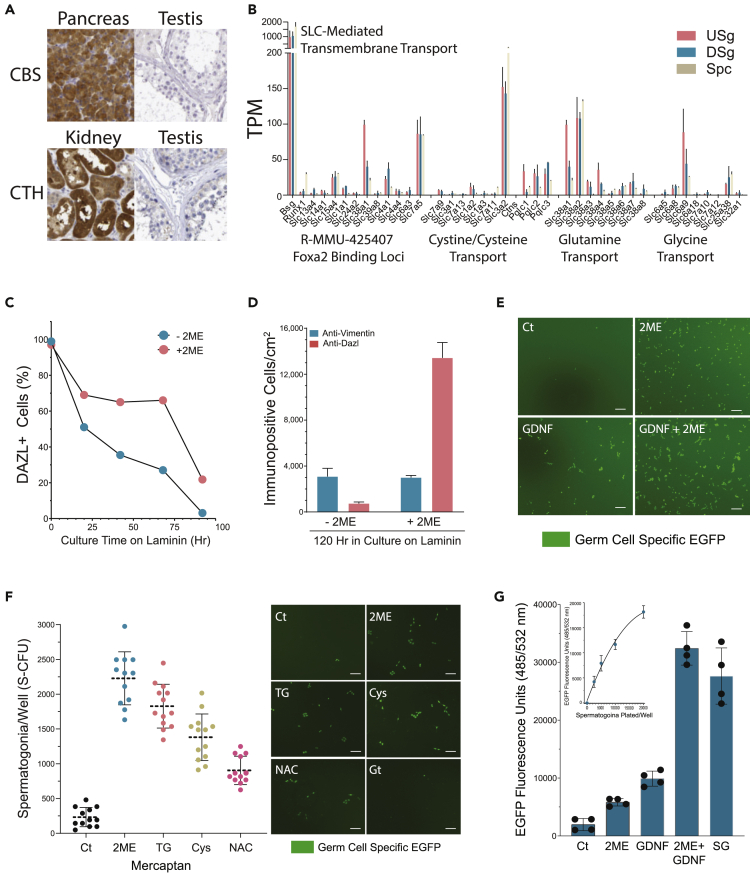
Robust glutathione-linked metabolic enzyme gene profiles in rat spermatogonial lines contrasted with relatively low levels of transcripts encoding cystathionine beta-synthase (Cbs) and cystathionine gamma-lyase (Cth) that generate cysteine intracellularly from homocysteine via the transsulfuration pathway for protein biosynthesis, protein unfolding, and glutathione biosynthesis for cellular redox balance and detoxification (Combs and DeNicola, 2019). Like relatively low Cth and Cbs levels that we observed as being conserved across rat, mouse, monkey, and human (Figures 5F and S7B), relatively low CBS and CTH levels were detected in human spermatogonia when compared with pancreatic and renal epithelia, respectively (Figure 7A; Human Protein Atlas).
Figure 7.
Cysteine-Like Factors Promote Spermatogonial Viability in Culture
(A) Antibody labeling profiles for CBS and CTH in human tissue sections modified from the Human Protein Atlas database (Pontén et al., 2008).
(B) Transcript profiles in rat USg, DSg, and Spc for genes linked to SLC-mediated transmembrane transport Reactome pathway gene set R-RNO-425407.
(C) Dazl+ cells/well scored over time on laminin with and without 2-mercaptoethanol (2ME) supplemented into the Control (Ct) DMEM:Hams F12 (1:1) culture medium containing 10% FBS. Representative of duplicate time course experiments (average values plotted, n = 2 wells/point).
(D) Relative numbers of Dazl+ (germ cell marker) and Vimentin+ (somatic cell marker) laminin-binding testis cells after 120 h in culture on laminin with and without 2ME supplemented into Ct medium. Mean, ±S.E.M. n = 3 wells/condition. Representative of duplicate experiments.
(E) Images of freshly isolated laminin-binding germ cells (tgGCS-Egfp+) after 120 h in culture on laminin without (−2ME) and with (+2ME) 2ME supplemented into Ct medium with (+) and without (−) 10 nM rat GDNF. Representative Images from duplicate experiments. Scale, 100 μm
(F) (Left) Effects of L-cysteine and L-cysteine-like mercaptans on the viability of a rat spermatogonial stem cell line (tgGCS-Egfp+ passage 16) based on spermatogonial colony forming unit (CFU) numbers scored/well after 120 h on laminin in: Ct, Control, or Ct plus 2ME, 50 μM 2-Mercaptoethanol; TG, 50 μM alpha-thioglycerol; Cys, 1 mM L-Cysteine; NAC, 1 mM N-Acetyl-L-Cysteine; Gt, 1 mM L-Glutathione (S.E.M., n = 12 counts/well; representative of duplicate experiments) (Hamra et al., 2007). (Right) Respective images of cultures described for left panel. Scale, 100 μm.
(G) Relative levels of Egfp extracted from tgGCS-Egfp+ spermatogonia (passage 14; 2000/0.96 cm2) after 120 h in culture on laminin with (+2ME) and without (−2ME) 50 μM 2ME supplemented into Ct medium with (+) and without (−) 10 nM rat GDNF. SG Medium (SG). Inset: Fluorescence Units (485/532nm) extracted from cultures 20 h after plating different numbers of tgGCS-Egfp+ germ cells in Ct medium on laminin (R = 0.907). Mean, ±S.E.M. n = 4 wells/condition. Representative of duplicate experiments.
Also see Figure S9.
Given low levels of spermatogonial Cbs and Cth in rodents and primates (Figures 5F and S7B), we formulated the hypothesis that spermatogonia require a mechanism(s) to uptake extracellular cysteine sources to promote germ cell growth and protect the germline from oxidative damage. Interestingly, we noticed Foxa2-binding loci were enriched in the SLC-mediated transmembrane transport gene set (R-MMU-425407) that included the cysteine uptake transporter gene Slc1a1 (Aoyama et al., 2006) (Table S4). Still, analysis of R-MMU-425407 gene profiles in rat USg demonstrated relatively low levels of Slc1a1 (Figure 7B) that did not support Slc1a1 representing a dominant source for cysteine uptake by spermatogonia in culture. Instead, the most abundantly expressed USg genes within R-MMU-425407 encoded Bsg (monocarboxylate transport chaperone), Slc15a4 (lysosomal His export), Slc38a1 (Gln uptake), Slc4a1 (HCO3−/Cl− exchange), and Slc7a5 (Leu/Gln exchange) (Figure 7B).
Analysis of additional cysteine/cystine transport gene profiles across spermatogonia from rats, mice, monkeys, and humans expressed relatively high levels of the Slc3a2 (Figure 7B; Table S2). Slc3a2 (alias CD98) functions in a complex with the Cystine/Glutamate exchanger, Slc7a11 (alias xCT) (Sato et al., 1999, 2005). Slc3a2/Slc7a11-mediated cystine uptake functions to fuel glutathione biosynthesis and buffer cell death by ferroptosis (Stockwell et al., 2017). Similarly, Slc3a2 functions in a complex with the related Leu/Gln exchanger, Slc7a5, that drives leucine uptake and mTor-dependent cell growth (Mastroberardino et al., 1998). Despite rat USg expressing relatively high levels for Slc3a2 and Slc7a5 for Leu uptake, as well as Slc38a1/2 and Slc6a9 for respective glutamine and glycine uptake (Figure 7B), rodent and primate spermatogonia displayed relatively low levels of cystine or cysteine uptake transporters, including low levels of the Slc7a11 in the face of elevated Nfe2l3 (mouse Nrf2) (Figures 7B and Tables S1 and S2) that feed into the Gss-, Gclc-, and Gpx4-dependent inactivation of lipid peroxides, as well as Sod1/Gpx1-dependent inactivation of ROS.
Spermatogonial culture medium is supplemented with glutathione, Sod1, catalase, and 2-mercaptoethanol (2ME) (Wu et al., 2009). Curiously, 2ME is also used to formulate culture media that promotes the growth of cancer and stem cell lines deficient in cystine uptake (Ishii et al., 1981; Ishii and Mann, 2014). Adding 2ME to culture medium generates “mixed disulfide” bonds with oxidized forms of cysteine (e.g. cystine) (Ishii et al., 1981). Cysteine- 2ME “mixed disulfides” are transported through an alternate L-system solute uptake pathway and then reduced intracellularly by Txn and Txnd as a mechanism to generate cysteine within cells (Willis and Schleich, 1995).
Supporting our hypothesis that spermatogonia require a mechanism(s) to uptake extracellular cysteine sources to promote germ cell growth, a standard FBS-containing culture medium effectively promoted survival and syncytial development of freshly isolated rat type A spermatogonia (Figures 7C–7E) and rat spermatogonial stem cell lines (Figures 7F, 7G, and S9A) only after supplementation with cysteine or cysteine-like mercaptans (i.e. 2ME, α-thioglycerol, N-acetyl-L-cysteine). With both freshly isolated and sub-cultured rat USg, we found that GDNF and 2ME synergistically stimulated germ cell viability on laminin and at levels comparable to SG Medium (Figures 7E and 7G). Standard FBS-containing culture medium, therefore, appeared to require importable pro-cysteine-like substrates that counteracted ferroptosis in the germline and effectively fueled GDNF-dependent spermatogonial growth.
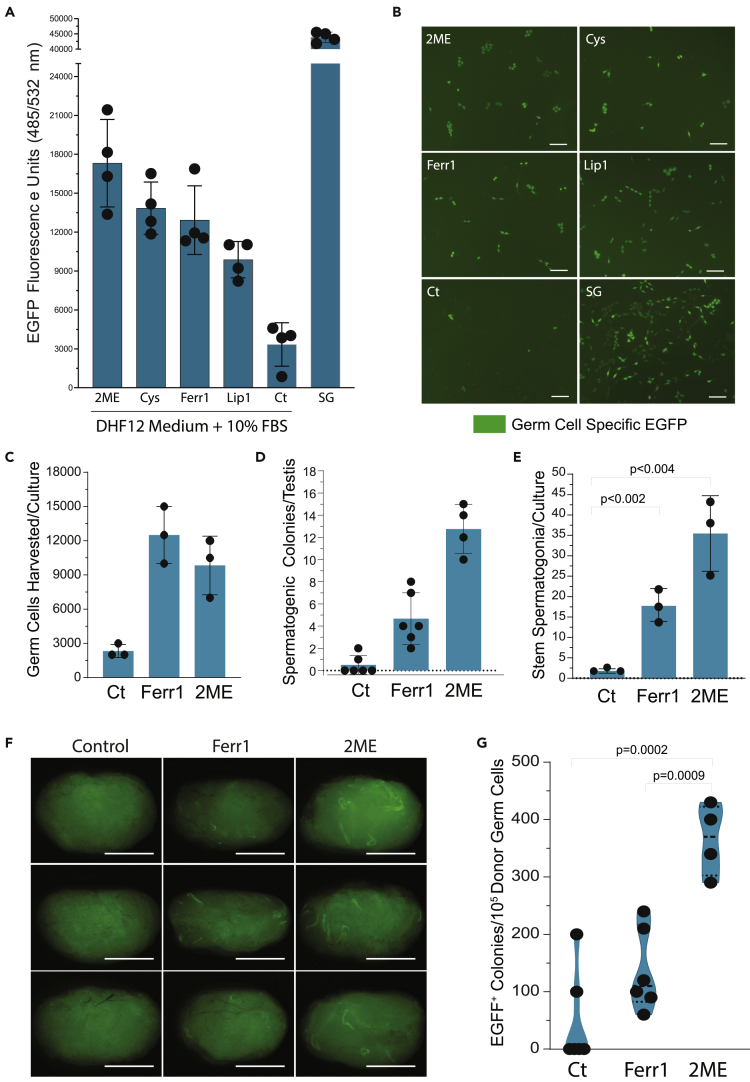
Given poor cystine-dependent viability displayed by undifferentiated spermatogonia (Figures 7C–7E), plus the reported dependency of intracellular glutathione levels on cystine uptake to prevent ferroptosis in absence of cysteine-generating transsulfuration pathways (Zhu et al., 2019), we tested the hypothesis that ferroptosis inhibitors would restore a healthy intracellular redox environment(s) (Schafer and Buettner, 2001) to rat USg in the Ct medium. Supporting this hypothesis, we found ferroptosis inhibitors ferrostatin-1 and liproxstatin-1 (Figures 8A and 8B), and co-culture with rat somatic testis cells (Figure S9B), each effectively promoted spermatogonial survival in cultures lacking L-cysteine or L-cysteine-like mercaptans.
Figure 8.
Cysteine-Like Factors Counteract Spermatogonial Stem Cell Ferroptosis
(A) Relative levels of Egfp extracted from tgGCS-Egfp+ spermatogonia (passage 16; 4000/0.96 cm2) after culture 120 h on laminin in the Control (Ct) 10% FBS-containing culture medium as described in panel 7G with and without 50 μM 2-Mercaptoethanol (2-ME), 1 mM L-Cysteine (Cys), 1 μM Ferristatin-1 (Ferr1), 0.1 μM Liproxstatin-1 (Lip1), or with 100% SG Medium (SG). Mean ±S.E.M. n = 4 wells/condition. Representative of duplicate experiments.
(B) Images of tgGCS-Egfp+ spermatogonial cultures described in panel 8A with and without 50 μM 2ME, 1 mM Cys, 1 μM Ferr1, 0.1 μM Lip1, or with SG Medium. Scale: 100 μm
(C) tgGCS-Egfp+ Germ Cells harvested per culture condition in Ct, Ferr1, or 2ME (average counts plotted/condition, n = 2–4 wells/condition), as described in panel 8B. One hundred twenty hours post-plating, 40,000 tgGCS-Egfp+ spermatogonia/9.6 cm2, n = 3 cultures.
(D) tgGCS-Egfp+ donor-derived spermatogenic colonies that developed by d32 post-transplantation of tgGCS-Egfp+ germ cells into recipient rat testes. Donor germ cells were harvested and transplanted 120 h after plating in each respective Ct, Ferr1, or 2ME medium at 0.5–4x103 cells/testis/rat, as described in panel 8C. Ct, 3 colonies/3,000 cells transplanted (n = 6 rats); Ferr1 = 28 colonies/20,000 cells transplanted (n = 6 rats); 2ME = 51 colonies/14,000 cells transplanted (n = 4 rats).
(E) Numbers of stem spermatogonia harvested per culture calculated by germ cell numbers harvested/culture in panel C x average spermatogonial stem cell concentration/number donor germ cells transplanted/condition (Ct, Ferr1, 2ME) in panel D.
(F) Testes form recipient rats described in panels 8D and 8E illustrating more donor-derived spermatogenesis (EGFP + tubules) generated by cultures with Ferr1 or 2ME in the culture medium versus Ct. Scale, 0.5 cm.
(G) Relative spermatogonial stem cell activity in cultures described in panel 8C based on mean numbers of donor-derived spermatogenic colonies formed/105 EGFP+ germ cells transplanted/testis obtained from study in panel 8D.
Also see Figure S9.
To quantify effects of L-cysteine-generating factors on USg, we measured relative numbers of rat spermatogonial stem cells/culture by in vivo spermatogenesis colony-forming assays (Hamra et al., 2004; Nagano, 2003). Spermatogenesis colony-forming assays were conducted by transplanting donor cultures into rat testes after 5 days on laminin in Ct medium with and without ferrostatin-1 or 2ME (Figures 8C–8G). Compared with Ct medium alone, Ct medium with ferrostatin-1, or Ct medium with 2ME, more effectively promoted spermatogonial stem cell maintenance in culture on laminin, as measured by greater relative numbers of tgGCS-EGFP+ germ cells harvested/culture (Figure 8C), greater tgGCS-EGFP+ donor-derived spermatogenic colonies produced/testis/culture (Figures 8D–8F), and greater relative spermatogonial stem cell activity based on numbers of EGFP+ spermatogenic colonies formed/testis/EGFP+ germ cell transplanted (Figure 8G).
Based on >20 times more total tgGCS-EGFP+ spermatogonial stem cells harvested/culture in medium with ferrostatin or 2ME versus control (p < 0.003; Figure 8E), and an ~7 times higher spermatogonial stem cell concentration/culture using medium with 2ME versus control (p < 0.0002; Figure 8G), rat USg are highly dependent on extracellularly provided sources of L-cysteine and/or pro-cysteine-like factors to balance their intracellular glutathione redox environment(s) (Stockwell et al., 2017).
Discussion
Given the robust regenerative capacity of rat spermatogonial stem cell lines (Chapman et al., 2015b; Wu et al., 2009), we exploited a rat culture system (Hamra, 2017) to analyze the genomic landscape in undifferentiated spermatogonia and compare it with spermatogonia undergoing premeiotic differentiation in vitro on laminin (Figures 1E, 1F, S1D, and S1F). Rat spermatogonial transcriptomes served as a springboard to functionally annotate conserved metabolic processes critical for spermatogonial stem cell viability and growth in vitro (Figure S2). Here, we found that conserved molecular signatures in rodent and primate spermatogonia point to the diversion of glycolytic intermediates into oxidative and non-oxidative pentose phosphate pathways (PPP) (Christodoulou et al., 2019). Diverting metabolism through the PPP in spermatogonia, conceivably, may be more compatible with protecting germlines from metabolic stress-induced cellular damage (Milanese et al., 2019). For example, glycolytic flux through the PPP may promote genomic stability under conditions that are more favorable for preserving DNA integrity during the long term maintenance of male germline stem cells (Helsel et al., 2017a), and during their transition from a slower cell cycle in USg to a faster cell cycle in DSg (Huckins, 1971a, 1971c; Lok et al., 1983).
Here, we further define a spermatogonial stemness gene signature that is conserved across rat, mouse, and human USg (n = 33, Figure 4D) and that includes Dusp6, Gfra1, Etv5, Tkt, Rest, Nanos2, and Farp1 as Foxa2-binding loci in the rat germline (Figures 5A–5D). Like in rat USg (Figure 1G and Table S2), conserved spermatogonial gene sets intersected with glutathione, NADPH, and pentose phosphate metabolism gene sets (Figures 4E and 4F, Table S2). Metabolically, Foxa-family transcription factors regulate responses to changes in nutrient levels in somatic cells across species (Bolukbasi et al., 2017; Carrano et al., 2009; Lantz et al., 2004). In humans, Foxa-family genes upregulate metabolic processes during starvation (Wolfrum et al., 2004), function as genome modifiers repressed by insulin receptor/daf-2 signaling (Puigserver and Rodgers, 2006), and influence fasting glucose levels in humans (Xing et al., 2010).
In mice, spermatogonial stem cells are represented by undifferentiated ID4+, Nanos2+, Gfra1+, Nanos3−, and Neurog3− As spermatogonia (Nakagawa et al., 2007, 2010; Oatley et al., 2011; Sada et al., 2009; Suzuki et al., 2009; Zheng et al., 2009). Here, in rats, we found Foxa2 to selectively mark populations of Gfra1+ and Gfra1− undifferentiated As spermatogonia that were also Snap91+ and Cd9+ (Figures 3B and 3C). In rats, similar to Foxa2, Snap91 transcripts co-purified with spermatogonial stem cells (Hamra et al., 2004, 2005). Accordingly, SNAP91 is localized to cytoplasm in human type A spermatogonia (von Kopylow et al., 2010). In rat seminiferous tubule whole mounts, Snap91 is a cytoplasmic marker enriched on endosome vesicle-like organelles in undifferentiated As and Apr spermatogonia (Gfra1+, Sall4+, Cd9+, Zbtb16+) (Abid et al., 2014) (Figures 3B and 3C). Moreover, CD9 is a cell surface marker enriched on undifferentiated As, Apr, and Aal type A spermatogonia in rats (Gfra1+, Zbtb16+; Figures 3B and 3C) (Abid et al., 2014; Kanatsu-Shinohara et al., 2004). In rats, only approximately 5% of total Snap91+ As spermatogonia were ErbbB3+ (Abid et al., 2014).
Here, the Foxa2+, Gfra1+, Snap91+, Cd9+, and Zbtb16+ As spermatogonia in rats further included the stage VIII-IX-specific Erbb3+ undifferentiated As spermatogonia (Figure S6DE). In contrast to robust Erbb3 labeling on a subset of undifferentiated stage-VIII-IX As spermatogonia, Erbb3 labeling was not detected in other USg or DSg types in vivo (Abid et al., 2014). Erbb3 was previously demonstrated to be essential for development of rat DSg from USg in vitro on laminin in SD Medium supplemented with recombinant Neuregulin-1, however, the Erbb3-deficient germlines effectively regenerated spermatogenesis in recipient testes (Chapman et al., 2015b). Consistent with strict localization of Erbb3 immunolabeling to rat undifferentiated As spermatogonia during late stage VIII and stage IX of spermatogenesis (coincides with the peak in RA accumulation during stages VIII-IX of a mouse seminiferous epithelial cycle (Hogarth et al., 2015)), we demonstrate robust induction of Erbb3 in rat spermatogonia during culture in the RA-containing SD Medium (Table 1, Figures 6A and S1C). In mice, Erbb3 transcripts are >5-fold enriched in GFP-ID4Bright versus GFP-ID4Dim spermatogonia (Helsel et al., 2017b), significantly change in abundance during spermatogonial differentiation (Tan et al., 2020), and total Erbb3 transcript abundance peaks in mouse testes on postnatal ~ d8-11 (Schultz et al., 2003). In humans, ERBB3 was found to be enriched in spermatogonia compared with spermatocyte/spermatid gene sets (Shami et al., 2020) and independently, was identified as being enriched in human early spermatocyte states and Sertoli cells (Young Adult Atlas) (Guo et al., 2018).
Based on their molecular profiles, the Gfra1+ and Gfra1− sub-populations of Foxa2+ As spermatogonia reported here represent undifferentiated As spermatogonia (Figures 3B and 3C) that are specified in parallel with induction of Gfra1 in the rat germline (Dazl+) between postnatal d4 to d8 (Figure 3A). Distinct reports demonstrate that Gfra1+ and Gfra1− testis cell fractions from mice each contain functional spermatogonial stem cells (Buageaw et al., 2005; Garbuzov et al., 2018). And, in mice, a key study reported that germline stem cells in a normal testis are GFRa1+ (Hara et al., 2014). Here, we make the unique observation, in seminiferous tubule whole mounts, that the Foxa2+, Gfra1−, Snap91+, and Cd9+ spermatogonia are exclusively undifferentiated As spermatogonia and therefore, appear to represent a distinct developmental/cellular state compared with the Gfra1+ As, Apr, and Aal spermatogonial populations (Figure 3F). Foxa2+ and Gfra1− As spermatogonia are found during all stages of a seminiferous epithelial cycle (Figure 3E), and so would predominantly not represent the stage VIII-dependent differentiating As spermatogonia (Huckins, 1971c)(Figure S6C). It should be noted that evidence for a primitive Gfra1− “State 0” USg population has been reported in human (Guo et al., 2018) and recently has been proposed to function as reserve germline stem cells (Caldeira-Brant et al., 2020).
Mouse type As spermatogonia develop into nascent syncytia of Aal progenitor spermatogonia characterized as Gfra1+ progenitor spermatogonia (Nakagawa et al., 2010). In mice, Rarg and Gfra1 localization is reported to be essentially mutually exclusive during an epithelial cycle, in that the “differentiation-primed” Aal spermatogonia in the mouse are Gfra1−, Rarg+ (Ikami et al., 2015). Upon Rarg induction, Gfra1 is downregulated in Aal progenitor spermatogonia as they effectively transition toward Kit+, Neruog3, and Rarg1− type A1 differentiating spermatogonia (Gely-Pernot et al., 2012; Ikami et al., 2015). The transient populations of Gfra1− and Rarg+ Aal progenitor spermatogonia are considered primed for differentiation into developmentally competent type A1 spermatogonia in the seminiferous epithelium of adult mice (Gely-Pernot et al., 2012; Ikami et al., 2015). In vivo, RA production peaks at stages VIII-IX and drives the development of Rarg+ progenitor spermatogonia into type A1 spermatogonia (Gely-Pernot et al., 2012). Here, in rat, we found Foxa1 to selectively mark populations of Rarg+, Gfra1+, Foxa2− type Aal spermatogonia (Figures 3E and S3A–S3C), which, based on mouse genetics (Gely-Pernot et al., 2012) and syncytia length in rat (Huckins, 1971c), seem to be primed for differentiation into type A1 spermatogonia (Gfra1−, Foxa1−, Foxa2−, Rarg−) (Figure 3F).
Curiously, transient sharp increases in Foxa1+ spermatogonia concentration along a spermatogenic wave predominantly occurred in peaks that either partially overlapped or that were fully separated by 0.5–2 mm valleys of tubule epithelium without Foxa1+ spermatogonia (1.0 ± 0.81mm/valley, n = 4 twin cohorts; Figure S3A). In a tubule fragment that contained two pairs of Foxa1+ spermatogonia cohorts, the subsegments containing each pair of Foxa1+ cohorts were ~3 cm apart (~1 rat spermatogenic wavelength) (Figure S3A) (Perey et al., 1961). The identification of heterogeneous populations of Foxa1+ and Foxa2+ USg (Gfra+) in the same subsegments of a rat spermatogenic wave provides new molecular markers for mapping the topography of germline stem cell clone development in the rat seminiferous epithelium (Abid et al., 2014).
In human testes, FOXA1, FOXA2, and FOXA3 were not significantly enriched in spermatogonial gene clusters. Still, state 0 and 1 clusters of human USg were significantly enriched in FOXP1, FOXP4, FOXD1, FOXG1, and FOXC2 (Guo et al., 2018). Enrichment for Foxp1 in USg (Figures 4B and 5D), together with enrichment for Foxa2-binding at consensus Foxp1- and Foxo1-binding elements in rat USg (Figure S8A), highlighted the potential for Foxa1 and Foxa2 to function as genetic modifiers at distinct developmental steps in the rat germline by competing for DNA binding in genes regulated by Forkhead Box family transcription factors (Figure S8F) (Goertz et al., 2011).
Based on conserved spermatogonial gene profiles linked to glutathione and pentose phosphate metabolism, and Foxa2's regulatory responses to metabolic stress signals in the soma of worms, flies, rodents, and primates (Panowski et al., 2007; Puigserver and Rodgers, 2006; Wolfrum et al., 2004; Wang et al., 2011), we hypothesize that rat USg coopted Foxa2-mediated genetic interactions to similarly help maintain germline stem cell integrity by counteracting metabolic stress (Wolfrum et al., 2004; Wang et al., 2011). By exploiting a rat culture system, we further report that the conserved germline gene networks enriched in spermatogonia predicted the functional expression of an anti-ferroptotic pro-cysteine-like factor uptake pathway in rat USg that selected against germline stem cell loss in vitro (Figure 8). Because cystine transporters are not abundantly expressed by rat USg in culture (Figures 7B and Tables S1 and S2), and standard eukaryotic cell culture medium contains cystine, but not cysteine, we propose a model analogous to that in L1210 mouse lymphoma cells (Ishii et al., 1981), wherein extracellular conversion of cystine into importable “cysteine-mercaptan disulfides” drives metabolic pathways that promote rat spermatogonial growth in culture.
Limitations of the Study
Comparisons between rodent and primate spermatogonial gene expression and molecular marker profiles in the current study are subject to evaluations being made between primary rat spermatogonial lines following subculture in vitro and more freshly isolated spermatogonia from mouse, monkey, and human testes. Thus, in vitro culture in rat spermatogonia, as well as time taken for testis cell isolation and histological processing protocols for mouse, monkey, and/or human specimens prepared under atmospheric conditions, may impact molecular profiles. The current study demonstrates Foxa2 is uniquely abundant in rat As spermatogonia and that Foxa2 binds to rat spermatogonial stemness genes, that included the gene encoding the non-oxidative pentose phosphate pathway enzyme Transketolase (Tkt) during rat spermatogonial culture in vitro. However, in the current study, a genetic interaction between Foxa2 and spermatogonial stemness genes or the ability of Foxa2 to function as a genetic modifier of germline metabolism by directly or indirectly regulating glutathione or pentose phosphate metabolism gene expression was not analyzed.
Resource Availability
Lead Contact
Further information and requests should be directed to and will be fulfilled by the Lead Contact, F. Kent Hamra (kent.hamra@utsouthwestern.edu).
Materials Availability
Rat spermatogonial stem cell lines are available for sharing from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
The accession number for RNA and DNA sequencing data reported in this paper is NCBI GEO: GSE163302, and is summarized in supplemental data Excel files Tables S1–S5.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by grants from The Eunice Kennedy Shriver National Institute of Child Health and Human Development, R01HD053889; The Office of the Director, R24OD011108; The National Institute on Drug Abuse, U44DA044885; The Department of Obstetrics and Gynecology's Reproductive Endocrinology and Infertility Fellowship Program (RF-21), UT Southwestern Medical Center, Dallas Texas; and GenomeDesigns Laboratroy, LLC., Richardson Texas. Chip Seq and RNA Seq were conducted by The McDermott Center Next Generation Sequencing (NGS) Core at UT Southwestern. Metabolite Profiling was conducted by the Children's Medical Research Institute Metabolomics Facility at UT Southwestern. We thank Audrey H. Nelson, Lauren G. Zacharias, and Hieu S. Vu for their help with experiments and Drs. R. Ann Word, Ralph J. DeBerardinis, and Bradley R. Cairns for their critical comments.
Author Contributions
F.K.H. conceptualized the study. F.K.H., D.P., M.K., X.C., and B.R.C. designed the experiments. D.P., A.P., K.M.C., M.K., A.T.M., A.E.W., and J.C. performed most of the experiments. J.A. helped perform immunofluorescent labeling studies and transplantation assays. P.K. helped to perform protein assays and supporting western blots. F.K.H., D.P., M.K., A.P., A.T.M., A.E.W., J.C., B.R.C., M.K., and X.C. performed data analysis and/or helped prepare figures and tables. F.K.H. and B.R.C. obtained funding and coordinated the study. F.K.H. wrote the paper.
Declaration of Interests
A.P. is an employee of GenomeDesigns Laboratory, LLC., and F.K.H. is a founder and consultant of GenomeDesigns Laboratory, LLC and a member of its scientific advisory board. A provisional patent application has been filled on portions of this work (Inventor: F.K.H.). D.P., M.K., A.T.M., A.E.W., K.M.C., J.C., J.A., P.K., X.C., and B.R.C. declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101880.
Supplemental Information
References
- Abid S.N., Richardson T.E., Powell H.M., Jaichander P., Chaudhary J., Chapman K.M., Hamra F.K. A-single spermatogonia heterogeneity and cell cycles synchronize with rat seminiferous epithelium stages VIII-IX. Biol. Reprod. 2014;90:32. doi: 10.1095/biolreprod.113.113555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K., Suh S.W., Hamby A.M., Liu J., Chan W.Y., Chen Y., Swanson R.A. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Ball R.L., Fujiwara Y., Sun F., Hu J., Hibbs M.A., Handel M.A., Carter G.W. Regulatory complexity revealed by integrated cytological and RNA-seq analyses of meiotic substages in mouse spermatocytes. BMC Genomics. 2016;17:628. doi: 10.1186/s12864-016-2865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkis I.M., Rubins N.E., White P., Furth E.E., Friedman J.R., Kaestner K.H. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat. Med. 2008;14:828–836. doi: 10.1038/nm.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolukbasi E., Khericha M., Regan J.C., Ivanov D.K., Adcott J., Dyson M.C., Nespital T., Thornton J.M., Alic N., Partridge L. Intestinal fork head regulates nutrient absorption and promotes longevity. Cell Rep. 2017;21:641–653. doi: 10.1016/j.celrep.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R.L., Avarbock M.R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. U S A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R.L., Zimmermann J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. U S A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buageaw A., Sukhwani M., Ben-Yehudah A., Ehmcke J., Rawe V.Y., Pholpramool C., Orwig K.E., Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol. Reprod. 2005;73:1011–1016. doi: 10.1095/biolreprod.105.043810. [DOI] [PubMed] [Google Scholar]
- Caldeira-Brant A.L., Martinelli L.M., Marques M.M., Reis A.B., Martello R., Almeida F., Chiarini-Garcia H. A subpopulation of human Adark spermatogonia behaves as the reserve stem cell. Reproduction. 2020;159:437–451. doi: 10.1530/REP-19-0254. [DOI] [PubMed] [Google Scholar]
- Carrano A.C., Liu Z., Dillin A., Hunter T. A conserved ubiquitination pathway determines longevity in response to diet restriction. Nature. 2009;460:396–399. doi: 10.1038/nature08130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K.M., Medrano G.A., Chaudhary J., Hamra F.K. NRG1 and KITL signal downstream of retinoic acid in the germline to support soma-free syncytial growth of differentiating spermatogonia. Cell Death Discov. 2015;1:15018. doi: 10.1038/cddiscovery.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K.M., Medrano G.A., Jaichander P., Chaudhary J., Waits A.E., Nobrega M.A., Hotaling J.M., Ober C., Hamra F.K. Targeted germline modifications in rats using CRISPR/Cas9 and spermatogonial stem cells. Cell Rep. 2015;10:1828–1835. doi: 10.1016/j.celrep.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou D., Kuehne A., Estermann A., Fuhrer T., Lang P., Sauer U. Reserve flux capacity in the pentose phosphate pathway by NADPH binding is conserved across kingdoms. iScience. 2019;19:1133–1144. doi: 10.1016/j.isci.2019.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Combs J.A., DeNicola G.M. The non-essential amino acid cysteine becomes essential for tumor proliferation and survival. Cancers (Basel) 2019;11:678. doi: 10.3390/cancers11050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danshina P.V., Geyer C.B., Dai Q., Goulding E.H., Willis W.D., Kitto G.B., McCarrey J.R., Eddy E.M., O'Brien D.A. Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol. Reprod. 2010;82:136–145. doi: 10.1095/biolreprod.109.079699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij D.G. The nature and dynamics of spermatogonial stem cells. Development. 2017;144:3022–3030. doi: 10.1242/dev.146571. [DOI] [PubMed] [Google Scholar]
- Garbuzov A., Pech M.F., Hasegawa K., Sukhwani M., Zhang R.J., Orwig K.E., Artandi S.E. Purification of GFRalpha1+ and GFRalpha1- spermatogonial stem cells reveals a niche-dependent mechanism for fate determination. Stem Cell Rep. 2018;10:553–567. doi: 10.1016/j.stemcr.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gely-Pernot A., Raverdeau M., Celebi C., Dennefeld C., Feret B., Klopfenstein M., Yoshida S., Ghyselinck N.B., Mark M. Spermatogonia differentiation requires retinoic acid receptor gamma. Endocrinology. 2012;153:438–449. doi: 10.1210/en.2011-1102. [DOI] [PubMed] [Google Scholar]
- Goertz M.J., Wu Z., Gallardo T.D., Hamra F.K., Castrillon D.H. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J. Clin. Invest. 2011;121:3456–3466. doi: 10.1172/JCI57984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Grow E.J., Mlcochova H., Maher G.J., Lindskog C., Nie X., Guo Y., Takei Y., Yun J., Cai L. The adult human testis transcriptional cell atlas. Cell Res. 2018;28:1141–1157. doi: 10.1038/s41422-018-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra F.K. Rattus norvegicus spermatogenesis colony-forming assays. Methods Mol. Biol. 2017;1463:185–203. doi: 10.1007/978-1-4939-4017-2_14. [DOI] [PubMed] [Google Scholar]
- Hamra F.K., Chapman K.M., Nguyen D., Garbers D.L. Identification of neuregulin as a factor required for formation of aligned spermatogonia. J. Biol. Chem. 2007;282:721–730. doi: 10.1074/jbc.M608398200. [DOI] [PubMed] [Google Scholar]
- Hamra F.K., Chapman K.M., Nguyen D.M., Williams-Stephens A.A., Hammer R.E., Garbers D.L. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc. Natl. Acad. Sci. U S A. 2005;102:17430–17435. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra F.K., Gatlin J., Chapman K.M., Grellhesl D.M., Garcia J.V., Hammer R.E., Garbers D.L. Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proc. Natl. Acad. Sci. U S A. 2002;99:14931–14936. doi: 10.1073/pnas.222561399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra F.K., Schultz N., Chapman K.M., Grellhesl D.M., Cronkhite J.T., Hammer R.E., Garbers D.L. Defining the spermatogonial stem cell. Dev. Biol. 2004;269:393–410. doi: 10.1016/j.ydbio.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Hara K., Nakagawa T., Enomoto H., Suzuki M., Yamamoto M., Simons B.D., Yoshida S. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell. 2014;14:658–672. doi: 10.1016/j.stem.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsel A.R., Oatley M.J., Oatley J.M. Glycolysis-optimized conditions enhance maintenance of regenerative integrity in mouse spermatogonial stem cells during long-term culture. Stem Cell Rep. 2017;8:1430–1441. doi: 10.1016/j.stemcr.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsel A.R., Yang Q.E., Oatley M.J., Lord T., Sablitzky F., Oatley J.M. ID4 levels dictate the stem cell state in mouse spermatogonia. Development. 2017;144:624–634. doi: 10.1242/dev.146928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilscher B., Hilscher W., Bulthoff-Ohnolz B., Kramer U., Birke A., Pelzer H., Gauss G. Kinetics of gametogenesis. I. Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res. 1974;154:443–470. doi: 10.1007/BF00219667. [DOI] [PubMed] [Google Scholar]
- Hogarth C.A., Arnold S., Kent T., Mitchell D., Isoherranen N., Griswold M.D. Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production. Biol. Reprod. 2015;92:37. doi: 10.1095/biolreprod.114.126326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins C. Cell cycle properties of differentiating spermatogonia in adult Sprague-Dawley rats. Cell Tissue Kinet. 1971;4:139–154. doi: 10.1111/j.1365-2184.1971.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Huckins C. The spermatogonial stem cell population in adult rats. 3. Evidence for a long-cycling population. Cell Tissue Kinet. 1971;4:335–349. doi: 10.1111/j.1365-2184.1971.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat. Rec. 1971;169:533–557. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- Huckins C. The spermatogonial stem cell population in adult rats. II. A radioautographic analysis of their cell cycle properties. Cell Tissue Kinet. 1971;4:313–334. doi: 10.1111/j.1365-2184.1971.tb01543.x. [DOI] [PubMed] [Google Scholar]
- Ikami K., Tokue M., Sugimoto R., Noda C., Kobayashi S., Hara K., Yoshida S. Hierarchical differentiation competence in response to retinoic acid ensures stem cell maintenance during mouse spermatogenesis. Development. 2015;142:1582–1592. doi: 10.1242/dev.118695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Bannai S., Sugita Y. Mechanism of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro. Role of the mixed disulfide of 2-mercaptoethanol and cysteine. J. Biol. Chem. 1981;256:12387–12392. [PubMed] [Google Scholar]
- Ishii T., Mann G.E. Redox status in mammalian cells and stem cells during culture in vitro: critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol. 2014;2:786–794. doi: 10.1016/j.redox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Ogonuki N., Inoue K., Miki H., Ogura A., Toyokuni S., Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Tanaka T., Ogonuki N., Ogura A., Morimoto H., Cheng P.F., Eisenman R.N., Trumpp A., Shinohara T. Myc/Mycn-mediated glycolysis enhances mouse spermatogonial stem cell self-renewal. Genes Dev. 2016;30:2637–2648. doi: 10.1101/gad.287045.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Toyokuni S., Shinohara T. CD9 is a surface marker on mouse and rat male germline stem cells. Biol. Reprod. 2004;70:70–75. doi: 10.1095/biolreprod.103.020867. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Yamamoto T., Toh H., Kazuki Y., Kazuki K., Imoto J., Ikeo K., Oshima M., Shirahige K., Iwama A. Aging of spermatogonial stem cells by Jnk-mediated glycolysis activation. Proc. Natl. Acad. Sci. U S A. 2019;116:16404–16409. doi: 10.1073/pnas.1904980116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M.L., de Rooij D.G., Page D.C. Amplification of a broad transcriptional program by a common factor triggers the meiotic cell cycle in mice. Elife. 2019;8:e43738. doi: 10.7554/eLife.43738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppula P., Zhang Y., Zhuang L., Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. (Lond) 2018;38:12. doi: 10.1186/s40880-018-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H., Brinster R.L. Technology insight: in vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat. Clin. Pract. Endocrinol. Metab. 2006;2:99–108. doi: 10.1038/ncpendmet0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E.W., Brosens J.J., Gomes A.R., Koo C.Y. Forkhead box proteins: tuning forks for transcriptional harmony. Nat. Rev. Cancer. 2013;13:482–495. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- Lantz K.A., Vatamaniuk M.Z., Brestelli J.E., Friedman J.R., Matschinsky F.M., Kaestner K.H. Foxa2 regulates multiple pathways of insulin secretion. J. Clin. Invest. 2004;114:512–520. doi: 10.1172/JCI21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond C.P., Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N. Y Acad. Sci. 1952;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Lok D., Jansen M.T., de Rooij D.G. Spermatogonial multiplication in the Chinese hamster. II. Cell cycle properties of undifferentiated spermatogonia. Cell Tissue Kinet. 1983;16:19–29. [PubMed] [Google Scholar]
- Mastroberardino L., Spindler B., Pfeiffer R., Skelly P.J., Loffing J., Shoemaker C.B., Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- Miki K., Qu W., Goulding E.H., Willis W.D., Bunch D.O., Strader L.F., Perreault S.D., Eddy E.M., O'Brien D.A. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl. Acad. Sci. U S A. 2004;101:16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanese C., Bombardieri C.R., Sepe S., Barnhoorn S., Payan-Gomez C., Caruso D., Audano M., Pedretti S., Vermeij W.P., Brandt R.M.C. DNA damage and transcription stress cause ATP-mediated redesign of metabolism and potentiation of anti-oxidant buffering. Nat. Commun. 2019;10:4887. doi: 10.1038/s41467-019-12640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan A.P., Kaestner K.H., Grau E., Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- Nagano M.C. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol. Reprod. 2003;69:701–707. doi: 10.1095/biolreprod.103.016352. [DOI] [PubMed] [Google Scholar]
- Nagano M., Avarbock M.R., Leonida E.B., Brinster C.J., Brinster R.L. Culture of mouse spermatogonial stem cells. Tissue Cell. 1998;30:389–397. doi: 10.1016/s0040-8166(98)80053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Nabeshima Y., Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev. Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Sharma M., Nabeshima Y., Braun R.E., Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakberg E.F. Spermatogonial stem-cell renewal in the mouse. Anat. Rec. 1971;169:515–531. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- Oatley M.J., Kaucher A.V., Racicot K.E., Oatley J.M. Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biol. Reprod. 2011;85:347–356. doi: 10.1095/biolreprod.111.091330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odet F., Duan C., Willis W.D., Goulding E.H., Kung A., Eddy E.M., Goldberg E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol. Reprod. 2008;79:26–34. doi: 10.1095/biolreprod.108.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odet F., Gabel S., London R.E., Goldberg E., Eddy E.M. Glycolysis and mitochondrial respiration in mouse LDHC-null sperm. Biol. Reprod. 2013;88:95. doi: 10.1095/biolreprod.113.108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odet F., Gabel S.A., Williams J., London R.E., Goldberg E., Eddy E.M. Lactate dehydrogenase C and energy metabolism in mouse sperm. Biol. Reprod. 2011;85:556–564. doi: 10.1095/biolreprod.111.091546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Dobrinski I., Brinster R.L. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31:461–472. doi: 10.1054/tice.1999.0060. [DOI] [PubMed] [Google Scholar]
- Orwig K.E., Shinohara T., Avarbock M.R., Brinster R.L. Functional analysis of stem cells in the adult rat testis. Biol. Reprod. 2002;66:944–949. doi: 10.1095/biolreprod66.4.944. [DOI] [PubMed] [Google Scholar]
- Panowski S.H., Wolff S., Aguilaniu H., Durieux J., Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- Perey B., Clermont Y., Leblond C.P. The wave of the seminiferous epithelium in the rat. Am. J. Anat. 1961;108:47–78. [Google Scholar]
- Pontén F., Jirström K., Uhlen M. The Human Protein Atlas--a tool for pathology. J. Pathol. 2008;4:387–393. doi: 10.1002/path.2440. [DOI] [PubMed] [Google Scholar]
- Puigserver P., Rodgers J.T. Foxa2, a novel transcriptional regulator of insulin sensitivity. Nat. Med. 2006;12:38–39. doi: 10.1038/nm0106-38. [DOI] [PubMed] [Google Scholar]
- Rato L., Alves M.G., Socorro S., Duarte A.I., Cavaco J.E., Oliveira P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012;9:330–338. doi: 10.1038/nrurol.2012.77. [DOI] [PubMed] [Google Scholar]
- Rausa F.M., Tan Y., Zhou H., Yoo K.W., Stolz D.B., Watkins S.C., Franks R.R., Unterman T.G., Costa R.H. Elevated levels of hepatocyte nuclear factor 3beta in mouse hepatocytes influence expression of genes involved in bile acid and glucose homeostasis. Mol. Cell. Biol. 2000;20:8264–8282. doi: 10.1128/mcb.20.21.8264-8282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada A., Suzuki A., Suzuki H., Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394–1398. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- Sato H., Shiiya A., Kimata M., Maebara K., Tamba M., Sakakura Y., Makino N., Sugiyama F., Yagami K., Moriguchi T. Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 2005;280:37423–37429. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- Sato H., Tamba M., Ishii T., Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Schultz N., Hamra F.K., Garbers D.L. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc. Natl. Acad. Sci. U S A. 2003;100:12201–12206. doi: 10.1073/pnas.1635054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shami A.N., Zheng X., Munyoki S.K., Ma Q., Manske G.L., Green C.D., Sukhwani M., Orwig K.E., Li J.Z., Hammoud S.S. Single-Cell RNA sequencing of human, macaque, and mouse testes uncovers conserved and divergent features of mammalian spermatogenesis. Dev. Cell. 2020;54:529–547 e512. doi: 10.1016/j.devcel.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascon S., Hatzios S.K., Kagan V.E. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Igarashi K., Aisaki K., Kanno J., Saga Y. NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc. Natl. Acad. Sci. U S A. 2010;107:3594–3599. doi: 10.1073/pnas.0908664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Sada A., Yoshida S., Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev. Biol. 2009;336:222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Tan K., Song H.W., Thompson M., Munyoki S., Sukhwani M., Hsieh T.C., Orwig K.E., Wilkinson M.F. Transcriptome profiling reveals signaling conditions dictating human spermatogonia fate in vitro. Proc. Natl. Acad. Sci. U S A. 2020;117:17832–17841. doi: 10.1073/pnas.2000362117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pelt A.M., de Rooij D.G. Retinoic acid is able to reinitiate spermatogenesis in vitamin A-deficient rats and high replicate doses support the full development of spermatogenic cells. Endocrinology. 1991;128:697–704. doi: 10.1210/endo-128-2-697. [DOI] [PubMed] [Google Scholar]
- von Kopylow K., Kirchhoff C., Jezek D., Schulze W., Feig C., Primig M., Steinkraus V., Spiess A.N. Screening for biomarkers of spermatogonia within the human testis: a whole genome approach. Hum Reprod. 2010;25 doi: 10.1093/humrep/deq053. 1104–1102. [DOI] [PubMed] [Google Scholar]
- Wang X., Vatamaniuk M.Z., Roneker C.A., Pepper M.P., Hu L.G., Simmons R.A., Lei X.G. Knockouts of SOD1 and GPX1 exert different impacts on murine islet function and pancreatic integrity. Antioxid. Redox Signal. 2011;14:391–401. doi: 10.1089/ars.2010.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D.C., Ruiz i Altaba A., Chen W.S., Hoodless P., Prezioso V.R., Jessell T.M., Darnell J.E., Jr. The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575–588. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Willis J.A., Schleich T. 13C-NMR spectroscopic studies of 2-mercaptoethanol-stimulated glutathione synthesis in the intact ocular lens. Biochim. Biophys. Acta. 1995;1265:1–7. doi: 10.1016/0167-4889(94)00195-k. [DOI] [PubMed] [Google Scholar]
- Wolfrum C., Asilmaz E., Luca E., Friedman J.M., Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- Wu Z., Falciatori I., Molyneux L.A., Richardson T.E., Chapman K.M., Hamra F.K. Spermatogonial culture medium: an effective and efficient nutrient mixture for culturing rat spermatogonial stem cells. Biol. Reprod. 2009;81:77–86. doi: 10.1095/biolreprod.108.072645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C., Cohen J.C., Boerwinkle E. A weighted false discovery rate control procedure reveals alleles at FOXA2 that influence fasting glucose levels. Am. J. Hum. Genet. 2010;86:440–446. doi: 10.1016/j.ajhg.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K., Wu X., Kaestner K.H., Wang P.J. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev. Biol. 2009;9:38. doi: 10.1186/1471-213X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Berisa M., Schworer S., Qin W., Cross J.R., Thompson C.B. Transsulfuration activity can support cell growth upon extracellular cysteine limitation. Cell Metab. 2019;30:865–876.e5. doi: 10.1016/j.cmet.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for RNA and DNA sequencing data reported in this paper is NCBI GEO: GSE163302, and is summarized in supplemental data Excel files Tables S1–S5.