Abstract
Virtual surgical planning (VSP) is becoming more widely used in maxillofacial reconstruction and can be surgeon-based or industry-based. Surgeon-based models require software training but allow surgeon autonomy. We evaluate the learning curve for VSP through a prospective cohort study in which planning times and accuracy of 7 otolaryngology residents with no prior VSP experience were compared to that of a proficient user after a single training protocol and 6 planning sessions for orbital fractures. The average planning time for the first session was 21 minutes 41 seconds ± 6 minutes 11 seconds with an average maximum deviation of 2.5 ± 0.8 mm in the lateral orbit and 2.3 ± 0.6 mm in the superior orbit. The average planning time for the last session was 13 minutes 5 seconds ± 10 minutes and 7 seconds with an average maximum deviation of 1.4 ± 0.5 mm in the lateral orbit and 1.3 ± 0.4 mm in the superior orbit. Novice users reduced planning time by 40% and decreased maximum deviation of plans by 44% and 43% in the lateral and superior orbits, respectively, approaching that of the proficient user. Virtual surgical planning has a quick learning curve and may be incorporated into surgical training.
Keywords: virtual surgical planning, computer aided surgery, surgical training, maxillofacial trauma, orbital fractures
Introduction
Computer-aided surgery (CAS) is gaining greater acceptance in maxillofacial reconstruction. Computer-aided surgery can be divided into 3 steps: (1) planning: also known as virtual surgical planning (VSP), (2) execution: carrying out the surgical plan (ie, models, guides, and patient specific implants), and (3) verification: confirming that the proposed plan has been achieved (ie, intraoperative navigation or computed tomography [CT]). This study focuses on VSP; the technique of using detailed CT imaging and planning software to generate a preoperative plan in a virtual environment.1–3 Virtual surgical planning modalities may be surgeon-based (ie, performed by the surgeon) or industry-based (ie, performed by the surgeon and an engineer via teleconferencing). While the surgeon-based model requires an upfront financial investment in planning software and surgeon training, it allows the individual surgeon to autonomously generate the plan. The industry-based model does not require a financial investment or surgeon training. However, engineer costs, prolonged planning time, and diminished surgeon input are limitations in this model.4–6 The industry-based model has gained a strong foothold, however as planning software becomes more user friendly, it is likely that surgeon-based workflows will become more common and gain greater acceptance.6 The aim of this study is to evaluate the learning curve for novice VSP software users. The authors hypothesize that VSP software can be learned quickly and effectively without the need for extensive training, making it a feasible tool to incorporate as part of resident surgical training.
Materials and Methods
Study Design
Institutional review board approval was obtained for this prospective study. Study participants included 7 otolaryngology residents (2 postgraduate year 1 [PGY-1], 3 PGY-2, 1 PGY-3, 1 PGY-5) at a single academic institution. The participants had no experience with VSP. The study was broken into 2 parts, a single training protocol where participants were educated on the use of the VSP tool (iPlan—Brainlab AG) and 6 planning sessions where the participants’ virtual surgical plans were evaluated for duration and accuracy. Only the planning sessions were used for data collection/analysis. The senior author (EBS), having previously created >500 plans, acted as a proficient user/control.
Training protocol
The training protocol was developed to educate inexperienced participants in a uniform fashion. It was completed individually and proctored by the same investigator (TYH). Each participant went through the training protocol at their own pace (range 30-60 minutes). The training protocol was broken into 2 parts: user interface training and case training.
User interface training
The user interface training involved viewing an online training manual and basic interaction with the user interface options on a workstation.
Case training
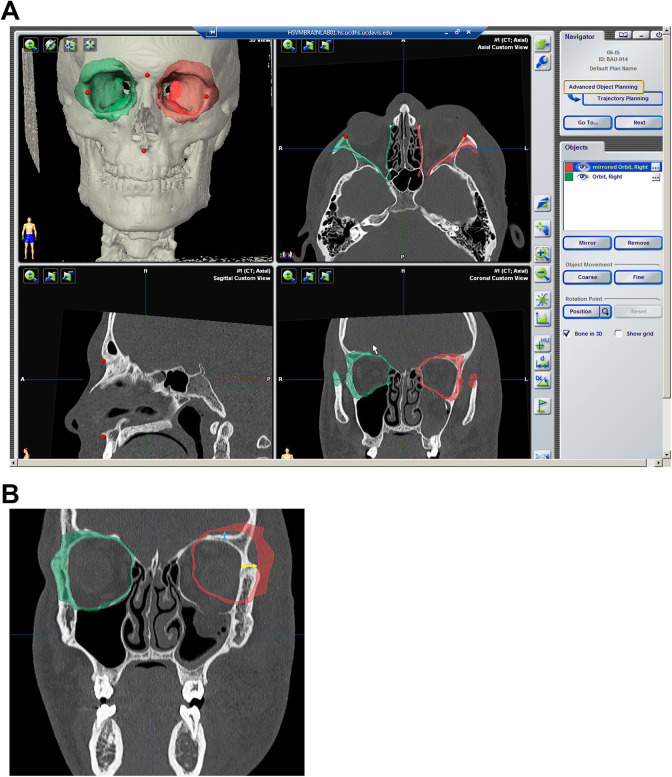
Case training involved planning a single orbital reconstruction. Four tasks were included: (1) placement of fiducial markers to be used for intraoperative registration, (2) segmentation of the uninjured right orbit, (3) mirroring of the uninjured right orbit across the midline plane, and (4) precise alignment of the uninjured mirrored orbit onto the contralateral injured orbit (Figure 1A).
Figure 1.
A, Example user interface for VSP demonstrating segmented unaffected orbit on the right and the mirrored orbit plan superimposed on the left fractured side. The red markers are fiducial points for intraoperative navigation. B, Coronal view: measurement of maximum deviation of mirrored orbit plan from bone superior (blue double-ended arrow) and lateral (yellow double-ended arrow) orbit. VSP indicates virtual surgical planning.
Planning sessions
Each participant independently completed 6 planning sessions. Each planning session required the participant to complete the same 4 tasks demonstrated in the training protocol. One case was provided to each participant every other day. Each case represented a similar but unique orbital fracture amenable to VSP. The order, setting, and timing of presentation were uniform and completed over an 11-day period. Recorded data points included (1) total time of each planning session, (2) accuracy of the mirroring task, and (3) the number of times each participant referred to the training manual per session. Accuracy was determined by comparing the largest deviation (in mm) of the mirrored orbit to the injured orbit. The measurements were taken along the orbital roof and lateral orbital wall, where there were no fractures (Figure 1B). These measurements were performed independently and averaged between 2 authors (TYH and RD) who were blinded to the participant and trial number.
Data Analysis
Ordinary least squares regression analysis with exponential fitting using RStudio (RStudio) was used for data analysis. To assess for proficiency and accuracy in the learning curve of VSP, the participants’ planning times and maximum deviation in the orbital roof and lateral orbital wall were compared with a proficient user’s data (EBS). A P value less than .05 was considered statistically significant. The data were plotted using Microsoft Excel.
Results
All participants completed the entire 6 case series. None of the participants referred to the BrainLab user manual during their planning session. The average planning time for the first case was 21 minutes 41 seconds ± 6 minutes 11 seconds with an average maximum deviation of 2.5 ± 0.8 mm in the lateral orbit and 2.3 ± 0.6 mm in the superior orbit (Table 1 and Figures 2 –4). With the completion of subsequent planning cases, regression analysis showed an overall downward trend in the planning time (P = .0037, R 2 = 0.19; Figure 2) and in the maximum deviation in the lateral orbit (P = .0149, R 2 = 0.14; Figure 3) and the superior orbit (P = .0301, R 2 = 0.11; Figure 4). The average planning time for the last case was 13 minutes 5 seconds ± 10 minutes and 7 seconds with an average maximum deviation of 1.4 ± 0.5 mm in the lateral orbit and 1.3 ± 0.4 mm in the superior orbit (Table 1 and Figures 2 –4). Overall, there was a 40% decrease in total planning time with a decrease of 44% and 43% in the maximum deviation of plans in the lateral and superior orbits, respectively.
Table 1.
The Planning Time and Maximum Deviation in the Lateral and Superior Orbits in Plans Created by Each Novice User Throughout the Study.
| Trial number | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Participant 1 | Superior deviation, mm | 3.1 | 2.3 | 2.1 | 4 | 2.7 | 2 |
| Lateral deviation, mm | 2.3 | 2.1 | 3.4 | 3 | 1.2 | 1.4 | |
| Planning time, seconds | 1517 | 1261 | 1153 | 889 | 572 | 554 | |
| Participant 2 | Superior deviation, mm | 2.9 | 2.3 | 2.6 | 2.4 | 2.4 | 1.4 |
| Lateral deviation, mm | 3.4 | 2.5 | 2.4 | 2.4 | 3.7 | 1.6 | |
| Planning time, seconds | 1671 | 1285 | 1295 | 1203 | 1738 | 2008 | |
| Participant 3 | Superior deviation, mm | 1.6 | 1.2 | 1.4 | 2.3 | 1.6 | 0.8 |
| Lateral deviation, mm | 1.5 | 1.1 | 1.1 | 0.8 | 0.2 | 0.6 | |
| Planning time, seconds | 1740 | 1228 | 389 | 534 | 371 | 383 | |
| Participant 4 | Superior deviation, mm | 2.4 | 2.6 | 1.8 | 1.6 | 1.3 | 1.6 |
| Lateral deviation, mm | 2.5 | 3.2 | 1.6 | 1.2 | 1.3 | 1.2 | |
| Planning time, seconds | 1198 | 662 | 567 | 589 | 410 | 468 | |
| Participant 5 | Superior deviation, mm | 1.6 | 1 | 1.7 | 2 | 1.2 | 1.5 |
| Lateral deviation, mm | 2.6 | 1.3 | 2.2 | 2.3 | 2 | 2 | |
| Planning time, seconds | 679 | 572 | 521 | 521 | 655 | 621 | |
| Participant 6 | Superior deviation, mm | 1.8 | 1.7 | 1.6 | 1 | 1.8 | 1.1 |
| Lateral deviation, mm | 1.6 | 1.3 | 2 | 1.2 | 1.4 | 2 | |
| Planning time, seconds | 1213 | 1061 | 662 | 755 | 565 | 775 | |
| Participant 7 | Superior deviation, mm | 2.4 | 1.2 | 1.2 | 0.8 | 1.4 | 1 |
| Lateral deviation, mm | 3.4 | 1.4 | 0.8 | 0.6 | 1.4 | 0.8 | |
| Planning time, seconds | 1092 | 807 | 830 | 740 | 1057 | 692 | |
| Average | Superior deviation ± SD, mm | 2.3 ± 0.6 | 1.8 ± 0.6 | 1.8 ± 0.5 | 2.0 ± 1.0 | 1.8 ± 0.6 | 1.3 ± 0.4 |
| Lateral deviation ± SD, mm | 2.5 ± 0.8 | 1.8 ± 0.8 | 1.9 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 1.0 | 1.4 ± 0.5 | |
| Planning time ± SD, seconds | 1301 ± 371 | 982 ± 299 | 774 ± 338 | 747 ± 241 | 767 ± 483 | 786 ± 554 |
Abbreviation: SD, standard deviation.
Figure 2.
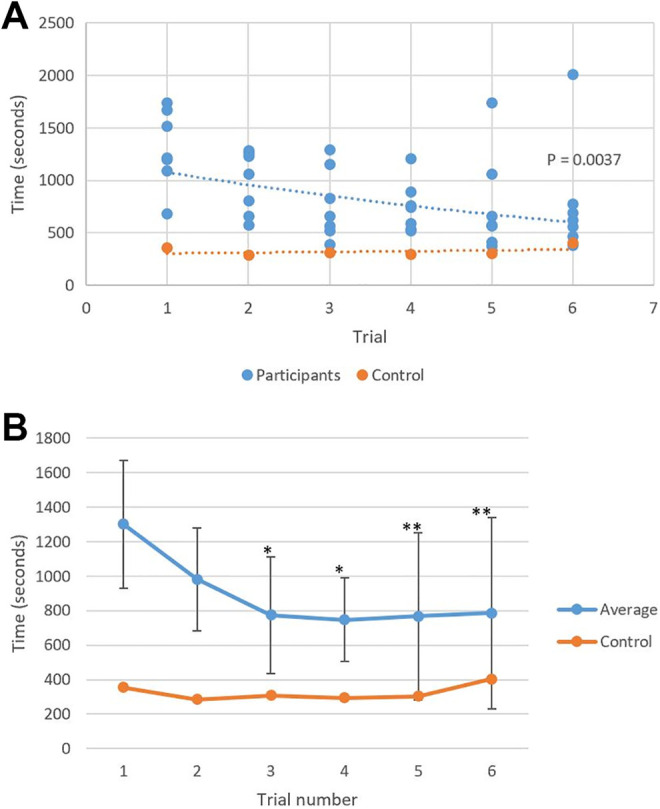
A, Exponential fitted regression analysis demonstrated a downward trend in the planning time with subsequent trials. Control: y = 298.04e 0.0224x. Participants: y = 1204.2e −0.114x. R 2 = 0.1925. P = .0037. B, The novice users’ average planning times approached a proficient user’s planning times (control). *Within 2 standard deviations; **within 1 standard deviation.
Figure 3.
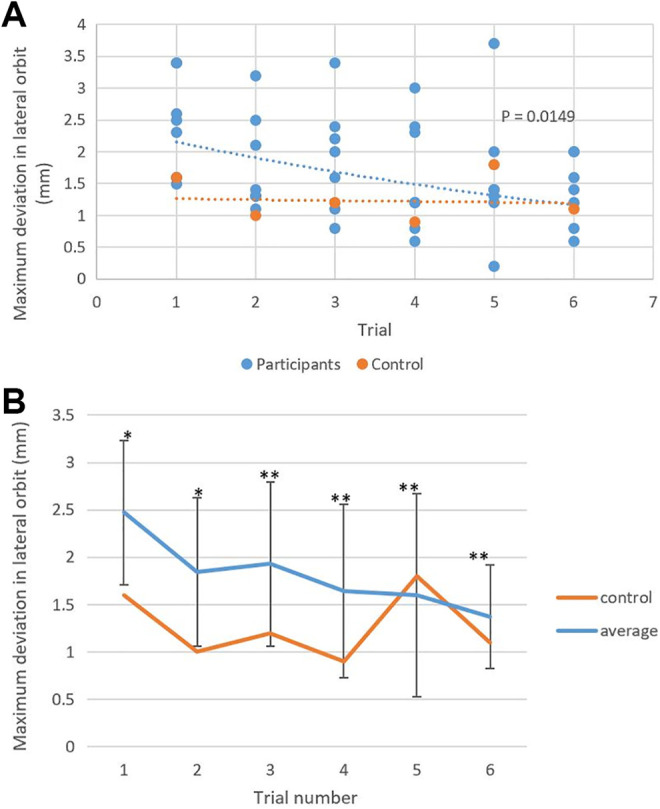
A, A scatter plot and exponential fitted regression analysis showed improvement in the maximum deviation in the lateral orbit in preoperative plans for orbital fractures with successive trials. Control: y = 1.2774e 0.011x. Participants: y = 2.4626e − 0.127x. R2 = 0.1393. P = .0149. B, There was no significant difference in the novice users’ average maximum deviation in the lateral orbit compared to the proficient user’s measurements for all the trials. *Within 2 standard deviations; **within 1 standard deviation.
Figure 4.
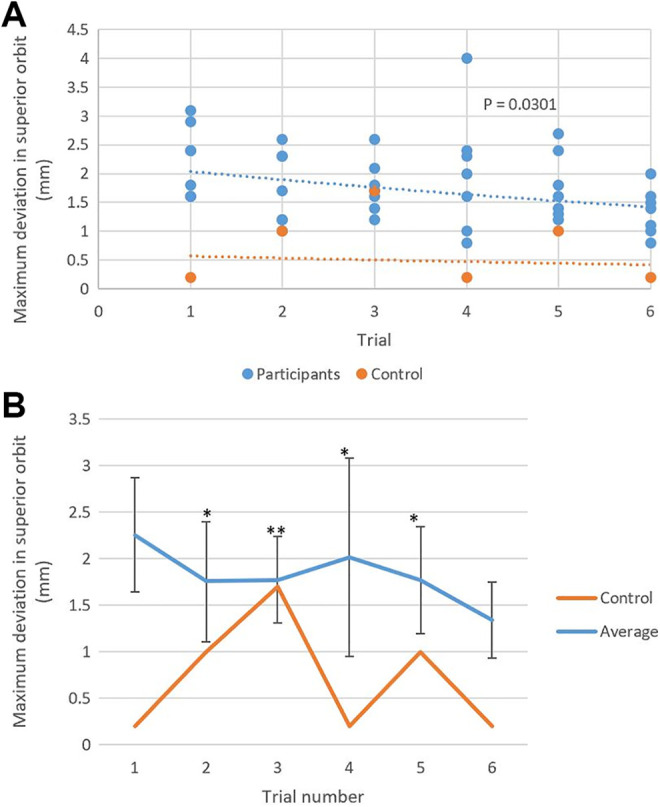
A, Exponential fitted regression analysis of the maximum deviation in the superior orbit in virtual preoperative plans decreased as the number of created plans by each novice user increased. Control: y = 0.6052e −0.061x. Participants: y = 2.2291e −0.08x. R 2 = 0.1122. P = .0301. B, There was variation in the novice users’ average maximum deviation in the superior orbit compared to the proficient user’s data. *Within 2 standard deviations; **within 1 standard deviation.
Discussion
Virtual surgical planning is an evolving tool in maxillofacial reconstruction that facilitates surgical repair and may optimize patient outcomes.7–9 Bly et al demonstrated that mirror image overlay in unilateral orbital fractures had a 5-fold decrease in revision surgeries and a 2-fold improvement in postoperative diplopia.7 The use of intraoperative surgical planning has been proposed to improve intraoperative accuracy and reduce the incidence of revision procedures.8 While this technology continues to grow, there is very little known about the learning curve for use of these tools. This study evaluated the learning curve for the use of iPlan software in orbital reconstruction by novice users.
The results of this study demonstrated a marked reduction in planning time from 21 minutes 41 seconds (± 6 minutes 11 seconds) to 13 minutes 5 seconds (±10 minutes and 7 seconds) after completion of 6 cases; a 40% reduction in time. Accuracy improved from 2.5 ± 0.8 mm to 1.4 ± 0.5 mm (lateral orbit) and 2.3 ± 0.6 mm to 1.3 ± 0.4 mm (superior orbit) after 6 cases; resulting in a 44% and 43% reduction, respectively. The average participant planning times and the maximum deviations in the lateral and superior orbits began to approach those of a proficient user (Figures 2 –4). It was noted that lateral orbit wall accuracy was improved more than the superior orbit. This difference may be reflective of the learning curve required to recognize facial asymmetry and geometric variability between the 2 orbits.10 Given the rapid improvements in total time and accuracy, as well as regression analysis with P values <.05, we conclude that the data demonstrate both statistically significant decreases and practical improvements in planning time and planning accuracy. While improvement is expected with repetition, our results suggest that novice users can approach the proficiency of a competent user after a relatively short timeframe with only 1 training session and 6 planning sessions. Following the study, participating residents were ultimately able to independently use VSP software to develop preoperative surgical plans.
A potential limitation with VSP is cost. However, Rodby et al demonstrated that VSP can decrease operative times while improving the accuracy of reconstruction; thus the overall cost savings may offset the technological cost.11 Resnick et al reported that VSP can significantly decrease planning time and overall planning cost associated with orthognathic surgery.12 Finally, Mendez et al demonstrated that surgeon-based VSP could potentially eliminate the costs and time associated with industry-based VSP.13
Limitations of this study include (1) use of a single medical center, (2) small sample size—likely resulting in low R2 values which may influence the fit of the data in the regression analysis, and (3) use of single VSP software.
Conclusion
There is a quick learning curve for VSP with iPlan software. After 1 training session and the completion of 6 cases, novice users reduced their planning time by 40% (from 21 minutes 41 seconds to 13 minutes 5 seconds). Accuracy was improved by 44% for the lateral orbit (2.5 ± 0.8 mm to 1.4 ± 0.5 mm) and 43% for the superior orbit (2.3 ± 0.6 mm to 1.3 ± 0.4 mm). Novice users were able to approach the proficiency of a competent user relatively rapidly, suggesting VSP software and application may be a valuable addition to resident surgical training.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tsung-yen Hsieh, MD  https://orcid.org/0000-0003-3883-8397
https://orcid.org/0000-0003-3883-8397
References
- 1. Essig H, Dressel L, Rana M, et al. Precision of posttraumatic primary orbital reconstruction using individually bent titanium mesh with and without navigation: a retrospective study. Head Face Med. 2013;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strong EB, Fuller SC, Wiley DF, Zumbansen J, Wilson MD, Metzger MC. Preformed vs intraoperative bending of titanium mesh for orbital reconstruction. Otolaryngol Head Neck Surg. 2013;149(1):60–66. [DOI] [PubMed] [Google Scholar]
- 3. Bell RB, Markiewicz MR. Computer-assisted planning, stereolithographic modeling, and intraoperative navigation for complex orbital reconstruction: a descriptive study in a preliminary cohort. J Oral Maxillofac Surg. 2009;67(12):2559–2570. [DOI] [PubMed] [Google Scholar]
- 4. Kirke DN, Owen RP, Carrao V, Miles BA, Kass JI. Using 3D computer planning for complex reconstruction of mandibular defects. Cancers Head Neck. 2016;1(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zweifel DF, Simon C, Hoarau R, Pasche P, Broome M. Are virtual planning and guided surgery for head and neck reconstruction economically viable? J Oral Maxillofac Surg. 2015;73(1):170–175. [DOI] [PubMed] [Google Scholar]
- 6. Efanov JI, Roy AA, Huang KN, Borsuk DE. Virtual surgical planning: the pearls and pitfalls. Plast Reconstr Surg Glob Open. 2018;6(1):e1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bly RA, Chang SH, Cudejkova M, Liu JJ, Moe KS. Computer-guided orbital reconstruction to improve outcomes. JAMA Facial Plast Surg. 2013;15(2):113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blumer M, Gander T, Kruse Gujer A, Seifert B, Rucker M, Lubbers HT. Influence of mirrored computed tomograms on decision-making for revising surgically treated orbital floor fractures. J Oral Maxillofac Surg. 2015;73(10):1982.e1981–1989. [DOI] [PubMed] [Google Scholar]
- 9. Day KM, Phillips PM, Sargent LA. Correction of a posttraumatic orbital deformity using three-dimensional modeling, virtual surgical planning with computer-assisted design, and three-dimensional printing of custom implants. Craniomaxillofac Trauma Reconstr. 2018;11(1):78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vehmeijer M, van Eijnatten M, Liberton N, Wolff J. A novel method of Orbtial floor reconstruction using virtual planning, 3-dimensional printing, and autologous bone. J Oral Maxillofac Surg. 2016;74(8):1608–1612. [DOI] [PubMed] [Google Scholar]
- 11. Rodby KA, Turin S, Jacobs RJ, et al. Advances in oncologic head and neck reconstruction: systematic review and future considerations of virtual surgical planning and computer aided design/computer aided modeling. J Plast Reconstr Aesthet Surg. 2014;67(9):1171–1185. [DOI] [PubMed] [Google Scholar]
- 12. Resnick CM, Inverso G, Wrzosek M, Padwa BL, Kaban LB, Peacock ZS. Is there a difference in cost between standard and virtual surgical planning for orthognathic surgery? J Oral Maxillofac Surg. 2016;74(9):1827–1833. [DOI] [PubMed] [Google Scholar]
- 13. Mendez BM, Chiodo MV, Patel PA. Customized “In-Office” three-dimensional printing for virtual surgical planning in craniofacial surgery. J Craniofac Surg. 2015;26(5):1584–1586. [DOI] [PubMed] [Google Scholar]