Abstract
Previous studies demonstrated that mitochondrial fission arguments the stemness of bone marrow-derived mesenchymal stem cells (BMSCs). Because mitophagy is critical in removing damaged or surplus mitochondrial fragments and maintaining mitochondrial integrity, the present study was undertaken to test the hypothesis that mitophagy is involved in mitochondrial fission-enhanced stemness of BMSCs. Primary cultures of rat BMSCs were treated with tyrphostin A9 (TA9, a potent inducer of mitochondrial fission) to increase mitochondrial fission, which was accompanied by enhanced mitophagy as defined by increased co-staining of MitoTracker Green for mitochondria and LysoTracker Deep Red for lysosomes, as well as the increased co-localization of autophagy markers (LC3B, P62) and mitochondrial marker (Tom20). A mitochondrial uncoupler, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) was used to promote mitophagy, which was confirmed by an increased co-localization of mitochondrial and lysosome biomarkers. The argumentation of mitophagy was associated with enhanced stemness of BMSCs as defined by increased expression of stemness markers Oct4 and Sox2, and enhanced induction of BMSCs to adipocytes or osteocytes. Conversely, transfection of BMSCs with siRNA targeting mitophagy-essential genes Pink1/Prkn led to diminished stemness of the stem cells, as defined by depressed stemness markers. Importantly, concomitant promotion of mitochondrial fission and inhibition of mitophagy suppressed the stemness of BMSCs. These results thus demonstrate that mitophagy is critically involved in mitochondrial fission promotion of the stemness of BMSCs.
Keywords: Bone marrow-derived mesenchymal stem cells, stem cell stemness, mitophagy, mitochondrial fission
Impact statement
How to maintain the stemness of bone marrow-derived mesenchymal stem cells (BMSCs) in the culture system is a long-standing question. The present research revealed that mitophagy is critically involved in mitochondrial fission promotion of the stemness of BMSCs. This finding thus provides a critical insight into stem cell propagation in cultures for their clinical application since maintenance of the stemness of stem cells is among the most important issues in stem cells and regenerative medicine. Therefore, manipulation of mitochondrial dynamics in the preparation of stem cells for clinical application would be an important undertaking for both experimental and clinical studies.
Introduction
Bone marrow-derived mesenchymal stem cells (BMSCs) are adult stem cells that can proliferate and differentiate into mature stromal cells, such as osteocytes,1 neurocytes, and cardiomyocytes.2,3 Studies have shown that BMSCs participate in bone repairing, neurogenesis, and myogenesis under given pathological conditions either by differentiating into functional cells or through paracrine signaling.4–7 A number of animal studies have confirmed BMSCs transplantation is a safe and efficient method to promote tissue repair.6 Over the last decade, BMSCs have been used in many clinical trials for the treatment of diverse diseases, such as nonunion,8 stroke,9 and cardiac infarct,10 showing good prospects for clinical applications.
The expansion of BMSCs is necessary for their clinical applications, since they are rare in bone marrow (BM).11 Nevertheless, BMSCs that cultured in vitro are prone to differentiate spontaneously.12–14 The reduction of the stemness of stem cells not only reduces the effectiveness of stem cell-based cell therapies, but also presents a new challenge in the safety of their use. Therefore, maintaining the stemness of BMSCs is of great significance for their clinical applications. In our previous study, we found mitochondrial fission, a process refers to the breakage of a single mitochondrion into multiple mitochondria, was crucial in maintaining the stemness of BMSCs.15 The underlying mechanisms are worth to be explored.
Mitophagy is an autophagic process by which impaired or surplus mitochondria are engulfed in autophagosomes and then degraded in lysosomes. This process is important in controlling mitochondrial numbers as well as mitochondrial quality.16,17 Studies in adult cardiomyocytes and HeLa cells have showed that mitochondrial fission promotes the translocation of Parkin to impaired mitochondria to initiate mitophagy, and inhibition of DRP1-mediated mitochondrial fission prevents mitophagy.18,19 Some studies have found that mitophagy-driven mitochondrial rejuvenation is closely related to the regulation of the fate of stem cells. For example, mitophagy in BMSCs was shown to play a vital role in protecting cells against the harshness of oxidative stress and irradiation, thus increasing the viability of stem cells.20–22 In addition, mitophagy was significantly enhanced during cell reprogramming into iPSCs.23,24 With inhibition of mitophagy, the adipogenic differentiation of MSCs was abolished.25 Here, we propose that mitophagy is involved in maintenance of the stemness of BMSCs.
The present study was undertaken to specifically test the hypothesis above. Our results showed that mitochondrial fission promoted mitophagy in BMSCs, and enhanced mitophagy help maintain the stemness of the BMSCs. The inhibition of mitophagy abolished mitochondrial fission maintained stemness of BMSCs. Thus, mitophagy is important in the maintenance of the stemness of BMSCs.
Materials and methods
Cell culture and treatment
BMSCs were obtained from four- to five-day-old Sprague–Dawley (SD) rats and isolated as described previously.15 In brief, after isolating the tibia and femur from the hind limbs of rats carefully, the cells in bone marrow were flushed using a 1 mL syringe containing Dulbecco’s modified Eagle’s medium-low glucose (DMEM-L) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (Natocor, AR). Cells were then seeded on plastic wells or flasks and cultured in the cell incubator (5% CO2, 37°C). Media was replaced two or three times a week. When cell colonies grew to 70%–80% confluency, cells were rinsed with phosphate-buffered saline (PBS) and then treated with 0.25% trypsin (Gibco, USA) (Sigma-Aldrich, USA). Afterwards, cells were resuspended in 10% FBS-supplemented media and 5–7 × 105 cells were plated to 25 cm2 flask. Only the third passage was used for further experiments. To promote mitochondrial fission, 1 μM TA9 (Sigma-Aldrich, USA) was added into the media for 24 h. To determine the effects of mitophagy on the stemness of BMSCs, cells were treated with 1 μM FCCP (Sigma-Aldrich, USA) for 4 h to promote mitophagy. Furthermore, for exploration of the role of mitophagy in regulating mitochondrial fission enhanced stemness, 50 nM Pink1 siRNA or Prkn siRNA was transfected into the cells to inhibit mitophagy.
Cell infection
Pink1 siRNA or Prkn siRNA was transfected into the cultured cells with jetPRIME (Invitrogen, USA) according to the manufacturer’s instructions. A mismatched siRNA (mm siRNA) was used as a negative control. All siRNA were purchased from RUIBO. Cells were used for further studies after transfection for 48 h.
Immunofluorescence staining
When BMSCs reached a confluence around 70% on coverslips, cells were washed with PBS, fixed with 4% PFA (Thermo Fisher, USA), and then permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, USA) at room temperature for 8 min, followed by incubation with 2% BSA (Cell Signaling Technology, USA) at 37°C for 30 min. Afterwards, cells were incubated overnight at 4°C with Oct4 antibody (1: 200; ab27985, Abcam, USA), Sox-2 antibody (1: 100; 6772–100, Bio Vision, USA), Tom20 antibody (1: 200; sc-17764, Santa Cruze), LC3B antibody (1: 200; 2775S, Cell Signaling), or P62 antibody (1: 200; ab56416, Abcam, USA). After washing with PBS for three times, BMSCs were then incubated with an Alexa Fluor 488 conjugated secondary antibody (1: 1000; 1869589, Thermo Fisher, USA) or Alexa Fluor 568 conjugated secondary antibody (1: 1000; 1793903, Thermo Fisher, USA) in the dark for 1 h and then incubated with DAPI at room temperature for 5 min. At last, a confocal microscope (Nikon Ti A1, Japan) was used to observe the fluorescence of cells. The mean fluorescence intensity (MFI) of Oct4 and Sox-2 and the co-localization of mitochondrial marker Tom20 and autophagy marker LC3B or P62 were assessed using ImageJ software.
Mitochondria and lysosomes imaging
Cells were seeded on glass bottom dishes and co-incubated with MitoTracker Green (Invitrogen, USA) and LysoTracker Deep Red (Invitrogen, USA) according to the manufacturer’s instructions. Briefly, freshly prepared MitoTracker Green or LysoTracker Deep Red solution was added to media to reach a final concentration of 1 μM and 0.5 μM, respectively. Cells were kept in the dark and maintained at 37°C for 20 min, then washed with FBS-free DMEM-L for three times. Live cell images of mitochondria and lysosomes were captured using a confocal microscope (Nikon Ti A1, Japan) equipped with oil immersion objective lens 60 × (λex: 488 nm; λem: 515–530 nm). Pictures were taken in a blinded fashion and at least 30 cells were counted in 3 independent experiments. The co-localization of mitochondria and lysosomes was assessed using ImageJ software. For mitochondrial length measurement, images were extracted to grayscale and inverted to show the specific fluorescence of mitochondria as black pixels. After sharpening the images, unified threshold was set to resolve individual cells and to measure mitochondrial length. Because the vast majority of mitochondrial mass shows an interconnected “network” in cells which makes the image hard to resolve, the resolvable mitochondrial length was always reported.
Oil red O and alizarin red S staining
When rat BMSCs reached a confluence around 95% on 6-well plates, cells were induced to adipocytes or osteocytes by culturing cells in the conditioned media. After two or three weeks, cells were rinsed twice with PBS and then fixed with freshly prepared 4% PFA at room temperature for 15 min. After three additional washes with PBS, cells were incubated with 0.5% Oil Red O solution or 0.1% Alizarin Red S (Cyagen, USA) at room temperature for 20–30 min. Then, cells were rinsed again with PBS for two to three times. Finally, the inverted phase-contrast microscope (Nikon, Tokyo, Japan) was used to capture images. The total area of lipid droplets or calcium nodus was assessed using ImageJ software.
qRT-PCR
Total RNA was extracted from BMSCs with the TRIzol reagent (TaKaRa, Japan) according to the manufacturer’s instructions. RNA concentration was quantified with a Gene Quant pro (Amersham Biosciences, GE healthcare, USA). Complementary DNAs (cDNAs) were synthesized with a SYBR Premix Ex Taq (TaKaRa, Japan) in MJ Mini personal thermal Cycler (Bio-Rad, USA). The amount of cDNA corresponding to 100 ng of RNA was amplified in triplicate with a total of 40 cycles (15 s at 95°C, 15 s at 56°C, and 60 s at 72°C) using a SYBR green PCR kit (Applied Biosystems) with the following primers: Pink1 forward. TATGAAGCCACCATGCC CAC; Pink1 reverse, GATCCTGCCGAGATATTCCAC; Prkn forward, TGTGTCAC AA-GGCTCAACGA; Prkn reverse, CCTGTTGTACTGCTCTTCTCCA; β-actin forward, TGTGTGGATTGGTGGCTCTA; β-actin reverse, GCTCAGTAACAGTCC GCCTA. The relative C values of Pink1 and Prkn were normalized to that of β-actin. Each test was performed in triplicate and repeated three times.
Statistical analysis
Data were obtained from at least three independent experiments. All data were analyzed by GraphPad prism 7.0 (GraphPad Software, USA) and presented as mean ± SEM. One way- or two way-ANOVA followed by Student’s t-test were used for analysis of differences. A value of P < 0.05 was considered statistically significant.
Results
Promotion of mitophagy by mitochondrial fission
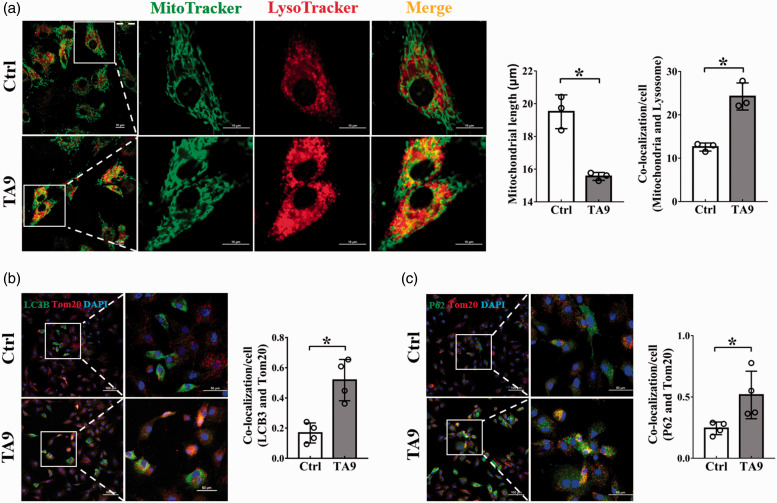
To investigate the effect of mitochondrial fission on mitophagy in BMSCs, we treated the cells with TA9 to specifically promote mitochondrial fission and then observed changes of mitophagy. An increased mitochondrial fragmentation was observed in cells treated with TA9 for 24 h, resulting in a significant reduction of the length of mitochondria (Figure 1(a)), a typical phenotype of mitochondrial fission. Along with the increased mitochondrial fission, mitophagy was greatly enhanced as indicated by an increased co-localization of MitoTracker Green for mitochondria and LysoTracker Deep Red for lysosomes (Figure 1(a)). Furthermore, an increased co-staining of autophagy markers (LC3B, P62) and mitochondrial marker (Tom20) confirmed the increase in mitophagy (Figure 1(b) and (c)).
Figure 1.
Effects of mitochondrial fission on mitophagy. BMSCs were treated with 1 μM TA9 for 24 h and visualized by confocal microscopy. (a) The length of mitochondria and the co-localization of mitochondria and lysosomes. Mitochondria in both groups were stained with freshly prepared MitoTracker Green and LysoTracker Deep Red for 20 min. Scale bars represent 10 μm; Original magnification: 1000× (left), 3000× (right). Mitochondrial length and the co-localization of mitochondria and lysosomes were analyzed by ImageJ software. Data were obtained from three separate experiments and more than 10 cells were analyzed in each experiment; (b, c) The co-localization of fluorescently stained autophagy markers (LC3, P62) and mitochondrial marker (Tom20) in BMSCs. Nucleus was stained with DAPI (blue). Scale bars represent 100 μm (left), 50 μm (right); Original magnification: 200× (left), 600× (right). The changes in mean fluorescence intensity (MFI) of LC3B, P62 and Tom20 were analyzed by ImageJ software. Each dot presents one independent experiment. Bar graph values are mean ± SEM. Data were compared using unpaired Student’s t-test. *Significantly different from control (P < 0.05). (A color version of this figure is available in the online journal.)
Maintenance of BMSCs stemness by mitophagy
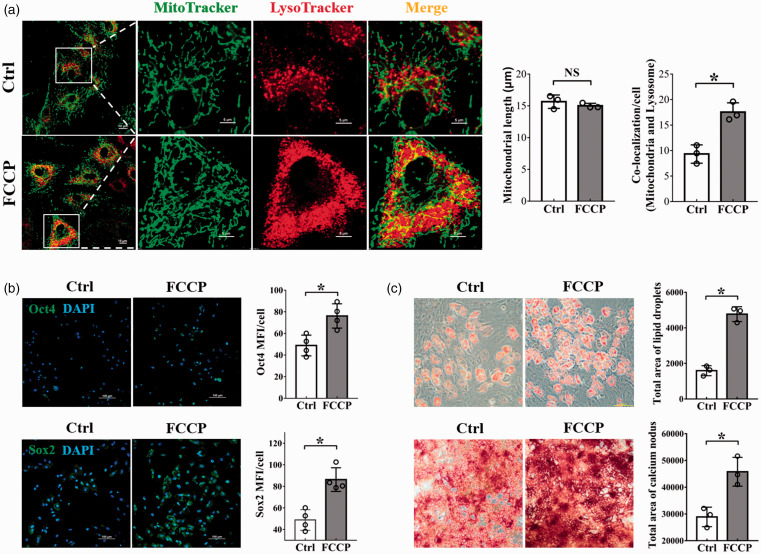
Treatment of BMSCs with a mitochondrial uncoupler, FCCP, for 4 h remarkably increased mitophagy detected by an increased co-localization of mitochondrial and lysosome biomarkers (Figure 2(a)). It was noticed that FCCP treatment did not change the length of mitochondria (Figure 2(a)), indicating FCCP-induced mitophagy was mitochondrial fission-independent. Under this condition of enhanced mitophagy without a concomitant mitochondrial fission, an enhanced expression of Oct4 and Sox2 (Figure 2(b)), as well as an increase in the potency of BMSCs induction to adipocytes or osteocytes were observed (Figure 2(c)), indicating a role of mitophagy in maintaining the stemness of BMSCs.
Figure 2.
The relationship between mitophagy and stemness maintenance. (a) The length of mitochondria and the co-localization of mitochondria and lysosomes. Mitochondria in both groups were stained with freshly prepared MitoTracker Green and LysoTracker Deep Red for 20 min. Scale bars represent 10 μm (left), 5 μm (right); Original magnification: 1000× (left), 4000× (right). Mitochondrial length and the co-localization of mitochondria and lysosomes were analyzed by ImageJ software. Morphometric analysis of mitochondrial length and the co-localization of mitochondria and lysosomes were performed from data obtained from three separate experiments, and more than 10 cells were analyzed in each experiment; (b) Representative images of fluorescently stained Oct4 and Sox2 (Green). Nucleus was stained with DAPI (blue). Scale bars represent 100 μm, original magnification: 200×. The changes in MFI of Oct4 and Sox2 were analyzed by ImageJ software. Data were obtained from four independent experiments; (c) Induction of BMSCs into adipocytes by culturing cells in adipogenic induction media for three weeks. The adipocytes were visualized by Oil Red O staining (top). Scale bars represent 100 μm, original magnification: 200×; Induction of BMSCs into osteocytes by culturing the cells in osteogenic induction media for two weeks. The osteocytes were visualized by Alizarin red S staining (bottom). Scale bars represent 500 μm, original magnification: 40×. Total area of lipid droplets and calcium nodus were analyzed by ImageJ software. Data were obtained from three separate experiments. Bar graph values are mean ± SEM. Data were compared using unpaired Student’s t-test. *Significantly different from control (P < 0.05). (A color version of this figure is available in the online journal.)
Inhibition of mitophagy by Pink1/Prkn gene silencing in BMSCs
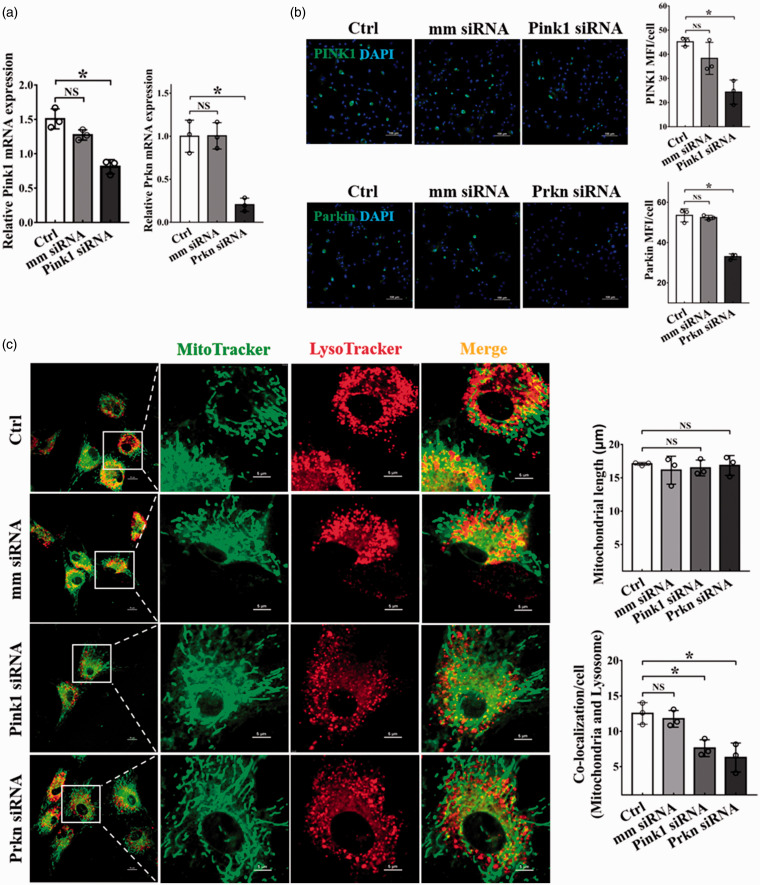
Transfection of BMSCs with siRNA targeting mitophagy essential genes Pink1/Prkn led to depression of Pink1/Prkn mRNA (Figure 3(a)) and protein levels (Figure 3(b)) in BMSCs. As expected, PINK1/Parkin reduction led to a decreased co-localization of mitochondrial and lysosome biomarkers without affecting mitochondrial length (Figure 3(c)), indicating that PINK1/Parkin reduction suppresses mitophagy in BMSCs.
Figure 3.
Decreased mitophagy by Pink1/Prkn gene silencing. BMSCs were transfected with 50 nM Pink1 siRNA or Prkn siRNA for 48 h, followed by qRT-PCR analyzation and immunofluorescence staining. (a) qRT-PCR determination of the mRNA expression of Pink1 and Prkn. Three independent experiments were carried out; (b) Representative images of fluorescently stained PINK1 and Parkin (Green), nucleus was stained with DAPI (blue). Scale bars represent 100 μm, original magnification: 200×. The changes in MFI of PINK1 and Parkin were analyzed by ImageJ software. Data were obtained from three independent experiments; (c) The length of mitochondria and the co-localization of mitochondria and lysosomes. Scale bars represent 10 μm (left), 5 μm (right); Original magnification: 1000× (left), 4000× (right). Images were analyzed by ImageJ software. Data were obtained from three independent experiments and more than 10 cells were analyzed in each experiment. Bar graph values are mean ± SEM. Data were compared using one way-ANOVA. *Significantly different from the control group (P < 0.05). (A color version of this figure is available in the online journal.)
Mitochondrial fission promotion of BMSCs stemness is mitophagy dependent
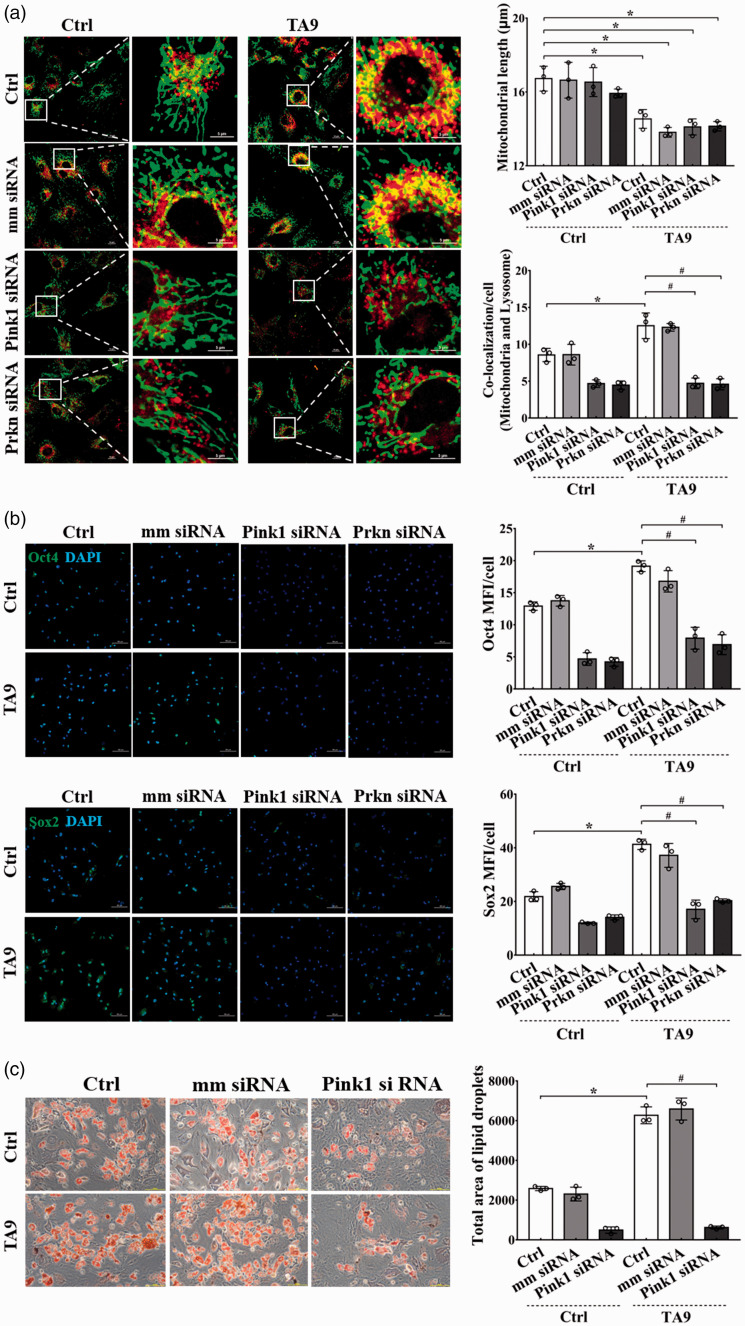
A mitochondrial fission promotion but mitophagy inhibition condition was generated by treating BMSCs with TA9 and concomitantly transfected with siRNA targeting Pink1/Prkn. As shown in Figure 4(a), the mitochondrial fragmentation and the decreased mitochondrial length occurred without mitophagy in the cells treated with TA9 along with transfection of Pink1/Prkn siRNA. In these cells, the expression of Oct4 and Sox2 was significantly depressed, and the induction of these BMSCs to adipocytes was blocked (Figure 4(b) and (c); Supplementary Figure 1(a) and (b)).
Figure 4.
Effects of mitochondrial fission on the stemness of BMSCs with mitophagy inhibition. BMSCs were treated with Pink1/Prkn siRNA for 24 h before next treatment with 1 μM TA9 for additional 24 h. Immunofluorescence and adipogenic induction were carried out to detect the stemness of stem cells. (a) The length of mitochondria and the co-localization of mitochondria and lysosomes. Scale bars represent 10 μm or 5 μm; Original magnification: 1000× or 3000×. Images were analyzed by ImageJ software. Morphometric analysis of mitochondrial length and the co-localization of mitochondria and lysosomes were performed from data obtained from three separate experiments, and more than 10 cells were analyzed in each experiment; (b) Representative image of fluorescently stained Oct4 and Sox2 (Green), nucleus was stained with DAPI (blue). Scale bars represent 100 μm; Original magnification 200×. Three independent experiments were carried out; (c) Induction of BMSCs into adipocytes by culturing cells in adipogenic induction media for three weeks. The adipocytes were visualized by Oil Red O staining. Scale bars represent 100 μm, original magnification: 200×. Images are analyzed by ImageJ software, each dot presents one independent experiment and bar graph values are mean ± SEM. Data were compared using two way-ANOVA. *Significantly different from the control group (P < 0.05). (A color version of this figure is available in the online journal.)
Discussion
The in vitro expansion of BMSCs is necessary for obtaining sufficient amounts of cells for clinical applications. However, BMSCs that cultured in vitro are prone to differentiate spontaneously, thus it is important to make efforts to maintain the stemness of BMSCs in cultures. Our previous studies found that mitochondrial fission contribute to maintain the stemness of stem cells,15 but the underlying mechanism is elusive. In the present study, we found that mitophagy is indispensable for mitochondrial fission maintenance of the stemness of BMSCs.
We first explored the relationship between mitophagy and mitochondrial fission in BMSCs. Mitochondrial fission was accelerated by TA9, and under this condition, mitophagy was remarkably augmented in these cells. This accompanied increase in mitophagy would result from the stimulation of accumulated mitochondrial fragments. It was found that mitochondrial fission and mitophagy shared some critical regulatory proteins involved in the two parallel cellular processes. For instance, DRP1 and FIS1, both participating in mitochondrial fission, function together with the LC3 adapters/receptors to promote mitophagy.19,26,27 Therefore, mitochondrial fission followed by mitophagy is an important sequential process to ensure the removal of damaged or fragmented mitochondria from the cells. The results here demonstrated that this sequential process is also important to maintain the stemness of BMSCs.Mitophagy could occur independent of mitochondrial fission.28 The result here using FCCP to promote mitophagy also showed that FCCP-augmented mitophagy was independent of mitochondrial fission. Taking an advantage of this distinguished approach, we examined the essential role of mitophagy in maintenance of the stemness of BMSCs. The results indeed showed that the enhancement of mitophagy induced by FCCP, without a concomitant mitochondrial fission, significantly increased the stemness of BMSCs. Conversely, inhibition of mitophagy blocked mitochondrial fission-enhanced stemness of BMSCs. During the process of mitophagy, PINK1 is accumulated at the outer membrane of mitochondria, which recruits E3 ubiquitin ligase Parkin, leading to clearage of impaired mitochondria by lysosomes.29–31 Gene silencing Pink1/Prkn suppressed mitophagy, and under this condition, stimulation of mitochondrial fission did not promote the stemness of BMSCs. Thus, it clearly demonstrated the critical role of mitophagy in mediating mitochondrial fission-enhanced stemness of BMSCs.
The association of mitophagy with the stemness of stem cells was also reported in other studies. An increase in mitophagy was observed in cells that were programmed to iPSCs,23 and inhibition of mitophagy impaired the stemness of MSCs.25 On the other hand, there were studies showing that mitophagy may also play a key role in cell differentiation during some particular developmental stages in mammalian cells. For example, a study in CD34+ hematopoietic progenitor cells (HPCs) showed that mitophagy would possibly contribute to cell differentiation as enhanced mitophagy was observed in erythroid differentiation in β-thalassemia.32 If the positive role of mitophagy in maintenance of stem cell stemness versus that in promotion of stem cell differentiation were considered to be contradictory, it would be a misinterpretation. As shown in the present study, augmented mitophagy in BMSCs was associated with enhanced stemness of these cells in cultures, but was also with promoted induction of these cells to adipocytes or osteocytes in response to different stimulatory signals. It thus is important to note that the fate of stem cells is not determined by mitophagy, but rather by its surrounding environment. Mitophagy would only enhance the intracellular machinery for whatever lineages of the stem cells.
The present study demonstrates that mitophagy is important in the maintenance of the stemness of BMSCs in cultures, and is essential in mediating mitochondrial fission-enhanced stemness of BMSCs. This understanding would provide a critical insight into stem cell propagation in cultures for their clinical application since maintenance of the stemness of stem cells is among the most important issues in stem cells and regenerative medicine. Therefore, manipulation of mitochondrial dynamics in the preparation of stem cells for clinical application would be an important undertaking for both experimental and clinical studies.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220964394 for Mitophagy promotes the stemness of bone marrow-derived mesenchymal stem cells by Xiaorong Feng, Wen Yin, Jialing Wang, Li Feng and Y James Kang in Experimental Biology and Medicine
Authors’ contributions
All authors participated in conceptualization and designing of this study. XRF and WY, performed the experimentation, data collection, and manuscript drafting; JLW, performed experimentation and participated writing; LF, provided consultation and supervision; YJK, funding acquisition, data reviewing and validating, and manuscript editing and final approval.
ACKNOWLEDGMENTS
The authors thank Fang Nan, Rui Li and Yanming Liu for providing technical assistance for cell imaging and flow cytometry analyses, and Jeffrey N. Harding for providing proofreading.
Footnotes
Declaration OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Sichuan University West China Hospital.
ORCID iD: Y James Kang https://orcid.org/0000-0001-8449-7904
SupplementAL MATERIAL: Supplemental material for this article is available online.
References
- 1.Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A 1998; 95:1142–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science 2000; 290:1775–9 [DOI] [PubMed] [Google Scholar]
- 3.Bittner RE, Schofer C, Weipoltshammer K, Ivanova S, Streubel B, Hauser E, Freilinger M, Hoger H, Elbe-Burger A, Wachtler F. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol 1999; 199:391–6 [DOI] [PubMed] [Google Scholar]
- 4.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999; 5:309–13 [DOI] [PubMed] [Google Scholar]
- 5.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 2004; 94:92–5 [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol 2005; 195:16–26 [DOI] [PubMed] [Google Scholar]
- 7.Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol 2011; 95:213–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dallari D, Rani N, Sabbioni G, Mazzotta A, Cenacchi A, Savarino L. Radiological assessment of the PRF/BMSC efficacy in the treatment of aseptic nonunions: a retrospective study on 90 subjects. Injury 2016; 47:2544–50 [DOI] [PubMed] [Google Scholar]
- 9.Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, Singh KK, Nair V, Sarkar RS, Gorthi SP, Hassan KM, Prabhakar S, Marwaha N, Khandelwal N, Misra UK, Kalita J, Nityanand S, Inve STSG. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke 2014; 45:3618–24 [DOI] [PubMed] [Google Scholar]
- 10.Diederichsen AC, Moller JE, Thayssen P, Videbaek L, Saekmose SG, Barington T, Kassem M. Changes in left ventricular filling patterns after repeated injection of autologous bone marrow cells in heart failure patients. Scand Cardiovasc J 2010; 44:139–45 [DOI] [PubMed] [Google Scholar]
- 11.Grisendi G, Anneren C, Cafarelli L, Sternieri R, Veronesi E, Cervo GL, Luminari S, Maur M, Frassoldati A, Palazzi G, Otsuru S, Bambi F, Paolucci P, Pierfranco C, Horwitz E, Dominici M. GMP-manufactured density gradient media for optimized mesenchymal stromal/stem cell isolation and expansion. Cytotherapy 2010; 12:466–77 [DOI] [PubMed] [Google Scholar]
- 12.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 2007; 110:1362–9 [DOI] [PubMed] [Google Scholar]
- 13.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 2006; 54:3254–66 [DOI] [PubMed] [Google Scholar]
- 14.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 2003; 17:160–70 [DOI] [PubMed] [Google Scholar]
- 15.Feng X, Zhang W, Yin W, Kang YJ. Feature article: the involvement of mitochondrial fission in maintenance of the stemness of bone marrow mesenchymal stem cells. Exp Biol Med 2019; 244:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol 2018; 20:1013–22 [DOI] [PubMed] [Google Scholar]
- 17.Lampert MA, Orogo AM, Najor RH, Hammerling BC, Leon LJ, Wang BJ, Kim T, Sussman MA, Gustafsson AB. BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation. Autophagy 2019; 15:1182–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by parkin. J Cell Biol 2010; 191:1367–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y, Lee HY, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol 2011; 301:H1924–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DW, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 2015; 6:8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy A, Stedman A, Deutsch E, Donnadieu F, Virgin HW, Sansonetti PJ, Nigro G. Innate immune receptor NOD2 mediates LGR5(+) intestinal stem cell protection against ROS cytotoxicity via mitophagy stimulation. Proc Natl Acad Sci U S A 2020; 117:1994–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan P, Yu XY, Xie XH, Chen CH, Zhang P, Yang C, Peng X, Wang YT. Mitophagy is a protective response against oxidative damage in bone marrow mesenchymal stem cells. Life Sci 2019; 229:36–45 [DOI] [PubMed] [Google Scholar]
- 23.Vazquez-Martin A, Van den Haute C, Cufi S, Corominas-Faja B, Cuyas E, Lopez-Bonet E, Rodriguez-Gallego E, Fernandez-Arroyo S, Joven J, Baekelandt V, Menendez JA. Mitophagy-driven mitochondrial rejuvenation regulates stem cell fate. Aging 2016; 8:1330–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naik PP, Birbrair A, Bhutia SK. Mitophagy-driven metabolic switch reprograms stem cell fate. Cell Mol Life Sci 2019; 76:27–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song BQ, Chi Y, Li X, Du WJ, Han ZB, Tian JJ, Li JJ, Chen F, Wu HH, Han LX, Lu SH, Zheng YZ, Han ZC. Inhibition of notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell Physiol Biochem 2015; 36:1991–2002 [DOI] [PubMed] [Google Scholar]
- 26.Pei S, Minhajuddin M, Adane B, Khan N, Stevens BM, Mack SC, Lai S, Rich JN, Inguva A, Shannon KM, Kim H, Tan AC, Myers JR, Ashton JM, Neff T, Pollyea DA, Smith CA, Jordan CT. AMPK/FIS1-Mediated mitophagy is required for Self-Renewal of human AML stem cells. Cell Stem Cell 2018; 23:86–100.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirihai OS, Song M, Dorn GW., 2nd. How mitochondrial dynamism orchestrates mitophagy. Circ Res 2015; 116:1835–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita SI, Kanki T. How autophagy eats large mitochondria: autophagosome formation coupled with mitochondrial fragmentation. Autophagy 2017; 13:980–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Springer W, Kahle PJ. Regulation of PINK1-Parkin-mediated mitophagy. Autophagy 2011; 7:266–78 [DOI] [PubMed] [Google Scholar]
- 30.Vazquez-Martin A, Cufi S, Corominas-Faja B, Oliveras-Ferraros C, Vellon L, Menendez JA. Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging 2012; 4:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Humbeeck C, Cornelissen T, Vandenberghe W. Ambra1: a Parkin-binding protein involved in mitophagy. Autophagy 2011; 7:1555–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu L, Xu W, Xu L, Kong Q, Fang J. Mitophagy is increased during erythroid differentiation in beta-thalassemia. Int J Hematol 2017; 105:162–73 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220964394 for Mitophagy promotes the stemness of bone marrow-derived mesenchymal stem cells by Xiaorong Feng, Wen Yin, Jialing Wang, Li Feng and Y James Kang in Experimental Biology and Medicine