Abstract
Interleukin (IL)-17A, a proinflammatory cytokine produced by T-helper (Th)17 cells, has been associated with autoimmune diseases. Type 1 diabetes (T1D) is caused either due to mutation of insulin gene or developed as an autoimmune disease. Studies have shown that IL-17A expression is upregulated in the pancreas in T1D patients and animal models. However, role or importance of IL-17A in T1D pathogenesis needs elucidation. Particularly, evidence for a direct injury of IL-17A to pancreatic β cells through activating IL-17 receptor A (IL-17RA) is lacking. Ins2Akita (Akita) mouse, a T1D model with spontaneous mutation in insulin 2 gene leading to β-cell apoptosis, was crossed with IL-17A-knockout mouse and male IL-17A-deficient Akita mice were used. Streptozotocin, a pancreatic β-cell-specific cytotoxin, was employed to induce a diabetic model in MIN6 cells, a mouse insulinoma cell line. IL-17A expression in the pancreas was upregulated in both Akita and streptozotocin-induced diabetic mice. IL-17A-knockout Akita mice manifested reduced blood glucose concentration and raised serum insulin level. IL-17A deficiency also decreased production of the proinflammatory cytokines tumor necrosis factor (TNF)-α, IL-1β, and interferon (IFN)-γ in Akita mice. IL-17RA expression in MIN6 cells was upregulated by IL-17A. IL-17A enhanced expression of TNF-α, IL-1β, IFN-γ, and inducible nitric oxide synthase (iNOS) and further increased streptozotocin-induced expression of the inflammatory factors in MIN6 cells. IL-17A exacerbated streptozotocin-induced MIN6 cell apoptosis and insulin secretion impairment. Blocking IL-17RA with anti-IL-17RA-neutralizing antibody reduced all these deleterious effects of IL-17A on MIN6 cells. Collectively, IL-17A deficiency alleviated hyperglycemia, hypoinsulinemia, and inflammatory response in Akita mice that are characteristic for T1D. IL-17A exerted an alone and synergistic destruction with streptozotocin to pancreatic β cells through IL-17RA pathway. Thus, the data suggest that targeting IL-17A and/or IL-17RA is likely to preserve remaining β-cell function and treat T1D.
Impact statement
The participation of interleukin (IL)-17A in diabetic pathogenesis is suggested in animal models of autoimmune diabetes and in patients with type 1 diabetes (T1D), but with some contradictory results. Particularly, evidence for a direct injury of IL-17A to pancreatic β cells is lacking. We showed that IL-17A deficiency alleviated diabetic signs including hyperglycemia, hypoinsulinemia, and inflammatory response in Ins2Akita (Akita) mice, a T1D model with spontaneous mutation in insulin 2 gene leading to β-cell apoptosis. IL-17A enhanced inflammatory reaction, oxidative stress, and cell apoptosis but attenuated insulin level in mouse insulin-producing MIN6 cells. IL-17A had also a synergistic destruction to MIN6 cells with streptozotocin (STZ), a pancreatic β-cell-specific cytotoxin. Blocking IL-17 receptor A (IL-17RA) reduced all these deleterious effects of IL-17A on MIN6 cells. The results demonstrate the role and the importance of IL-17A in T1D pathogenesis and suggest a potential therapeutic strategy for T1D targeting IL-17A and/or IL-17RA.
Keywords: Interleukin-17A, diabetes, inflammation, IL-17 receptor A, Ins2Akita mice, MIN6 cells
Introduction
Inflammation has been associated with initiation and progression of diabetes mellitus both in humans and animal models.1–3 Diabetes mellitus includes type 1 diabetes (T1D) and type 2 diabetes (T2D). T1D is caused either due to mutation of insulin gene4 or developed as an autoimmune disease.5 T1D is characterized by pancreatic β-cell destruction. Pancreatic β cells are responsible for producing insulin that regulates glucose metabolism. When β cells are less or absent due to either genetic mutation or autoimmune destruction, the insulin released in response to high blood glucose level is either very low or absolutely absent. This causes low levels of insulin and high levels of glucose in the blood, a signature of T1D.6 In the autoimmune diabetic processes, immune cells, particularly CD4+ and CD8+ T cells and macrophages, progressively infiltrate islets in the pancreas and secrete proinflammatory cytokines into the environment of pancreatic insulin-producing β cells, which can result in insulitis, β-cell destruction, and impairment of insulin production.2 Several proinflammatory cytokines have been shown to play important roles in developing T1D at the levels of both immune responses and targeting β cells. Tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and interferon (IFN)-γ are the most likely cytokines acting in synergy during inflammation of pancreatic β cells, leading to β-cell destruction.7
IL-17 is a key proinflammatory cytokine produced by T-helper type 17 (Th17) cells, a CD4+ T cell subset. The IL-17 family contains six structurally related cytokines, IL-17A through IL-17F. IL-17A, the prototypical member of this family, has received much attention for its proinflammatory role in autoimmune diseases.8 Studies have shown that IL-17A/IL-17 expression is upregulated both in the pancreas9 and in circulating lymphocytes10,11 in patients with T1D. Nonobese diabetic (NOD) mouse is one of the most commonly used models in studying autoimmunity of T1D.12 Diabetic NOD mice have elevated IL-17 expression in the pancreas, and the islet-reactive Th17 cells promote pancreatic inflammation, but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells.13IL-17 single-deficient NOD mice show delayed onset of diabetes and reduced severity of insulitis, but cumulative incidence of longstanding diabetes in the IL-17-deficient mice is similar to that in wild-type mice.14 Further, IL-17A silencing does not protect NOD mice from autoimmune diabetes.15 In contrast, IL-17A deficiency ameliorates hyperglycemia and insulitis induced by streptozotocin (STZ),16 a pancreatic β-cell-specific cytotoxin. Thus, the role or the importance of IL-17A in T1D occurrence and development needs to be further evaluated. Particularly, direct evidence supporting the involvement of IL-17A in T1D predisposition caused by insulin gene mutation is lacking.
Ins2Akita (Akita) mouse carries a spontaneous mutation in insulin 2 (Ins2) gene. This mutation causes incorrect folding of insulin protein, and leads, subsequently, to endoplasmic reticulum (ER) stress and β-cell apoptosis that results in T1D.12,17 It is relatively unclear whether IL-17A influences T1D processes induced by the insulin gene mutation. In addition, although a direct destruction to pancreatic β cells by the proinflammatory cytokines TNF-α, IL-1β and IFN-γ has been reported,18,19 the direct effect of IL-17A on pancreatic β cells is poorly identified. Intracellular signal transduction of IL-17A depends on the presence of the receptor heterodimer complex formed by the subunits IL-17 receptor A (IL-17RA) and IL-17RC.20IL-17RA is expressed in rat insulin-producing INS-1E cells and upregulated by combined IL-1β and IFN-γ treatment.9 Inducible nitric oxide (NO) synthase (iNOS)-mediated NO release is related to inflammatory destruction to pancreatic β cells.21 Nevertheless, it still needs elucidation that IL-17A directly activates IL-17RA to promote inflammatory reaction and oxidative stress leading to pancreatic β-cell apoptosis. Thus, in the present study, firstly, IL-17A-deficient Akita mice were used to assess the role of IL-17A in T1D development caused by the insulin gene mutation. Secondly, IL-17A level was evaluated in the pancreas in STZ-induced T1D mice. Thirdly, a direct and synergistic effect of IL-17A and STZ on pancreatic β cells was examined by using MIN6 cells, a mouse insulinoma cell line. Finally, IL-17RA expression was tested in MIN6 cells, and anti-IL-17RA-neutralizing antibody was applied to the cells to determine whether IL-17A effect is mediated by IL-17RA. This study provides further evidence for IL-17A participation in T1D development and a potential therapeutic strategy for T1D targeting IL-17A or/and IL-17RA.
Materials and methods
Animal models
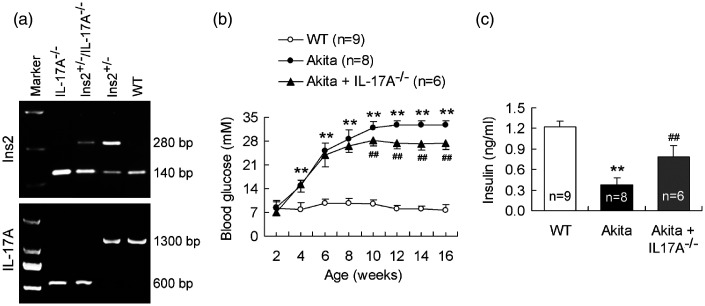
Akita mice of Ins2+/− genotype with C57BL/6 background were used as T1D model (from Model Animal Research Center of Nanjing University, Nanjing, China). Age-matched wild-type (WT) littermates with C57BL/6 background were served as control of the Akita diabetic model. As described, Akita mice of Ins2+/− genotype that develop insulin-dependent T1D are characterized by hyperglycemia with an onset of 3 to 4 weeks of age and a peak at the age of 10 to 12 weeks.6,12IL-17A-knockout mice on a C57BL/6 background were kindly provided by Dr. Shuo Yang (Nanjing Medical University, Nanjing, China). To examine the effect of IL-17A gene ablation on development of T1D, we prepared the mice with the genotype of Ins2+/−/IL-17A−/− by cross-breeding of Ins2+/− with IL-17A−/− mice (referred to the method Chavali et al.6). Firstly, female Ins2+/− mice were crossed with male IL-17A−/− mice and heterozygous mice of Ins2+/−/IL-17A+/− genotype were obtained in F1 generation. Next, female Ins2+/−/IL-17A+/− mice were crossed with male IL-17A−/− mice and Ins2+/−/IL-17A−/− mice were obtained in F2 generation. Male Ins2+/−/IL-17A−/− mice were collected as IL-17A-deficient Akita diabetic model and male Ins2+/− Akita diabetic mice were used as its control. The genotypes of mice were identified at three to four-week age by polymerase chain reaction (PCR) amplification (Figure 1).
Figure 1.
IL-17A deficiency reduces blood glucose concentration and raises serum insulin level in Akita mice. (a) Genotypes of WT, Ins2+/−, IL-17A−/−, and Ins2+/−/IL-17A−/− mice. The mice generated by cross-breeding of Ins2+/− with IL-17A−/− mice were identified at three to four weeks of age for the genotypes. PCR amplification of Ins2 and IL-17A genes indicates WT mice with the bands of 140 bp and 1300 bp, Ins2+/− mice (Akita mice) with the bands of 140 bp, 280 bp, and 1300 bp, IL-17A−/− mice with the bands of 140 bp and 600 bp, and Ins2+/−/IL-17A−/− mice with the bands of 140 bp, 280 bp, and 600 bp. (b) Knockout of IL-17A reduces blood glucose concentration in Akita mice. Glucose concentration in blood was monitored weekly at 4:00 p.m. up to the age of 16 weeks. (c) Knockout of IL-17A raises serum insulin level in Akita mice. Serum was obtained from 12-week age of mice. **P < 0.01 versus WT mice; ##P < 0.01 versus Akita mice.
STZ-induced diabetic model was performed as described previously.22 Briefly, STZ (Sigma, St. Louis, MO, USA) was freshly dissolved in 0.01 M citrate buffer (pH 4.5), which was intraperitoneally injected in male C57BL/6 mice daily at a dose of 50 mg/kg body weight for five consecutive days. Control animals received only citrate buffer (vehicle) intraperitoneally. The mice were euthanized on day 14 after first STZ injection for testing IL-17A level in the pancreas.
All the mice were housed in groups of three to four animals at a temperature of 21 ± 1°C with a 12-h light-dark cycle (light from 6:00 a.m. to 6:00 p.m.). The animals were provided food and water ad libitum. All the animal experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
Blood glucose monitoring
Blood glucose levels of the mice were monitored weekly at 4:00 pm beginning from the age of 2 weeks up to the age of 16 weeks. The measurement was conducted by punctuation of the tail using a syringe cannula connected to a blood glucose meter (Roche, Basel, Switzerland).
Cell culture and drug exposure
Mouse insulinoma MIN6 cells (purchased from Guangzhou Jennio Biotech Co., Ltd, Guangzhou, China) were maintained at 37°C in a 5% CO2 atmosphere in Dulbecco's modified Eagle medium (DMEM) containing 25 mM glucose, 10% fetal calf serum, 3.7 g/L sodium bicarbonate, 100 U/mL penicillin, and 100 mg/mL streptomycin (Gibco, Grand Island, NY, USA). When MIN6 cells in six-well plates reached 70% confluence, the cells were pretreated with anti-IL-17RA-neutralizing antibody (500 ng/mL) or isotype antibody (IgG, 500 ng/mL) (both from R&D Systems, Minneapolis, MN, USA) for 2 h and then STZ (Sigma, St Louis, MO, USA; 5 mM) and IL-17A (R&D Systems, Minneapolis, MN, USA; 100 ng/mL) was added to the media alone or in combination, which was incubated for 24 h. The MIN6 cells and supernatants were then collected, respectively, for subsequent analysis.
Quantitative reverse transcriptase PCR
Total RNA was isolated from the pancreas of mice at the age of 12 weeks or from MIN6 cells using Trizol (Invitrogen, Carlsbad, CA, USA). The RNA of 5 µg was reversely transcribed to cDNA with a Transcriptor cDNA Synthesis Kit (Roche, Basel, Switzerland) according to the manufacturer's instructions. Quantitative determination of gene expression was performed on a Rotor-Gene 3000 Real-time Cycler (Corbett Research, Sydney, Australia) using a two-step cycling protocol. β-actin was used to normalize gene expression. qRT-PCR was conducted with cDNA in duplicate 20-μl reactions using the Universal SYBR Green Master Mix (Roche, Basel, Switzerland). The reactions were incubated at 95°C for 10 min. A polymerase chain reaction cycling protocol consisting of 15 s at 95°C and 1 min at 60°C for 40 cycles was used for quantification. Relative expression levels were calculated according to Livak and Schmittgen,23 and values were normalized to respective WT or control samples. The sequences of the primers used for qRT-PCR experiments were as follows: IL-17A, 5′-TGGACTCTGAGCCGCA-3′ and 5′-GGCGGACAATAGAGGA-3′; TNF-α, 5′-CCACCACGCTCTTCTGT-3′ and 5′-ATCTGAGTGTGGGGTC-3′; IL-1β, 5′-CTTCCTTGTGCAAGTGT-3′ and 5′-CAGGTCATTCTCATCAC-3′; IFN-γ, 5′-TCAAGTGGCATAGATGT-3′ and 5′-TGGCTCTGCAGGATTTT-3′; and β-actin, 5′-CGTTGACATCCGTAAA-3′ and 5′-TAGAGCCACCAATCCA-3′.
Immunoblotting analysis
Proteins from MIN6 cell samples were extracted with RIPA buffer (Thermo Scientific, Waltham, MA, USA). The protein samples (20–40 μg) were separated by SDS-PAGE gels and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% milk in TBS/0.1% Tween-20 and incubated with anti-IL-17RA, anti-iNOS (both in 1:200; Abcam, Cambridge, UK), anti-caspase-3 (1:1000; Cell Signaling Technology, Danvers, MA, USA), or anti-β-actin (1:2000; Sigma, St Louis, MO, USA). Appropriate IRDye 800-conjugated secondary antibodies (1:5000; Rockland Immunochemicals, Gilbertsville, PA, USA) were used to visualize the protein bands on an Odyssey laser scanning system (LI-COR Inc., Lincoln, NE, USA).
Enzyme-linked immunosorbent assay
Serum was obtained from mice at 12 weeks of age for assessment of the levels of insulin, IL-17A, TNF-α, IL-1β, and IFN-γ. The pancreas was separated from STZ-treated mice on the 14th day after first STZ injection for measurement of IL-17A level. The supernatants of MIN6 cell cultures were collected at the indicated time point for test of insulin level. All the samples were assayed using commercially available ELISA kits (all from eBioscience, San Diego, CA, USA; but insulin test kit from Millipore, Billerica, MA, USA). Insulin concentration in the supernatants of MIN6 cell cultures was normalized to total cellular proteins.
Annexin V-FITC/propidium iodide flow cytometry
Fluorescence-activated cell sorting (FACS) is a laser-based biophysical technology that allows simultaneous multiparametric analysis. MIN6 cell death was measured using Annexin V-FITC Apoptosis Detection Kit (Roche, Basel, Switzerland) following the manufacturer's procedure. Briefly, MIN6 cells were stained with a combination of Annexin V-FITC and PI. The cells were then analyzed by a two-laser flow cytometry system and at least 10,000 events of single cells per sample were collected on a FACS Calibur flow cytometer with CellQuest software (BD Biosciences, San Jose, CA, USA). Depending on fluorescence intensity of Annexin V-FITC and PI, the populations can be distinguished into double negative (healthy) cells, Annexin-V positive (early apoptotic) cells, and double positive (late apoptotic and necroptotic) cells.24 Percentage of Annexin-V positive plus double positive cells in total cells was statistically analyzed as an assessment of cell death in this study.
Statistical analysis
Data were expressed as means ± standard deviations. Statistical analyses were performed with the Statistics Package for Social Science (SPSS, 12.0). The data were subjected to one-way analysis of variance, followed by Student–Newman–Keul’s test to compare the data of all groups relative to each other. Differences were considered statistically significant at P < 0.05.
Results
IL-17A deficiency reduces blood glucose concentration and raises serum insulin level in Akita mice
The cross-breeding mice of Ins2+/− with IL-17A−/− mice were detected to identify IL-17A-deficient Akita mice with the genotype of Ins2+/−/IL-17A−/− (Figure 1). Blood glucose concentration of mice was monitored weekly beginning from 2-week age until 16-week age. Akita mice showed a significantly increased blood glucose concentration at 4-week age and a peak value at 12-week age until 16-week age in comparison with WT mice (Figure 1). Importantly, IL-17A-knockout Akita mice manifested a significant decrease in blood glucose concentration from 10-week age until 16-week age compared with Akita mice (Figure 1). In addition, insulin level in serum was remarkably lower in Akita mice than in WT mice at the tested age of 12 weeks (Figure 1). The IL-17A-deficient Akita mice were elevated in serum insulin level with respect to the IL-17A-intact Akita mice (Figure 1). The data showed that IL-17A deficiency mitigated diabetic signs in Akita mice.
IL-17A deficiency decreases proinflammatory cytokine production in Akita mice
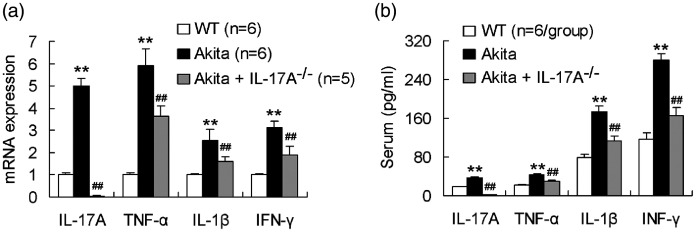
The pancreas was taken from 12-week-age mice for test of mRNA levels of the proinflammatory cytokines IL-17A, TNF-α, IL-1β, and IFN-γ. As indicated in Figure 2, mRNA expression of all the four proinflammatory cytokines was significantly upregulated in the pancreas of Akita mice compared with those of WT mice. Akita mice with IL-17A gene ablation were downregulated in expression of all the four proinflammatory cytokines in the pancreas compared with Akita mice without IL-17A gene deficiency (Figure 2). Similarly, levels of IL-17A, TNF-α, IL-1β, and IFN-γ in serum were significantly higher in Akita mice than in WT mice (Figure 2). IL-17A knockout diminished serum levels of all the four proinflammatory cytokines in Akita mice (Figure 2). The results showed that IL-17A deficiency attenuated proinflammatory cytokine production in Akita mice.
Figure 2.
IL-17A deficiency decreases proinflammatory cytokine production in Akita mice. (a) IL-17A deficiency downregulates expression of the proinflammatory cytokines IL-17A, TNF-α, IL-1β, and IFN-γ in the pancreas of Akita mice. The pancreas of mice at the age of 12 weeks was isolated and measured for the gene expression of the proinflammatory cytokines. (b) IL-17A deficiency diminishes serum levels of the proinflammatory cytokines in Akita mice. The serum was obtained from 12-week-age mice. **P < 0.01 versus WT mice; ##P < 0.01 versus Akita mice.
IL-17A enhances STZ-induced inflammatory responses in MIN6 cells and anti-IL-17RA-neutralizing antibody reduces this effect
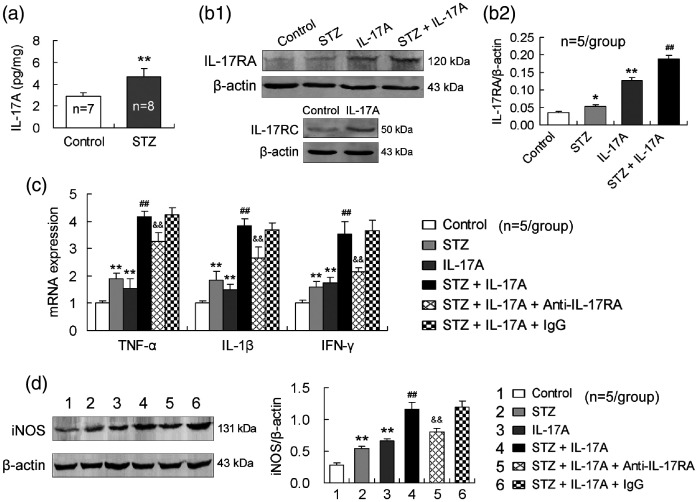
Firstly, IL-17A content in the pancreas was increased in STZ-treated mice relative to that in vehicle-injected control mice (Figure 3), suggesting that diabetes induced IL-17A production in pancreatic tissue. To show role of IL-17A in diabetes, we conducted in vitro experiments using MIN6 cells, a mouse insulin-producing β-cell line. MIN6 cells expressed IL-17RA and this expression was upregulated by the treatment with STZ or IL-17A alone (Figure 3). The combined treatment with STZ and IL-17A further upregulated IL-17RA expression in MIN6 cells (Figure 3), suggesting a synergistic effect of IL-17A with STZ on IL-17RA expression. In addition, IL-17RC was also expressed in MIN6 cells and upregulated by IL-17A (Figure 3), confirming that the heterodimer receptor consisting of IL-17RA and IL-17RC exists on MIN6 cells.
Figure 3.
IL-17A enhances STZ-induced inflammatory responses in MIN6 cells and anti-IL-17RA-neutralizing antibody reduces this effect. (a) STZ increases IL-17A production in the pancreas. STZ was intraperitoneally injected daily at a dose of 50 mg/kg body weight for five consecutive days. On the 14th day after first STZ injection, IL-17A level in the pancreas was assessed by ELISA. (b1,b2) IL-17A upregulates IL-17RA and IL-17RC expression in MIN6 cells. The cells were exposed to STZ (5 mM) or IL-17A (100 ng/mL) alone or to the combination of STZ and IL-17A for 24 h. (c) IL-17A enhances STZ-induced expression of the proinflammatory cytokines in MIN6 cells and anti-IL-17RA-neutralizing antibody diminishes this effect. MIN6 cells were exposed to STZ (5 mM) or IL-17A (100 ng/mL) alone or to the combination of STZ and IL-17A for 24 h. The anti-IL-17RA-neutralizing antibody (500 ng/mL) or isotype antibody (IgG, 500 ng/mL) was applied to the media 2 h earlier. (d) The anti-IL-17RA-neutralizing antibody reduces IL-17A effect of enhancing STZ-induced iNOS expression in MIN6 cells. The design of the experiment was similar to that of (c). *P < 0.05, **P < 0.01 versus control; ##P < 0.01 versus STZ; &&P < 0.01 versus STZ + IL-17A + IgG.
Furthermore, mRNA expression of all the three proinflammatory cytokines, TNF-α, IL-1β, and IFN-γ, was upregulated in MIN6 cells by the treatment with STZ or IL-17A alone and further upregulated by the combination of STZ and IL-17A (Figure 3). More importantly, in the presence of anti-IL-17RA-neutralizing antibody in MIN6 cells, the upregulated gene expression of TNF-α, IL-1β, or IFN-γ by STZ and IL-17A combination was reduced in comparison with the presence of isotype antibody (IgG) (Figure 3). Further, to show that oxidative stress is implicated in IL-17A destruction to pancreatic β cells, we measured iNOS protein expression levels in MIN6 cells. As expected, iNOS expression was upregulated by the treatment with STZ or IL-17A alone and further upregulated by the combined treatment with STZ and IL-17A. Similarly, anti-IL-17RA-neutralizing antibody reduced the upregulated iNOS expression in MIN6 cells induced by the combination of STZ and IL-17A with respect to isotype antibody (IgG) (Figure 3).
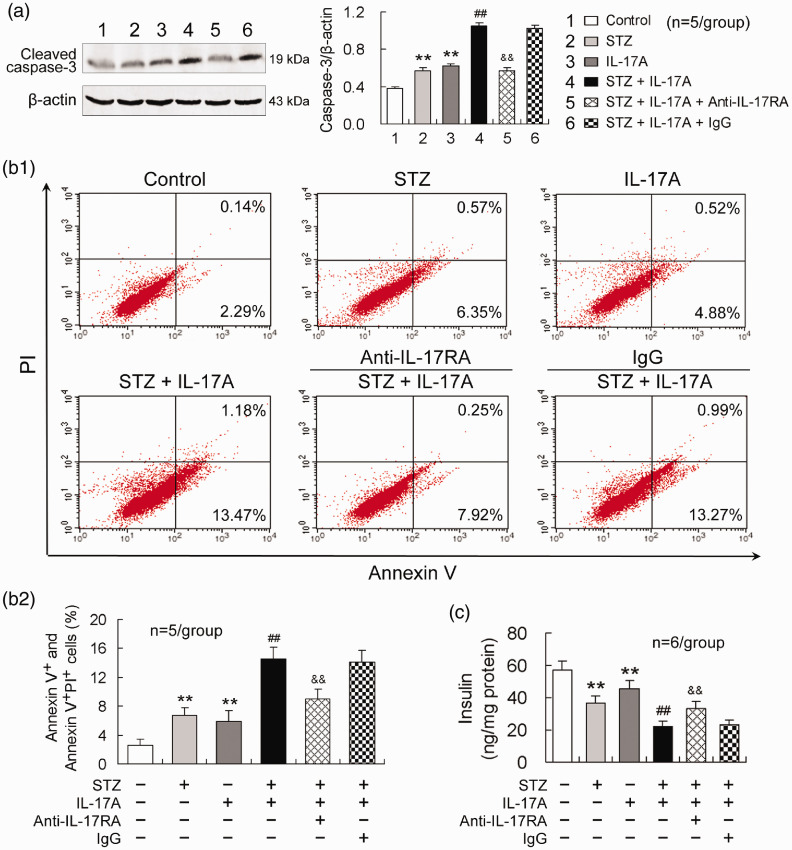
IL-17A exacerbates STZ-induced MIN6 cell apoptosis and insulin impairment, and blocking IL-17RA reduces these effects
Apoptosis-mediated loss of pancreatic β cells correlates with the development of diabetes mellitus.12 Therefore, we assessed apoptosis of insulin-producing MIN6 cells. Compared with control, either STZ or IL-17A treatment alone enhanced caspase-3 activity in MIN6 cells (Figure 4). The combined treatment with STZ and IL-17A further increased caspase-3 activity relative to STZ treatment alone (Figure 4). Blocking IL-17RA with anti-IL-17RA-neutralizing antibody abolished IL-17A effect of enhancing caspase-3 activity in MIN6 cells (Figure 4). Furthermore, Annexin V/PI flow cytometric analysis showed that apoptotic and necroptotic cell populations in MIN6 cells were increased by the treatment with either STZ or IL-17A and further increased by the combined treatment with STZ and IL-17A. Blocking IL-17RA in the presence of STZ and IL-17A reduced the percentage of the dead cells in MIN6 cells compared with isotype antibody (IgG) treatment (Figure 4).
Figure 4.
IL-17A exacerbates STZ-induced MIN6 cell apoptosis and insulin impairment and blocking IL-17RA reduces these effects. (a) IL-17A enhances STZ-induced caspase-3 activity in MIN6 cells and anti-IL-17RA antibody abolishes this effect. (b1) A representative image of FACS analysis with Annexin V-FITC and PI staining. The double negative cell population is healthy, Annexin V positive cells are early apoptotic, and double positive cells are late apoptotic and necroptotic cell populations. (b2) A statistical histogram from the data like (b1). The Annexin V positive cells plus double positive cells were statistically analyzed as dead cells. (c) Blocking IL-17RA reduces IL-17A effect of attenuating insulin production in MIN6 cells. The supernatants of MIN6 cell cultures were collected at 24 h after the treatments and determined for insulin level by ELISA. The insulin concentration in the supernatants of MIN6 cell cultures was normalized to total cellular proteins. The design of the experiments was similar to that in Figure 3. **P < 0.01 versus control; ##P < 0.01 versus STZ; &&P < 0.01 versus STZ + IL-17A + IgG. (A color version of this figure is available in the online journal.)
To demonstrate that β-cell apoptosis leads to insulin production impairment, a major property of diabetes mellitus, we examined insulin levels in supernatants of MIN6 cell cultures. Either STZ or IL-17A treatment diminished insulin level and combination of STZ and IL-17A further decreased insulin level in supernatants of MIN6 cell cultures (Figure 4). Blocking IL-17RA with anti-IL-17RA-neutralizing antibody in the combination of STZ and IL-17A elevated insulin level in MIN6 cells relative to isotype antibody (IgG) treatment (Figure 4).
Discussion
Akita mice spontaneously develop into pronounced insulin dependent diabetes with an onset of three to four weeks of age and exhibit characteristic signs, including hyperglycemia and hypoinsulinemia.12 The hyperglycemia is robust at the age of 10 to 12 weeks.6 In this study, Akita mice initiated the elevated blood glucose concentration at 4-week age, reached the peak at 12-week age, and kept the high values up to 16-week age, the finally observed age. Simultaneously, insulin level in serum of Akita mice was significantly decreased at the observed 12-week age. These data confirm that Akita mice represent the properties of T1D. Importantly, IL-17A-deficient Akita mice manifested the significantly reduced blood glucose concentration and the increased serum insulin level in comparison with IL-17A-intact Akita mice, although both the values did not return to normal control levels. The results demonstrate that IL-17A deficiency mitigates diabetic development induced by the insulin gene mutation. In support of our present results, Tong et al.16 show that IL-17A deficiency ameliorates STZ-induced hyperglycemia, insulitis, and splenic CD8+ cell number. On the other hand, Joseph et al.15 indicate that IL-17A silencing does not protect NOD mice from autoimmune diabetes. The inconsistent results may be explained by the different diabetic animal models. In Akita mice, ER stress is a major cause for pancreatic β-cell apoptosis.12,17IL-17A induces ER stress in macrophages and IL-17A inhibition attenuates ER stress and neuroinflammation.25 Accordingly, we propose that IL-17A deficiency may reduce ER stress in Akita mice and thereby can improve T1D signs.
Furthermore, both the expression of IL-17A, TNF-α, IL-1β, and IFN-γ in the pancreas and the concentrations in serum were increased in Akita mice, demonstrating that enhanced inflammatory reaction occurs in T1D development caused by the insulin gene mutation. Importantly, IL-17A knockout attenuated the production of TNF-α, IL-1β, and IFN-γ in Akita mice, suggesting that IL-17A is closely related to the inflammatory reaction driven by the proinflammatory cytokines in T1D development. In NOD mice, which can develop spontaneous T1D similar to humans, insulitis appears at around third or fourth week of their age.12 During this prediabetic stage, the islets of the pancreas become infiltrated by CD4+ and CD8+ lymphocytes, though natural killer and B cells are also present. The infiltration of innate immune cells into the islets attracts adaptive CD4+ and CD8+ T cell subsets into the islets starting from approximately 4–6 weeks of age, which are both required for diabetes development. Insulitis leads to the destruction of β cells, while the onset of overt diabetes usually appears when approximately 90% of the pancreatic insulin is lost at around 10–14 weeks, although diabetes can develop up to 30 weeks of age.12 It is clear that the proinflammatory cytokines TNF-α, IL-1β, and IFN-γ are produced mainly by innate immune cells such as macrophages and monocytes and by adaptive immune cells such as Th1 lymphocytes. In patients with T1D, TNF-α, IL-1β, and IFN-γ are increased in splenocytes and peripheral blood mononuclear cells.26 In STZ-induced diabetic mouse model, TNF-α, IL-1β, and IFN-γ are elevated in blood, CD3+ lymphocytes infiltrate into pancreatic islets, and periinsular apoptotic cells are observed.27 Overexpression of proinflammatory cytokines in immune competent cells in T1D results in insulitis by infiltration of macrophages and T lymphocytes into islet tissue, leading to β-cell death through the action of TNF-α and IFN-γ.28 More directly, Diaz-Ganete et al.29 induced T1D in mice by administration of a cytokine cocktail containing IL-1β, IFN-γ, and TNF-α. These data strongly show that the proinflammatory cytokines, which derived most possibly from innate immune cells and Th1 cells, are implicated in inflammation leading to pancreatic β-cell death and T1D development. Notably, the long-held view that autoimmune T1D is primarily driven by a Th1 response has been challenged by the discovery of Th17 cells.15 Honkanen et al.10 have reported an upregulation of Th17 immunity in peripheral blood T cells from children with T1D. This is characterized by increased IL-17 secretion and expression in vitro. Also, circulating memory CD4 cells from children with T1D show the same pattern of IL-17 mRNA upregulation, indicating IL-17 pathway activation in vivo.10 We propose that IL-17A exerts a synergistic effect with innate and Th1 immune responses in T1D development, since IL-17A deficiency reduced the proinflammatory cytokine production and alleviated diabetic manifestations. The explanation is supported by the other report that IL-17/IFN-γ receptor double-deficient NOD mice have an apparent decline in longstanding diabetes onset in comparison with IL-17 single-deficient NOD mice.14
Studies have suggested that STZ is preferably absorbed by insulin-secreting β cells and induces diabetes associated with inflammatory changes of pancreatic islets involving T cells and macrophages.30 STZ cytotoxicity is mediated by reactive oxygen species (ROS), reactive nitric oxide species (NO/RNS), and inflammatory responses.31 In this study, STZ induced IL-17A production in the pancreas. Further, exogenously added IL-17A upregulated TNF-α, IL-1β, IFN-γ, and iNOS expression and enhanced STZ-induced expression of the inflammatory factors in MIN6 cells. The findings suggest that IL-17A increase in pancreatic tissue induced by STZ can further promote inflammatory reaction and oxidative stress of β cells, implying a synergistic effect of IL-17A with STZ. It has been shown that TNF-α, IL-1β, or IFN-γ exerts a direct inflammatory injury to pancreatic β cells18,19 and IL-17 has a synergistic effect in combination with the proinflammatory cytokines.9,21,32 We propose that in addition to the synergistic effect, IL-17A can also directly act on pancreatic β cells to enhance inflammatory response and oxidative stress. Both IL-17RA and IL-17RC were expressed in MIN6 cells and upregulated by IL-17A in this study. IL-17A induces positive regulator of its own signaling pathway, resulting in a complex feedback loop that can amplify inflammatory response.8 Accordingly, our present results suggest that IL-17A-induced IL-17R expression, as reported by other authors,33 may be an outcome of regulation by IL-17A itself or/and cellular signals. Although IL-17 signals through the heterodimeric receptor of IL-17RA and IL-17RC,34,35 lack of either IL-17RA or IL-17RC in the heterodimer completely abrogates IL-17 function.36 In the present study, blocking IL-17RA attenuated but not completely abolished the deleterious effects of IL-17A and STZ combination on pancreatic β cells, suggesting that only IL-17A synergistic effect may be reversed by IL-17RA blockage. Arif et al.9 have reported that IL-17RA expressed in rat insulin-producing INS-1E cells is upregulated by combined IL-1β and IFN-γ treatment. They think that the upregulated IL-17RA expression by IL-1β and IFN-γ may trigger IL-17A through IL-17RA to exacerbate apoptosis initiated by IL-1β and IFN-γ, leading to increased β-cell death. Our present results suggest that IL-17RA expression is regulated directly by IL-17A in pancreatic β cells and therefore IL-17A can directly affect survival of the cells via IL-17RA pathway.
Indeed, IL-17A increased MIN6 cell apoptosis and further enhanced STZ-induced cell apoptosis. The pro-apoptotic effect of IL-17A was blocked by anti-IL-17A antibody. Furthermore, IL-17A impaired insulin production in MIN6 cells, demonstrating a consequence due to MIN6 cell death. This effect of IL-17A was also reduced by the blockage of IL-17RA. The findings confirm that IL-17A exacerbates STZ-induced β-cell death by acting on IL-17RA. The other reports have presented that IL-17A/IL-17 augments apoptosis in mouse, rat, or human β-cell line (or murine β cells) induced by the combined treatments with IL-1β and IFN-γ or TNF-α and IFN-γ.9,32 Our current results provide further evidence showing that IL-17A has a synergistic effect with STZ in promoting β-cell death and insulin impairment via IL-17RA pathway.
In summary, IL-17A deficiency alleviated hyperglycemia and hypoinsulinemia and also reduced TNF-α, IL-1β, and IFN-γ production in Akita mice. These data suggest that IL-17A is involved in T1D pathogenesis by enhancing inflammatory reaction. Further, STZ induced IL-17A production in pancreatic tissue of mice in vivo. Exogenously added IL-17A enhanced STZ-induced TNF-α, IL-1β, IFN-γ and iNOS expression in mouse insulin-producing MIN6 cells, and the effect of IL-17A was blocked by anti-IL-17RA-neutralizing antibody. Simultaneously, IL-17A exacerbated STZ-induced apoptosis and insulin reduction in MIN6 cells. Blocking IL-17RA abolished the IL-17A deleterious effects. Accordingly, we propose that IL-17A augments inflammatory reaction and oxidative stress in pancreatic β cells through IL-17RA pathway and thereby aggravates β-cell apoptosis and insulin impairment that are characteristic for T1D. Thus, targeting IL-17A or/and IL-17RA is likely to preserve remaining β-cell function and treat T1D.
Footnotes
Authors' contributions: QAW, ZWW, and LQH participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript; QAW and CX conducted the experiments; QAW wrote the manuscript and LQH edited the manuscript. All authors read and approved the final manuscript.
Declaration OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China [grant numbers 81900873, 81770973].
ORCID iD: Qing-Huai Liu https://orcid.org/0000-0003-1351-7343
References
- 1.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011; 11:98–107 [DOI] [PubMed] [Google Scholar]
- 2.Bending D, Zaccone P, Cooke A. Inflammation and type one diabetes. Int Immunol 2012; 24:339–46 [DOI] [PubMed] [Google Scholar]
- 3.Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep 2013; 13:435–44 [DOI] [PubMed] [Google Scholar]
- 4.Stoy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, Greeley SA, Patch AM, Ellard S, Steiner DF, Hattersley AT, Philipson LH, Bell GI. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A 2007; 104:15040–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010; 464:1293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavali V, Nandi SS, Singh SR, Mishra PK. Generating double knockout mice to model genetic intervention for diabetic cardiomyopathy in humans. Mouse genetics. London: Humana Press, 2014, pp. 385–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K, Li F, Cui Y, Cui C, Cao Z, Xu K, Han S, Zhu P, Sun Y. The association between depression and type 1 diabetes mellitus: inflammatory cytokines as ferrymen in between? Mediators Inflamm 2019; 2019:2987901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity 2019; 50:892–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arif S, Moore F, Marks K, Bouckenooghe T, Dayan CM, Planas R, Vives-Pi M, Powrie J, Tree T, Marchetti P, Huang GC, Gurzov EN, Pujol-Borrell R, Eizirik DL, Peakman M. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated beta cell death. Diabetes 2011; 60:2112–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, Ilonen J, Knip M, Otonkoski T, Vaarala O. IL-17 immunity in human type 1 diabetes. J Immunol 2010; 185:1959–67 [DOI] [PubMed] [Google Scholar]
- 11.Kumar P, Natarajan K, Shanmugam N. High glucose driven expression of proinflammatory cytokine and chemokine genes in lymphocytes: molecular mechanisms of IL-17 family gene expression. Cell Signal 2014; 26:528–39 [DOI] [PubMed] [Google Scholar]
- 12.Al-Awar A, Kupai K, Veszelka M, Szűcs G, Attieh Z, Murlasits Z, Török S, Pósa A, Varga C. Experimental diabetes mellitus in different animal models. J Diabetes Res 2016; 2016:9051426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol 2009; 39:216–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuriya G, Uchida T, Akazawa S, Kobayashi M, Nakamura K, Satoh T, Horie I, Kawasaki E, Yamasaki H, Yu L. Double deficiency in IL-17 and IFN-γ signalling significantly suppresses the development of diabetes in the NOD mouse. Diabetologia 2013; 56:1773–80 [DOI] [PubMed] [Google Scholar]
- 15.Joseph J, Bittner S, Kaiser FM, Wiendl H, Kissler S. IL-17 silencing does not protect nonobese diabetic mice from autoimmune diabetes. J Immunol 2012; 188:216–21 [DOI] [PubMed] [Google Scholar]
- 16.Tong Z, Liu W, Yan H, Dong C. Interleukin-17A deficiency ameliorates streptozotocin-induced diabetes. Immunology 2015; 146:339–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi J, Kobayashi H, Okuda H, Harada KH, Takeda M, Fujimoto H, Yamane S, Tanaka D, Youssefian S, Inagaki N, Koizumi A. β-cell-specific overexpression of adiponectin receptor 1 does not improve diabetes mellitus in Akita mice. PLoS One 2018; 13:e0190863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pirot P, Cardozo AK, Eizirik DL. Mediators and mechanisms of pancreatic beta-cell death in type 1 diabetes. Arq Bras Endocrinol Metabol 2008; 52:156–65 [DOI] [PubMed] [Google Scholar]
- 19.Burke SJ, Lu D, Sparer TE, Karlstad MD, Collier JJ. Transcription of the gene encoding TNF-α is increased by IL-1β in rat and human islets and β-cell lines. Mol Immunol 2014; 62:54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology 2011; 134:8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miljkovic D, Cvetkovic I, Momcilovic M, Maksimovic-Ivanic D, Stosic-Grujicic S, Trajkovic V. Interleukin-17 stimulates inducible nitric oxide synthase-dependent toxicity in mouse beta cells. Cell Mol Life Sci 2005; 62:2658–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu AW, Liu QH, Wang JL. Blocking IL-17A alleviates diabetic retinopathy in rodents. Cell Physiol Biochem 2017; 41:960–72 [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25:402–8 [DOI] [PubMed] [Google Scholar]
- 24.Pietkiewicz S, Schmidt JH, Lavrik IN. Quantification of apoptosis and necroptosis at the single cell level by a combination of imaging flow cytometry with classical annexin V/propidium iodide staining. J Immunol Methods 2015; 423:99–103 [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Liu Q, Shi H, Jiang X, Wang S, Lu Y, Zhang J, Huang X, Yu A. Interleukin 17A exacerbates ER-stress-mediated inflammation of macrophages following ICH. Mol Immunol 2018; 101:38–45 [DOI] [PubMed] [Google Scholar]
- 26.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005; 54:S97–107 [DOI] [PubMed] [Google Scholar]
- 27.Shehata AM, Quintanilla-Fend L, Bettio S, Singh CB, Ammon HP. Prevention of multiple low-dose streptozotocin (MLD-STZ) diabetes in mice by an extract from gum resin of boswellia serrata (BE). Phytomedicine 2011; 18:1037–44 [DOI] [PubMed] [Google Scholar]
- 28.Ammon HPT. Boswellic extracts and 11-keto-ß-boswellic acids prevent type 1 and type 2 diabetes mellitus by suppressing the expression of proinflammatory cytokines. Phytomedicine 2019; 63:153002. [DOI] [PubMed] [Google Scholar]
- 29.Diaz-Ganete A, Baena-Nieto G, Lomas-Romero IM, Lopez-Acosta JF, Cozar-Castellano I, Medina F, Segundo C, Lechuga-Sancho AM. Ghrelin's effects on proinflammatory cytokine mediated apoptosis and their impact on β-Cell functionality. Int J Endocrinol 2015; 2015:235727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimoto H, Hirase T, Miyazaki Y, Hara H, Ide-Iwata N, Nishimoto-Hazuku A, Saris CJ, Yoshida H, Node K. IL-27 inhibits hyperglycemia and pancreatic islet inflammation induced by streptozotocin in mice. Am J Pathol 2011; 179:2327–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nahdi AMTA, John A, Raza H. Elucidation of molecular mechanisms of Streptozotocin-Induced oxidative stress, apoptosis, and mitochondrial dysfunction in rin-5F pancreatic β-Cells. Oxid Med Cell Longev 2017; 2017:7054272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grieco FA, Moore F, Vigneron F, Santin I, Villate O, Marselli L, Rondas D, Korf H, Overbergh L, Dotta F, Marchetti P, Mathieu C, Eizirik DL. IL-17A increases the expression of proinflammatory chemokines in human pancreatic islets. Diabetologia 2014; 57:502–11 [DOI] [PubMed] [Google Scholar]
- 33.Ganesan R, Rasool M. Interleukin 17 regulates SHP-2 and IL-17RA/STAT-3 dependent Cyr61, IL-23 and GM-CSF expression and RANKL mediated osteoclastogenesis by fibroblast-like synoviocytes in rheumatoid arthritis. Mol Immunol 2017; 91:134–44 [DOI] [PubMed] [Google Scholar]
- 34.Ely LK, Fischer S, Garcia KC. Structural basis of receptor sharing by interleukin 17 cytokines. Nat Immunol 2009; 10:1245–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y, Ota N, Peng I, Refino CJ, Danilenko DM, Caplazi P, Ouyang W. IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol 2010; 184:4307–16 [DOI] [PubMed] [Google Scholar]
- 36.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol 2006; 177:36–9 [DOI] [PubMed] [Google Scholar]