Abstract
SARS-CoV-2 infection, resulting in Coronavirus disease 2019 (COVID-19), has significantly affected the entire world. It was labelled a pandemic by World Health Organization. Although it commonly produces respiratory symptoms, neurological features have been described. Neurological manifestations may vary from non-specific symptoms such as headache, dizziness, myalgia and/or fatigue, olfactory or taste dysfunction to specific syndromes including meningitis, stroke, acute transverse myelitis and Guillain-Barre syndrome. This review describes potential pathogenetic mechanisms and neurological manifestations of COVID-19 along with its management. Considering structural and pathogenetic similarity of SARS-CoV-2 with SARS-CoV and MERS viruses, we compared their neurological manifestations and mentioned few features expected in COVID-19 in future. Interestingly, many COVID-19 cases may present with pure neurological manifestations at onset with non-neurological features manifesting few days later and we propose the term “Neuro-COVID syndrome” for such cases. Awareness of neurological manifestations may facilitate its management and improve outcome in such patients.
Keywords: Coronavirus, COVID-19, Neuro-COVID syndrome, neuroinvasion, neurological manifestations, SARS-CoV-2
Introduction
An outbreak of atypical pneumonia caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was initially reported in December 2019 from Wuhan in China [1]. The resulting disease is now termed as coronavirus disease 2019 (COVID-19). SARS-CoV-2 is a novel beta coronavirus which shares structural and genetic homology to SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), but is relatively more contagious with a longer incubation period, shorter serial interval, and lower case fatality rate. Its high person-to-person transmissibility resulted in a rapid rise in number of cases, and on March 11, 2020, WHO declared COVID-19 a pandemic with serious health concerns [2]. Owing to a high proportion of cases being asymptomatic or mildly symptomatic, the complete denominator remains unknown. While literature is replete with pulmonary manifestations of COVID-19, its neurological complications are being increasingly recognized. This review discusses the current state of knowledge about neuropathogenesis and neurological manifestations in COVID-19 along with its management and compares it to SARS-CoV and MERS outbreaks, to obtain further insight into its neuropathogenesis and additional neurological manifestations expected.
Methods
We searched PubMed database from April 1, 2020, to November 15, 2020, for articles published in English from October 1990 to November 15, 2020, along with references from the relevant articles. The search terms used included “Coronavirus,” “SARS,” “MERS,” “COVID-19,” “SARS-CoV-2,” “SARS-CoV” alone or in combination with “neurology,” “neuroinvasion,” “meningoencephalitis,” “encephalopathy,” “stroke,” “multiple sclerosis,” “acute disseminated encephalomyelitis (ADEM),” “polyneuropathy,” “Guillain–Barre syndrome (GBS),” “neuromuscular disorder,” and “myopathy.”
Pathogenesis
SARS-CoV-2 is an enveloped, non-segmented, single-stranded RNA virus of betacoronaviridae family. While commonly reported human CoV infections cause self-limiting flu-like symptoms, SARS-CoV and MERS-CoV globally infected nearly 10,500 people in past two decades, with case fatality rate of approximately 10% and 36%, respectively [1]. CoVs possess a genomic RNA of 29.9 kilobase pairs in a nucleocapsid core and a phospholipid bilayer with embedded virulent surface proteins including spike (S), hemagglutinin-esterase (HE), membrane (M), and envelope (E) proteins.
The dynamics of SARS-CoV-2 are currently unknown, but a non-human origin like SARS and MERS has been speculated. Various epidemiological studies propose bats its natural zoonotic reservoir. However, intermediate hosts of SARS-CoV-2, fuelling spill-over to humans, remains unknown, with pangolins, livestock, and zoonotic or aquarian animals being the suspected ones [3–5]. Animal-to-human and human-to-human transmission are potential sources of COVID-19 spread with respiratory droplets from coughing and sneezing being the most common mode. The Centers for Disease Control and Prevention, China, suggested an incubation period of 3 to 7 days, with a maximum up to 2 weeks [2].
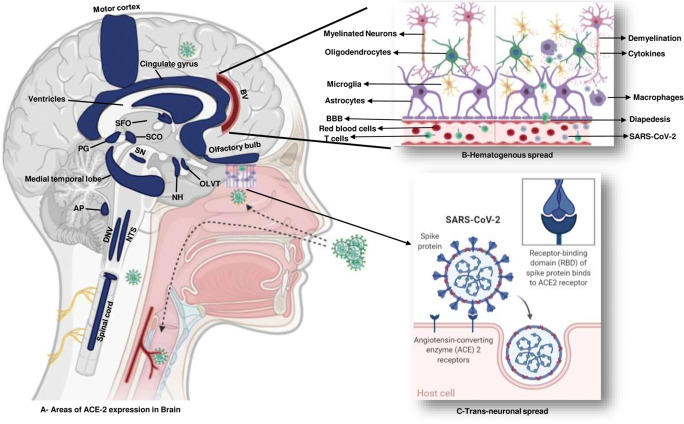
The SARS-CoV-2, similar to SARS-CoV, uses angiotensin-converting enzyme 2 (ACE2) receptor to enter host cells, although with twenty times higher affinity. ACE2 receptor is ubiquitously expressed and has been detected in adipose tissue, heart, brain, lung, vascular endothelium, liver, and naso-oral mucosa, thereby making these organs vulnerable [3]. In brain, ACE2 are expressed in neurons, astrocytes, and oligodendrocytes with high concentration found in the motor cortex, posterior cingulate cortex, middle temporal gyrus, sympathetic pathways in brainstem, substantia nigra, ventricles, circumventricular organs, thalamus, and olfactory bulb (Fig. 1) [3, 4]. SARS-CoV-2 uses S1 subunit of spike protein to attach to host’s ACE2 receptors and S2 subunit for fusion followed by endocytosis. Once inside the cell, uncoated RNA translates various proteins via sub-genomic RNA, followed by assembly of viral particle buds and release from host cells [3, 4].
Fig. 1.
Schematic diagram depicting high ACE-2-containing brain regions with mechanisms of neuroinvasion by SARS-CoV-2. a Brain regions with high ACE-2 expression. b Hematogenous spread through blood brain barrier. c Transcribrial path from olfactory receptor to brain, followed by activation of inflammation. AP area postrema, BV blood vessels, DNV dorsal motor nucleus of vagus, NH neurohypophysis, NTS nucleus tractus solitarius, OLVT organum vasculosum of the lamina terminalis, PG pineal gland, SCO subcommissural organ, SFO subfornical organ, SN substantia nigra
In addition to direct cellular invasion, indirect mechanisms like inflammatory response and cytokine storm also contribute to the pathogenesis of SARS-CoV-2. Although both cell-mediated and humoral immunity are essential to contain CoV infections, uncontrolled immune response may often be harmful [3]. SARS-CoV-2 activates both innate and cellular immunities resulting into “cytokine storm” with various pathological effects including increased levels of inflammatory cytokines; activation of T lymphocytes, macrophages, and endothelial cells; and stimulation of complement and coagulation cascade leading to disseminated intravascular coagulation and later multiorgan dysfunction syndrome (MODS) [5].
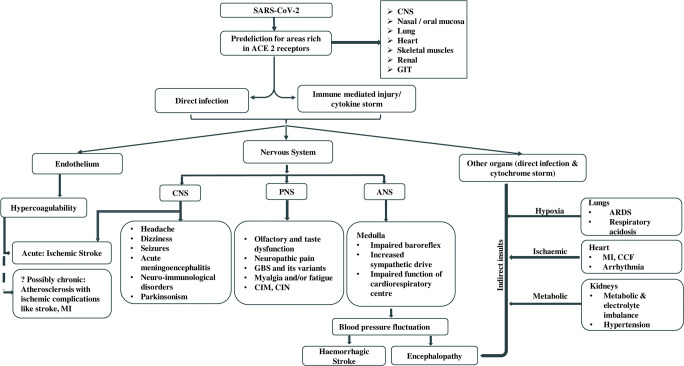
Detection of neuropathological findings in brain tissue of CoV-infected patients favors their neurotropic and neuroinvasive nature [6, 7]. Several theories have been postulated for the virus entry into nervous system, such as direct entry of the virus through ACE2 receptor, hematogenous spread, and transneuronal spread (Fig. 1). It may infect vascular endothelium and cross blood–brain barrier (BBB) or infect leukocytes that pass through BBB to reach brain tissue, with the latter known as the “Trojan horse mechanism” [4]. Transneuronal spread of virus may occur through transcribrial path from olfactory receptor neurons via olfactory nerve and olfactory bulb to reach hippocampus and nearby brain regions, as shown in animal studies [4, 6]. This hypothesis is supported by orbitofrontal hypometabolism in 18fluoro-2-deoxy-d-glucose (18FDG) positron emission tomography computed tomography (PET-CT) brain scan in a COVID-19-positive patient [8]. Once inside the brain, CoVs could affect brain tissue either by directly damaging the neurons after entering through ACE2 receptors or due to misdirected host immune response in susceptible individuals [3, 4]. Additionally, involvement of other essential organs by SARS-CoV-2 may affect the nervous system indirectly due to resulting hypoxia, blood pressure fluctuations, metabolic and electrolyte imbalances (Fig. 2) [4].
Fig. 2.
Possible pathophysiological effects and resulting neurological manifestations of SARS-CoV-2. SARS-CoV-2 can invade and damage susceptible organs expressing ACE2 receptors either by direct infection or immune-mediated mechanism via cytokine storm, leading to manifestations resulting from central, peripheral, and autonomic nervous system involvement. ACE2 angiotensin-converting enzyme 2, ANS autonomic nervous system, ARDS acute respiratory distress syndrome, CCF congestive cardiac failure, CIM critical illness myopathy, CIN critical illness neuropathy, CNS central nervous system, GBS Guillain–Barre syndrome, GIT gastrointestinal tract, MI myocardial infarction, PNS peripheral nervous system
Clinical features
While non-neurological manifestations of COVID-19 illness including fever (77–89%), cough (61–81%), shortness of breath (3–26%), sore throat (10%), and gastrointestinal symptoms (5–9%) are commonly reported, more than one third hospitalized COVID-19 cases may have neurological manifestations [1, 9–11]. Neurological features, including myalgia and/or fatigue, dizziness, headache, anosmia, ageusia, and altered mental status, are relatively common in severe disease, elderly population, and in patients with preexisting comorbidities [4, 11, 12]. They may appear due to central and/or peripheral nervous system involvement, either early or later in the course of COVID-19 illness.
CNS involvement
Manifestations of central nervous system (CNS) involvement are reported in up to 25% of COVID-19 cases [11]. While common non-specific symptoms include dizziness, headache, and altered mentation, several CNS syndromes including meningoencephalitis, cerebrovascular events, seizures, and CNS neuro-immunological disorders have also been reported (Table 1).
Table 1.
Neurological features in COVID-19 reported to date, SARS and MERS illnesses. Expected neurological manifestations of COVID-19 are in italics
| Neurological features | SARS-CoV | MERS | SARS-CoV2 |
|---|---|---|---|
| A. Central nervous system | |||
| 1. Infective | • Acute encephalitis [13, 14] |
• Acute encephalitis [1] • Bickerstaff encephalitiswith GBS [15] |
• Acute necrotizing hemorrhagicencephalitis [16, 17] • Meningoencephalitis [21] • Rhombencephalitis [22] |
| 2. Vascular | • Ischemic stroke [23] | • Ischemic stroke[1] |
• Ischemic stroke with large vesselocclusion [11, 24] • Intracerebral hemorrhage,subarachnoidhemorrhage [25, 26] • Microvascular occlusivedisorder [34] |
| 3. Immunological |
• ADEM [35] |
• ADEM[15] |
• Post infectious ADEM [38, 39] • Acute transverse myelitis [40–43] • Anti-NMDA encephalitis [44] • Generalized and diaphragmaticmyoclonus [45, 46] • Opsoclonus and ocularflutter [47] |
| 4. Extrapyramidal features | • Parkinsonism [48] | • Parkinsonism [49, 50] | |
| 5. Miscellaneous (includingnon-specific features) |
• Alzheimer’s like dementia [48] • Schizophrenia like illness [48] |
• Headache, confusion, dizziness,seizures [1, 5, 10, 11, 24, 51–54] • Alzheimer and schizophrenialike illness expected in future |
|
| B. Peripheral nervous system | |||
| 1. Neuropathy | • CIN [1] |
• GBS [15] • CIN [15] • Sensory neuropathy [15] |
• Olfactory and tastedysfunction [1, 11, 52, 55–57] • GBS [58] • MFS and polyneuritiscranialis [58] • Facial diplegia [58] • Sensory neuropathy • CIN [59] |
| 2. Neuromuscular junction | • Myasthenia gravis [60] | ||
| 3. Myopathy | • CIM [1] |
• Myositis/myalgia [1, 10, 11, 52] • Rhabdomyolysis [1] • CIM [59] |
|
ADEM acute disseminated meningoencephalitis, CIM critical illness myopathy, CIN critical illness neuropathy, COVID-19 coronavirus disease 2019, CVST cerebral venous sinus thrombosis, GBS Guillain–Barre syndrome, MERS-CoV Middle East respiratory syndrome coronavirus, MFS Miller–Fisher syndrome, MS multiple sclerosis, NMDA N-methyl-D-aspartate, PRES posterior reversible encephalopathy syndrome, SARS-CoV severe acute respiratory syndrome coronavirus
Dizziness
Dizziness is a non-specific symptom encountered in several neurological and non-neurological disorders [5]. It is the most common neurological feature described in COVID-19, involving up to 17% cases, especially those in ICU due to severe illness [11, 24].
Headache
Although most series describe headache in 6–13% of COVID-19 cases [1, 10, 11, 51, 61], it was reported in 82% cases in a French study [62]. Its pathophysiology and character may differ according to phase of COVID-19 illness. While acute headache related to flu-like illness, migraine, and tension-type headache predominate in initial days of illness, headache resulting from hypoxia and systemic inflammation due to cytokine storm may occur later in the course [61]. Headache may also be a sentinel sign in COVID-19-related meningitis and venous sinus thrombosis [24]. A recent cross-sectional study of 130 hospitalized COVID-19 patients revealed that headache was bilateral, of severe intensity with frontal predominance and oppressive quality among three-fourth patients. In 62% patients, headache occurred within 24 h of illness. Nearly all (94%) cases fulfilled ICHD-3 criteria for headache attributed to systemic viral infection. While migrainous phenotype was reported in one-fourth cases, nearly half the cases had tension-type headache [63]. Interestingly, presence of headache in patients with COVID-19 pneumonia has been associated with a shorter course of non-neurological systemic illness, but disabling headache often persisted [64].
Altered mentation
Impairment in the level or content of consciousness may involve up to 9% of hospitalized COVID-19 patients, especially severe cases [11, 21]. Agitation and confusion affect more than two-third cases admitted in ICU and nearly one-third patients can manifest executive dysfunction after discharge [53]. MRI brain in 13 cases with undiagnosed encephalopathy revealed leptomeningeal enhancement in eight (62%), ischemic stroke in three (23%) cases, while bilateral fronto-temporal hypoperfusion in eleven (84%) cases [53]. Various factors contributing to altered mentation in COVID-19 include toxic-metabolic encephalopathy resulting from cytokine storm with systemic hyperinflammation, cerebrovascular events, seizures, para- or postinfectious immune-mediated CNS syndromes and a possible CNS infection by SARS-CoV-2 [11]. In addition, new-onset immune-mediated psychotic symptoms have also been described in COVID-19 cases [65].
Meningitis or meningoencephalitis
In 33 meningoencephalitis cases reported in association with SARS-CoV-2 infection, varied manifestations have been described including delirium/altered sensorium (71%), aphasia/dysarthria (53%), headache (34%), seizures/status epilepticus (34%), focal neurological deficits (18%), and myoclonus (9%) with a single patient having parkinsonian syndrome (3%) [18, 21, 49, 62, 66].
Three case reports described more fulminant course of COVID-19-associated encephalitis in patients of acute hemorrhagic necrotizing encephalopathy and rhombencephalitis [18, 20, 21]. Meningeal signs (including nuchal rigidity, Kerning, and Brudzinski signs) along with extensor plantar response were present among all. Commonly reported viral and bacterial screening was negative. While only a single case showed presence of SARS-CoV-2 in cerebrospinal fluid (CSF) [18], three cases had increased anti-S1 IgM antibodies with markedly elevated inflammatory markers in CSF [62], thereby suggesting a role of para-/postinfectious autoimmune response. All cases made good recovery with antiviral with or without immunosuppressants (high-dose methylprednisolone, intravenous immunoglobulins, plasmapheresis).
Cerebrovascular events
Ischemic and hemorrhagic arterial stroke, cortical venous sinus thrombosis (CVST), as well as intracranial vasculitis-induced microvascular occlusive disorder have been reported in COVID-19 cases, with ischemic stroke being most common. Of 214 COVID-19 cases in a series, six (2.8%) developed stroke including five ischemic and one hemorrhagic stroke [11]. Another series of 221 hospitalized COVID-19 cases reported 13 (5.8%) stroke cases including 11 (5%) ischemic and one (0.5%) case each of hemorrhagic stroke and CVST. Stroke commonly affects elderly patients with severe COVID-19 illness along with vascular risk factors including hypertension, diabetes, and prior stroke [11, 24]. Although ischemic stroke usually appears later in the course of COVID-19 illness with a mean duration being 12 days, early occurrence has also been reported. COVID-19-related ischemic stroke often result from large vessel occlusion and may be muti-territorial [24, 67, 68]. Several cases of COVID-19-associated CVST have been reported. They likely present in the second week of infection with no sex predilection, with headache being the most common symptom followed by focal neurological deficit. Neuroimaging shows non-arterial infarct or hemorrhage [28, 29, 69].
Increased risk of arterial ischemic stroke or CVST in SARS-CoV-2 infection suggests a pro-coagulant state, which may result from either blood flow stasis, especially in critically ill and immobilized patients or due to hypercoagulability and direct endothelial damage via ACE-2 receptors [68]. A highly elevated C-reactive protein (CRP) and D-dimer in COVID-19 patients suggest hyperinflammation and hypercoagulable state, respectively [24]. This in turn stimulates endothelial and mononuclear cells and facilitates tissue factor expression leading to excess free thrombin generation, which results in platelet activation and thrombosis [70]. Thrombocytopenia along with raised CRP and D-dimer in COVID-19-associated stroke cases suggest possibility of underlying virus-associated microangiopathy [71]. Significantly, elevated D-dimer and fibrin degradation product (FDP) along with prolonged PT and APTT on admission suggest poor survival in COVID-19 pneumonia [70]. Elevated antiphospholipid antibodies reported in COVID-19-related ischemic stroke lacks significance due to transient elevation seen in several infections and critically ill patients [67, 72].
ACE2 receptors in circumventricular organ and endothelial cells are essential in modulating cerebral autoregulation, cerebral blood flow, and central autonomic activity. Neuroinvasion of SARS-CoV-2 at these locations may disrupt cerebral autoregulation leading to blood pressure fluctuations resulting in intracerebral and/or subarachnoid hemorrhage reported in COVID-19 cases. Additionally, hyperinflammatory state resulting from cytokine storm along with sympathetic over activity may lead to aneurysm formation and/or trigger its rupture [25, 26].
Extensive intracranial vasculitis resulting in local micro-thrombosis and micro-hemorhages has recently been reported in a 68-year-old male patient suffering from SARS-CoV-2, who had persistent impaired sensorium after sedation stoppage. Brain MRI showed multiple areas of restricted diffusion along with scattered hypointensities in SWI sequence. CSF was positive for SARS-CoV-2 RNA. This might have resulted from a systemic pro-coagulant state along with local and systemic inflammation–related endothelium damage and eventual immune-mediated vascular injury [34].
Cerebral autoregulation dysfunction has been implicated in the development of posterior reversible encephalopathy syndrome (PRES), reported in nine COVID19 cases to date, all aged above 60 except a young male of 38. Two-third patients had preexisting hypertension and diabetes. Six patients presented with acute respiratory distress syndrome followed by acute kidney injury (AKI) with rapid elevation of blood pressure preceding PRES. Persistent confusion, lethargy, focal neurological deficits, and seizures despite improvement in other non-neurological parameters helped diagnose PRES. All patients improved with symptomatic management of seizure along with strict control of blood pressure [30–33, 73].
Seizures
Seizures and/or status epilepticus (convulsive or non-convulsive) appears likely as SARS-CoV-2 may involve CNS either directly or indirectly related to hypoxia, metabolic and electrolyte imbalances [4]. Few reports describe clinical or electrophysiological evidence of new onset seizures or new-onset refractory status epilepticus (NORSE) in COVID-19 patients [18, 54, 74], but larger studies failed to detect additional risk of acute symptomatic seizures [11, 53]. Breakthrough seizures are commonly reported in epileptic patients developing COVID-19 [4]. Reduced seizure threshold in COVID-19 patients may appear even in absence of overt inflammatory features, resulting in new-onset seizure or status epilepticus or reappearance of well-controlled seizures [30–33, 73, 75].
Generalized, both positive and negative, myoclonus primarily involving craniofacial region and upper extremities have been reported in three COVID-19 cases, lacking evidence of metabolic or hypoxic disturbance, any offending drug or known autoimmune disorders [45]. In addition to being spontaneous, the jerks worsened with voluntary movement and tactile and auditory stimuli with an exaggerated startle response. Available MRI brain and CSF study reports were unremarkable. Based on its character, myoclonus seemed to be of brainstem origin, likely postinfectious or immune-mediated in nature especially when immunomodulation with pulse methylprednisolone and plasma exchange led to significant clinical improvement [37]. Sequential appearance of hyposmia, hypersomnia, and generalized myoclonus in these cases indicate possible transneuronal spread of SARS-CoV-2 through first- and second-order olfactory pathway to involve hypothalamus and brainstem [45].
CNS neuroimmunological disorders
Although human CoVs have been implicated in multiple sclerosis (MS) [48], association of SARS-CoV-2 with MS is lacking. MRI brain in a 54-year-old male, presenting with COVID-19 pneumonia and seizures, revealed T2 and FLAIR hyperintense lesions involving bilateral periventricular white matter, corpus-callosum, cervico-medullary junction, and cervico-dorsal spinal cord, lacking diffusion restriction or contrast enhancement. CSF examination failed to detect SARS-CoV-2 or confirm MS. Since demyelinating changes along with virus particles have been detected in autopsied brain tissue in SARS-CoV, a possibility of SARS-CoV-2-related demyelinating pathology can be speculated in this case. SARS-CoV-2 infection–related hyperinflammatory state might cause glial activation and CNS demyelination. Another hypothesis proposes demyelination related to a delayed immune response, after viremia had settled, and hence failure to detect SARS-CoV-2 in CSF [39].
At least four COVID-19-related acute transverse myelitis (ATM) have been reported to date with patients presenting with acute onset symmetrical sensorimotor quadri- or paraparesis with bladder/bowel disturbances. None of these cases manifested features related to cerebrum, optic nerve, cerebellar pathways, or brainstem involvement. Three of them developed neurological symptoms in the second week of illness, with the fourth case manifesting on the 3rd day of illness [40–43]. MRI study revealed longitudinally extensive transverse myelitis involving the entire length of cord in first two [40], multifocal transverse myelitis in third [42], and brainstem involvement in the fourth case [40]. Although SARS-CoV-2 could not be detected in CSF examination, it revealed lymphocytic pleocytosis with hyperproteinorrachia in one of the four cases [42]. Relevant autoimmune panel in serum and CSF and serological screening for neurotropic viruses were negative [40, 42, 43]. Considering the onset of myelitis symptoms in the second week of illness along with significant response to immunomodulation, immune-mediated pathology appears likely in these cases [43]. Nevertheless, SARS-CoV-2 directly invading the spinal cord cannot be ruled out, especially when ACE2 receptors have been detected on the surface of spinal cord neurons [76]. A 23-year-old COVID-19 patient with substance abuse disorder, presenting with fever and neuropsychiatric symptoms followed by dysphagia, dyskinesia, hyponatremia, and autonomic dysfunction, showed positive anti-NMDA receptor antibodies and negative SARS-CoV-2 in CSF. Patient showed clinical improvement with high-dose dexamethasone and intravenous immunoglobulin [44].
Movement disorders
Probable Parkinson’s disease has been reported in a 70-year-old male 2 months following SARS-CoV-2 infection with asymmetrical reduction of 18F-FDOPA uptake in basal ganglia and good response to pramipexole and biperiden. COVID-19-associated immune activation in the olfactory system leading to alpha-synuclein misfolding and development of Parkinsonian features has been speculated in such cases [49, 50]. Reports of COVID-19-associated other movement disorders including ocular flutter, opsoclonus, tremors, myoclonus, and ataxia have also been reported [47, 77, 78]. Most of them manifested in the second or third week of illness, making postinfectious or para-infectious autoimmune phenomenon the likely pathogenesis.
Peripheral nervous system and muscle involvement
PNS involvement has been reported in nearly 10% of COVID-19 patients, with smell and taste impairment being most common [11].
Smell and taste impairment
Several series report smell and taste dysfunction in 5–90% COVID-19 cases, with females outnumbering males [1, 11, 52, 55, 57]. They are more commonly reported in European cases as compared to Asian, probably related to genetic variability in distribution and expression of ACE2 receptors or mutation in virus strain. Nearly 80% cases lacking nasal obstruction or discharge report smell impairment. Although olfactory dysfunction in 88% cases appears simultaneously or after clinical onset of COVID-19, it may be the sentinel feature in 12% cases. Although recovery in smell and taste dysfunction may take longer in some cases, near complete resolution has been seen in 25–80% cases within 2 weeks [52, 57]. High prevalence of anosmia and ageusia in absence of nasal obstruction or rhinorrhoea suggest possible sensory neural loss along with trans-nasal transmission, as the underlying cause of the olfactory dysfunction rather than the conductive mechanism seen in most cases of postviral olfactory loss [65, 71, 79].
GBS and cranial neuropathy
To date, at least 73 cases of COVID-19-related GBS and its variants have been reported [58]. Flaccid-areflexic weakness (paraparesis/quadriparesis) with/without sensory symptoms was the major clinical form (70%), with 10% cases having features suggestive of Miller–Fisher syndrome (MFS). Other variants reported include facial diplegia (6.8%), polyneuritis cranialis (2.7%), and pharyngeal-cervical-brachial variant (1.3%) [58]. Pupillary sparing oculomotor nerve palsy and isolated ophthalmoplegia involving right abducens have also been reported in two separate cases [80]. Interestingly, early and severe respiratory compromise was observed in one fourth patients, probably related to involvement of brainstem respiratory center [81]. While neurological features in most cases appeared after first week of illness suggestive of postinfectious immune-mediated pathology, a single case showed pure neurological onset favoring a para-infectious autoimmune pathology.
More than four-fifth (81.8%) cases satisfied electrophysiological criteria for AIDP, with AMSAN and AMAN reported in 12.7% and 5.4% cases, respectively. Of 23 patients (31%), who underwent brain and/or spinal cord MRI, contrast enhancement of nerve roots were seen at the level of the brachial plexus in two (8%), lumbosacral plexus in four (17%), cauda equina in four (17%), spinal nerves roots in eight (34%), and cranial nerves (especially third, sixth, seventh, eighth) in five (21%) cases. While CSF SARS-CoV-2 RNA was absent in all tested cases, more than two-third (71%) cases showed albumin-cytological dissociation. A single case of classic sensorimotor GBS along with one of the cases with MFS tested positive for serum anti-GM1 antibodies anti-GD1b, respectively. More than 70% of patients showed a good prognosis, mostly after treatment with intravenous immunoglobulin [58].
Muscle involvement
Myalgia and/or fatigue is one of the most common symptoms in COVID-19, reported in 10–74% cases [1, 10, 11, 53]. Skeletal muscle damage with increased serum creatinine kinase levels above 200 U/L had been seen in 11% of hospitalized COVID-19 cases, especially in patients having concurrent liver and renal dysfunction [10]. Rhabdomyolysis has been reported in four COVID-19 cases [1, 82, 83], presenting with weakness, pain, and tenderness in lower limbs. Presence of ACE2 receptors in skeletal muscle indicate possible direct muscle infection by SARS-CoV-2 but immune-related pathology has also been speculated [5, 11]. Although not reported to date, severe COVID-19 cases requiring prolonged ICU stay may develop critical illness myopathy or neuropathy.
Neuromuscular junction
Postinfectious myasthenia gravis associated with SARS-CoV-2 has recently been reported in a 21-year-old female presenting with subacute onset, fluctuating diplopia with unilateral ptosis, developing 4 weeks after COVID-19 infection. Acetylcholine receptor antibodies in serum were elevated, and good clinical recovery occurred following treatment with IVIG and pyridostigmine. The probable molecular mimicry between the SARS-CoV-2 proteins and acetylcholine receptor might have activated the immune response [60].
CIN and CIM
In a cohort of 19 critically ill COVID-19 patients manifesting difficulty to wean from mechanical ventilation, nerve conduction study (NCS) and electromyography (EMG) studies revealed presence of critical illness neuropathy (CIN) and critical illness myopathy (CIM) in four and seven cases, respectively. Both CIN and CIM has also been reported in SARS-CoV previously [59].
Interestingly, many COVID-19 cases may present with pure neurological manifestations including headache, dizziness, stroke, GBS, and meningoencephalitis, at the onset of illness with non-neurological features manifesting few days later [11, 21, 38, 58, 81, 84]. Although the current case definition of COVID-19 illness includes a primary respiratory involvement, several cases have been reported with neurological illness being the earliest manifestation of COVID-19. While headache and dizziness are commonly reported at the onset in 26% patients in a series [63], stroke or GBS as the initial manifestation of SARS-CoV-2 infection has also been reported in three cases each [58, 84]. We propose the term “neuro-COVID syndrome” for such cases and physicians must be vigil to facilitate its management and due precautions should be taken to avoid cross-infection. We speculate a possibility of high neurotropism of SARS-CoV-2 as well as predilection of the immune-mediated response to primarily target the central and/or peripheral nervous system and its vascularity, as the plausible reasons for neuro-COVID syndrome. Thus, SARS-CoV-2 infection should be considered in the list of differentials in cases with new-onset unexplained CNS and/or PNS involvement.
Investigations
General investigations to rule out other systemic complications
A neutrophil-lymphocyte ratio (NLR) > 3 has been reported an independent predictor for neurological complications [72]. Serum electrolytes along with renal and liver functions should be tested regularly to detect MODS and rule out metabolic encephalopathy [11]. Derangement in coagulation profile including D-dimer is an independent predictor of systemic vascular complications including stroke [6, 70]. Assays of muscle enzymes including creatinine phophokinase(CPK) and lactate dehydrogenase along with acute phase reactants like serum ferritin and CRP is advisable. While patients with predominant CNS manifestations had lower lymphocyte and platelet counts along with elevated blood urea nitrogen, those with muscle symptoms had significantly high serum CPK (> 200 U/L), neutrophil, CRP, and D-dimer levels with reduced lymphocyte count [11].
Investigations for neurological manifestations
Efforts should be taken to minimize the transmission of SARS-CoV-2 infection in considering investigations requiring shifting patients away from his current ward.
Neuroimaging
COVID-19 patients presenting with unexplained altered sensorium, confusion or agitation, focal neurological deficit indicating an acute stroke, meningoencephalitis, and acute myelitis should undergo neuroimaging, preferably MRI of the region of interest. To date, several neuroimaging findings have been reported in COVID-19 patients (Table 2).
Table 2.
Reported neuroimaging features in COVID-19 cases
| Systems involved | Syndromic manifestations | CT brain/PET-CT | MRI |
|---|---|---|---|
| Meninges ± brain parenchyma |
• Acute meningoencephalitis [18, 21] • Meningitis/encephalitis [18] • Rhombencephalitis/myelitis [22] |
• Leptomeningeal enhancement • T2 hyperintense signal changes in upper pons, limbic lobes, medial thalami and subcortical cerebral white matter • Lateral ventriculitis along with right medial temporal encephalitis • T2 hyperintense lesion in the right inferior cerebellar peduncle, extending to involve a small portion of the upper cord, associated with swelling and microhemorrhages |
|
| Cortical and subcortical |
• Acute hemorrhagic necrotizing encephalopathy [16, 17] • Hypoxic ischemic encephalopathy [62] • Hypoactive orbitofrontal cortex in anosmia [8] |
• Symmetric hypodensities involving bilateral medial thalami • Diffuse swelling of the brain stem. • Hypometabolism of the left orbitofrontal cortex |
• Hemorrhagic rim enhancing lesions involving bilateral thalami, medial temporal lobes, and sub-insular regions on post contrast coronal T1-weighted images • T2 and FLAIR hyperintensities with diffusion restriction involving frontal, parietal, temporal, occipital, insular and cingulate gyrus • Right cerebral hemispheric restricted diffusion and cerebral edema and spinal edema • Hyperintensities on T2/FLAIR in splenium • Multiple clusters of lesions in the deep cerebral white matter. Cyst-like areas of varied sizes, some with hemorrhagic foci and peripheral rims of restricted diffusion. • Multifocal and confluent areas of signal change in the cerebral hemispheric, white matter with extensive micro hemorrhages in the subcortical regions • Extensive, confluent, and largely symmetrical areas throughout brainstem, limbic and insular lobes, superficial subcortical white matter and deep gray matter. Clusters of microhemorrhages, restricted diffusion and peripheral rim enhancement • Brain stem swelling with symmetrical hemorrhagic lesions in the brain stem, amygdalae, putamen, and thalamic nuclei • Multifocal confluent lesions in internal and external capsules, splenium and deep white batter of cerebral hemispheres with multiple microhemorrhages and extensive prominent medullary veins. • Components of brachial and lumbosacral plexus showed increased signal and enhancement |
| Cerebrovascular system |
• Intracerebral hemorrhage, subarachnoid hemorrhage [24, 25] |
• Loss of gray-white differentiation at occipital and parietal lobes or hypo dense lobar lesion. • Hyper dense appearance of MCA vessel • SAH centered in the posterior fossa, including the fourth ventricle leading to hydrocephalus • CT angiogram showed right-sided ruptured dissecting posterior-inferior cerebellar artery aneurysm • New bilateral confluent hypodensities in occipital/parieto-occipital/white matter • Patchy lucencies in the bilateral frontoparietal white matter and posterior limb of the left internal capsule • CT angiogram showed a filling defect in the right transverse sinus and jugular bulb suggestive of venous sinus thrombosis |
• Cortical microhemorrhages • Absence of normal flow void in right transverse sinus • Occlusion of proximal MCA • Left temporoparietal hemorrhagic venous infarction with left transverse and sigmoid sinus thrombosis • Confluent T2 hyperintensity in the same regions without diffusion restriction or susceptibility hypointensity. • Extensive dural venous sinus thrombosis involving the straight sinus, torcula, left transverse and sigmoid sinus, extending into the jugular vein, as well as right transverse sinus, superior sagittal sinus, and left vein of Labbe. |
| Brainstem | • Acute rhombencephalitis [22] | - | • T2 hyperintensities involving cerebellar peduncles |
| Spinal cord | • Acute transverse myelitis [5, 39, 40, 42] | - |
• T2 hyperintensities involving upper cervical and lower dorsal spinal cord • Longitudinally extensive transverse myelitis involving the entire length of the spinal cord |
| Nerve root | • GBS [58] | • Contrast-enhanced lesions in caudal nerve roots and facial nerve |
CT computed tomography, CVST cortical venous sinus thrombosis, GBS Guillain–Barre syndrome, MCA middle cerebral artery, MRI magnetic resonance imaging, FLAIR fluid-attenuated inversion recovery, PET-CT positron emission tomography computed tomography, PRES posterior reversible encephalopathy syndrome, SAH subarachnoid hemorrhage
CSF
To date, CSF RT-PCR or ELISA has detected SARS-CoV-2 in only three COVID-19 cases, who presented with meningoencephalitis, GBS, and brain microvascular occlusive disorders, respectively [18, 34, 86]. Albumino-cytological dissociation in CSF may help diagnose GBS [58]. CSF protein may be elevated in COVID-19-related para- or postinfectious central or peripheral neuroimmunological disorders. Lypmhocytic pleocytosis with elevated CSF protein has been found in COVID-19-related meningoencephalitis [18, 21, 38] and transverse myelitis [40, 42]. However, positive immunological parameters in CSF including IgG index and oligoclonal bands in any COVID-19-related case has not been reported to date.
EEG
Various electroencephalography (EEG) findings including focal or diffuse delta-theta slowing, symmetrical or asymmetrical sporadic epileptiform discharges, lateralized periodic discharges with overriding fast activity, generalized sharp waves with spikes, triphasic waves, and repetitive focal rhthmic bursts of nonconvulsive status epilepticus have been reported in COVID-19 patients presenting with altered mental status, delirium, or encephalopathy, and continuous EEG monitoring is advisable in these patients to diagnose NCSE [53, 75, 87].
NCS and EMG
Electrophysiological studies are largely reserved for managing rapidly progressive neuromuscular disorders including GBS [58], myasthenic crisis, inflammatory myopathy, and critical illness neuropathy [59, 88]. NCS in COVID-19-related GBS cases revealed predominant demyelinating involvement in five, axonal in five, and mixed pattern in a single case [58].
Treatment
While more than 80% of COVID-19 cases remain either asymptomatic or develop self-limiting mild features, symptomatic management including oxygen therapy remains the mainstay in managing severe cases. Mechanical ventilation is indicated in respiratory failure refractory to oxygen therapy and hemodynamic support for septic shock [1, 4]. A variety of antiviral medications targeting different steps of viral replication cycle and convalescent plasma are undergoing evaluation [89].
Treatment of COVID-19-related neurological illness
In view of paucity of data regarding management of COVID-19-related neurological manifestations, syndrome-specific therapy along with supportive management employed during pre-COVID-19 time is advocated. Additionally, regular monitoring and manangement of metabolic and electrolyte derangements along with prevention and/or treatment of secondary infections may improve outcome.
Stroke
Antiplatelet therapy with aspirin or clopidogrel have been tried in six and anticoagulation with enoxaparin in five ischemic stroke cases, but three cases in former and one in the latter group failed to survive [1]. The interim guidance of the International Society of Thrombosis and Haemostasis (ISTH) proposed a widespread use of prophylactic low molecular weight heparin (LMWH) in all hospitalized COVID-19 patients lacking contraindication (active bleeding and platelet count below 25 × 109/L) [90], and it should be used with caution in ischemic stroke patients in view of possible risk of intracranial hemorrhage or hemorrhagic transformation of acute infarct [67].
Neuroimmunological disorders involving CNS and PNS
Use of IVIg may be preferred over plasma exchange as the former aids in managing ARDS by improving passive immunity and modulating immune response [89]. Additionally, plasma exchange procedure carries a substantial risk of cross-infection to healthcare workers. Immunomodulation with oral [40, 41] or intravenous [40, 42] steroids, intravenous immunoglobulin [40], and/or plasma exchange [39] led to remarkable clinical improvement most of COVID-19-related GBS and ATM cases reported to date. Although steroid administration during initial stage of infection may increase viral replication and delay development of adaptive immunity, timed and titrated usage may be safe and beneficial in meningoencephalitis and CNS demyelinating disorders [38, 39].
Seizures
Along with initiation of commonly used antiepileptic drugs (AEDs), management of primary pathology causing seizure is important [74, 75]. Immunomodulation with pulse methylprednisolone and plasma exchange led to significant clinical improvement in generalized myoclonus in three cases, possibly resulting from immune-mediated pathology [45].
Meningitis and/or meningoencephalitis
Although lacking concrete evidence, several antivirals including lopinavir-ritonavir and hydroxychloroquine [75] have been tried with some success in COVID-19-related meningitis and/or meningoencephalitis. Symptomatic therapy for raised intracranial pressure including mannitol may improve outcome [66].
Rhabdomyolysis
Since aggressive fluid administration may aggravate hypoxia in patients with ARDS, small boluses of intravenous fluid (approximately 250 ml) with monitoring of clinical status and oxygen saturation levels along with serum creatinine and CPK is warranted [82].
Treatment of preexisting chronic neurological illness during COVID-19 pandemic
Patients with chronic neurological conditions especially elderlies; those with comorbid illnesses, including diabetes and chronic heart, lung, liver, or kidney illness; or on immunomodulators may develop severe COVID-19 illness [4]. While data on change in behavior of preexisting chronic neurological illnesses and need to modify their management is lacking, these patients must be followed closely [91]. Management of preexisting chronic stroke, neuroimmunological disorders, Parkinson’s disease, epilepsy, migraine, and neuromuscular disorders may remain similar to pre-COVID time. Ongoing immunomodulation should be continued with regular monitoring for drug-related adverse effects, as discontinuation may trigger a relapse [89]. These patients must religiously practice social distancing and hand hygiene to prevent exposure to SARS-CoV-2 [4, 91]. In addition to known offending medications, hydroxychloroquine may worsen myasthenia gravis and should be avoided [4].
Conclusion
COVID-19 may present with a wide spectrum of neurological manifestations. Although neurological manifestations are common in severe cases, a high suspicion should be exercised to diagnose patients with pure neurological presentation (“neuro-COVID syndrome”) at onset of COVID-19 illness.
Abbreviations
- ACE2
Angiotensin-converting enzyme 2 receptor
- ADEM
Acute disseminated encephalomyelitis
- ATM
Acute transverse myelitis
- CNS
Central nervous system
- COVID-19
Coronavirus disease 2019
- CoVs
Coronaviruses
- CSF
Cerebrospinal fluid
- CVST
Cortical venous sinus thrombosis
- EEG
Electroencephalography
- GBS
Guillain–Barre syndrome
- IVIG
Intravenous immunoglobulin
- MERS-CoV
Middle East respiratory syndrome coronavirus
- MS
Multiple sclerosis
- NCS
Nerve conduction study
- PRES
Posterior reversible encephalopathy syndrome
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
Author contributions
Dr. Ishita Desai: writing first draft, design, and creating figures. Dr. Rajat Manchanda: writing first draft, design, and creating figures. Dr. Niraj Kumar: Conception, design, writing first draft, review, and critique. Dr. Ashutosh Tiwari: review and critique. Dr. Mritunjai Kumar: review and critique.
Data Availability
Not applicable.
Compliance with ethical standards
Conflict of interest
None.
Ethical approval
None.
Disclosures
Drs. Desai I, Manchanda R, Kumar N, Tiwari A and Kumar M report no disclosures relevant to the manuscript.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ishita Desai and Rajat Manchanda contributed equally to this work.
References
- 1.Galassi G, Marchioni A. Facing acute neuromuscular diseases during COVID-19 pandemic: focus on Guillain–Barré syndrome. Acta Neurol Belg. 2020;120(5):1067–1075. doi: 10.1007/s13760-020-01421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie M, Chen Q. Insight into 2019 novel coronavirus — an updated interim review and lessons from SARS-CoV and MERS-CoV. Int J Infect Dis. 2020;94:119–124. doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4):1–17. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: A literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonck K, Garrez I, De Herdt V, Hemelsoet D, Laureys G, Raedt R, et al. Neurological manifestations and neuro-invasive mechanisms of the severe acute respiratory syndrome coronavirus type 2. Eur J Neurol. 2020;27(8):1578–1587. doi: 10.1111/ene.14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coolen T, Lolli V, Sadeghi N, Rovai A, Trotta N, Taccone FS, et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020;95(14):e2016–e2027. doi: 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
- 8.Karimi-Galougahi M, Yousefi-Koma A, Bakhshayeshkaram M, Raad N, Haseli S. 18FDG PET/CT scan reveals hypoactive orbitofrontal cortex in anosmia of COVID-19. Acad Radiol. 2020;27(7):1042–1043. doi: 10.1016/j.acra.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL et al (2020) Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ 368(January):1–7 [DOI] [PMC free article] [PubMed]
- 10.Borges do Nascimento IJ, Cacic N, Abdulazeem HM, von Groote TC, Jayarajah U, Weerasekara I, et al. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med. 2020;9(4):941. doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, Griffin MR, Metersky ML, Musher DM, Restrepo MI, Whitney CG. Diagnosis and treatment of adults with community-acquired pneumonia. Am J Respir Crit Care Med. 2019;200(7):E45–E67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morfopoulou S, Brown JR, Davies EG, Anderson G, Virasami A, Qasim W, Chong WK, Hubank M, Plagnol V, Desforges M, Jacques TS, Talbot PJ, Breuer J. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016;375(5):497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 14.Jacomy H, Fragoso G, Almazan G, Mushynski WE, Talbot PJ. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology. 2006;349(2):335–346. doi: 10.1016/j.virol.2006.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, Ahn JY, Kim MK, Choi JP. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;5(564):201187. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon L, Varley J, Gontsarova A, Mallon D, Tona F, Muir D, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):1–8. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L, Zhang M, Wang J, Gao J (2020) Sars-Cov-2: Underestimated damage to nervous system. Travel Med Infect Dis [Internet] 36(January):101642 [DOI] [PMC free article] [PubMed]
- 20.Etemadifar M, Salari M, Murgai AA, Hajiahmadi S. Fulminant encephalitis as a sole manifestation of COVID-19. Neurol Sci. 2020;41(11):3027–3029. doi: 10.1007/s10072-020-04712-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav Immun. 2020;87:33. doi: 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong PF, Craik S, Newman P, Makan A, Srinivasan K, Crawford E, Dev D, Moudgil H, Ahmad N. Lessons of the month 1: A case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin Med. 2020;20(3):293–294. doi: 10.7861/clinmed.2020-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umapathi T, Kor AC, Venketasubramanian N, Lim CCT, Pang BC, Yeo TT, Lee CC, Lim PL, Ponnudurai K, Chuah KL, Tan PH, Tai DYH, Ang SPB. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J Neurol. 2004;251(10):1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fatima N, Saqqur M, Qamar F, Shaukat S, Shuaib A. Impact of COVID-19 on neurological manifestations: an overview of stroke presentation in pandemic. Neurol Sci. 2020;41(10):2675–2679. doi: 10.1007/s10072-020-04637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Saiegh F, Ghosh R, Leibold A, Avery MB, Schmidt RF, Theofanis T, et al. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. 2020;91(8):846–848. doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- 27.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7(5):1691. doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein DE, Libman R, Kirsch C, Arora R. Cerebral venous thrombosis: atypical presentation of COVID-19 in the young. J Stroke Cerebrovasc Dis. 2020;29(8):104989. doi: 10.1016/j.jstrokecerebrovasdis.2020.104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dakay K, Cooper J, Bloomfield J, Overby P, Mayer SA, Nuoman R, et al. Cerebral venous sinus thrombosis in COVID-19 infection: a case series and review of the literature. J Stroke Cerebrovasc Dis. 2020;30(1):105434. doi: 10.1016/j.jstrokecerebrovasdis.2020.105434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gómez-Enjuto S, Hernando-Requejo V, Lapeña-Motilva J, Ogando-Durán G, Fouz-Ruiz D, Domingo-García J, Rodríguez-García E, Cemillán-Fernández CA (2020) Verapamil as treatment for refractory status epilepticus secondary to PRES syndrome on a SARS-Cov-2 infected patient. Seizure [Internet] 80(January):157–8 [DOI] [PMC free article] [PubMed]
- 31.Kishfy L, Casasola M, Banankhah P, Parvez A, Jan YJ, Shenoy AM et al (2020) Posterior reversible encephalopathy syndrome (PRES) as a neurological association in severe Covid-19. J Neurol Sci [Internet] 414(January):116943 [DOI] [PMC free article] [PubMed]
- 32.Parauda SC, Gao V, Gewirtz AN, Parikh NS, Merkler AE, Lantos J, et al. Posterior reversible encephalopathy syndrome in patients with COVID-19. J Neurol Sci. 2020;416:117019. doi: 10.1016/j.jns.2020.117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaya Y, Kara S, Akinci C, Kocaman AS (2020) Transient cortical blindness in COVID-19 pneumonia; a PRES-like syndrome: case report. J Neurol Sci [Internet] 413(January):116858 [DOI] [PMC free article] [PubMed]
- 34.Saitta L, Molin A, Villani F, Insorsi A, Roccatagliata L, Inglese M, et al. Brain microvascular occlusive disorder in COVID-19: a case report. Neurol Sci. 2020;41:3401–3404. doi: 10.1007/s10072-020-04795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boucher A, Desforges M, Duquette P, Talbot PJ. Long-term human coronavirus-myelin cross-reactive T-cell clones derived from multiple sclerosis patients. Clin Immunol. 2007;123(3):258–267. doi: 10.1016/j.clim.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray RS, Brown B, Brain D, Cabirac GF. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol. 1992;31(5):525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart JN, Mounir S, Talbot PJ. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191(1):502–505. doi: 10.1016/0042-6822(92)90220-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pilotto A, Odolini S, Stefano Masciocchi S, Comelli A, Volonghi I, Gazzina S, et al. Steroid-responsive encephalitis in Covid-19 disease. Ann Neurol. 2020;2:1–5. doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020;162(7):1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.AlKetbi R, AlNuaimi D, AlMulla M, AlTalai N, Samir M, Kumar N. Acute myelitis as a neurological complication of Covid-19: a case report and MRI findings. Radiol Case Rep. 2020;15(9):1591–1595. doi: 10.1016/j.radcr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S (2020) Acute myelitis after SARS-CoV-2 infection: a case report. 10.1101/2020.03.16.2003510
- 42.Munz M, Wessendorf S, Koretsis G, Tewald F, Baegi R, Krämer S, et al. Acute transverse myelitis after COVID-19 pneumonia. J Neurol. 2020;267(8):2196–2197. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarma D, Bilello LA. A case report of acute transverse myelitis following novel coronavirus infection. Clin Pract Cases Emerg Med. 2020;4(3):321–323. doi: 10.5811/cpcem.2020.5.47937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panariello A, Bassetti R, Radice A, Rossotti R, Puoti M, Corradin M, et al. Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: a case report. Brain Behav Immun. 2020;87:179–181. doi: 10.1016/j.bbi.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rábano-Suárez P, Bermejo-Guerrero L, Méndez-Guerrero A, Parra-Serrano J, Toledo-Alfocea D, Sánchez-Tejerina D, et al. Generalized myoclonus in COVID-19. Neurology. 2020;95(6):e767–e772. doi: 10.1212/WNL.0000000000009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borroni B, Gazzina S, Dono F, Mazzoleni V, Liberini P, Carrarini C, Russo M, Pontolillo M, Vecchiet J, Onofrj M, Bonanni L. Diaphragmatic myoclonus due to SARS-CoV-2 infection. Neurol Sci. 2020;41(12):3471–3474. doi: 10.1007/s10072-020-04766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright D, Rowley R, Halks-Wellstead P, Anderson T, Wu TY. Abnormal saccadic oscillations associated with severe acute respiratory syndrome coronavirus 2 encephalopathy and ataxia. Mov Disord Clin Pract. 2020;7(8):980–982. doi: 10.1002/mdc3.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(19):8913–8921. doi: 10.1128/JVI.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pilotto A, Masciocchi S, Volonghi I, del Zotto E, Magni E, De Giuli V et al (2020) The clinical spectrum of encephalitis in COVID-19 disease: the ENCOVID multicentre study. J Infect Dis. 10.1093/infdis/jiaa609
- 50.Cohen ME, Eichel R, Steiner-Birmanns B, Janah A, Ioshpa M, Bar-Shalom R, Paul JJ, Gaber H, Skrahina V, Bornstein NM, Yahalom G. A case of probable Parkinson’s disease after SARS-CoV-2 infection. Lancet Neurol. 2020;19(10):804–805. doi: 10.1016/S1474-4422(20)30305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J', Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q, Gendrin V, et al. Features of anosmia in COVID-19. Med Mal Infect. 2020;50(5):436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dono F, Carrarini C, Russo M, De Angelis MV, Anzellotti F, Onofrj M, et al. New-onset refractory status epilepticus (NORSE) in post SARS-CoV-2 autoimmune encephalitis: a case report. Neurol Sci. 2020;3:1–4. doi: 10.1007/s10072-020-04846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130(7):1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bagheri SHR, Asghari AM, Farhadi M, Shamshiri AR, Kabir A, Kamrava SK, et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med J Islam Repub Iran. 2020;34:62. doi: 10.34171/mjiri.34.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M (2020) Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 10.1007/s00415-020-10124-x [DOI] [PMC free article] [PubMed]
- 59.Cabañes-Martínez L, Villadóniga M, González-Rodríguez L, Araque L, Díaz-Cid A, Ruz-Caracuel I, Pian H, Sánchez-Alonso S, Fanjul S, del Álamo M, Regidor I. Neuromuscular involvement in COVID-19 critically ill patients. Clin Neurophysiol. 2020;131(12):2809–2816. doi: 10.1016/j.clinph.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huber M, Rogozinski S, Puppe W, Framme C, Höglinger G, Hufendiek K et al (2020) Postinfectious onset of myasthenia gravis in a COVID-19 patient. Front Neurol 11(October):1–5 [DOI] [PMC free article] [PubMed]
- 61.Belvis R. Headaches during COVID-19: my clinical case and review of the literature. Headache. 2020;60(7):1422–1426. doi: 10.1111/head.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benameur K, Agarwal A, Auld SC, Butters MP, Webster AS, Ozturk T, Howell JC, Bassit LC, Velasquez A, Schinazi RF, Mullins ME, Hu WT. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis. 2020;26(9):2016–2021. doi: 10.3201/eid2609.202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trigo López J, García-Azorín D, Planchuelo-Gómez Á, García-Iglesias C, Dueñas-Gutiérrez C, Guerrero ÁL. Phenotypic characterization of acute headache attributed to SARS-CoV-2: an ICHD-3 validation study on 106 hospitalized patients. Cephalalgia. 2020;40(13):1432–1442. doi: 10.1177/0333102420965146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caronna E, Ballvé A, Llauradó A, Gallardo VJ, María Ariton D, Lallana S, et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020;40(13):1410–1421. doi: 10.1177/0333102420965157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrando SJ, Klepacz L, Lynch S, Tavakkoli M, Dornbush R, Baharani R, et al. COVID-19 Psychosis: A potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? Psychosomatics. 2020;61(5):551–555. doi: 10.1016/j.psym.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020;88:945–946. doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY et al (2020) Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry 0(0):8–11 [DOI] [PMC free article] [PubMed]
- 68.Naeimi R. Ghasemi–Kasman M. Update on cerebrovascular manifestations of COVID-19. Neurol Sci. 2020;41(12):3423–3435. doi: 10.1007/s10072-020-04837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grau AJ, Buggle F, Becher H, Zimmermann E, Spiel M, Fent T, Maiwald M, Werle E, Zorn M, Hengel H, Hacke W. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia. Clinical and biochemical studies. Neurology. 1998;50(1):196–203. doi: 10.1212/WNL.50.1.196. [DOI] [PubMed] [Google Scholar]
- 70.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doo FX, Kassim G, Lefton DR, Patterson S, Pham H, Belani P (2021) Rare presentations of COVID-19: PRES-like leukoencephalopathy and carotid thrombosis. Clin Imaging [Internet] 69(January):94–101 [DOI] [PMC free article] [PubMed]
- 74.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;2(3):e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vollono C, Rollo E, Romozzi M, Frisullo G, Servidei S, Borghetti A, Calabresi P. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure. 2020;78:109–112. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nemoto W, Yamagata R, Nakagawasai O, Nakagawa K, Hung WY, Fujita M et al (2020) Effect of spinal angiotensin-converting enzyme 2 activation on the formalin-induced nociceptive response in mice. Eur J Pharmacol [Internet] 872(July 2019):172950 [DOI] [PubMed]
- 77.Diezma-Martín AM, Morales-Casado MI, García-Alvarado N, Vadillo Bermejo A, López-Ariztegui N, Sepúlveda Berrocal MA. Temblor y ataxia en COVID-19. Neurología. 2020;35(6):409–410. doi: 10.1016/j.nrl.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schellekens MMI, Bleeker-Rovers CP, Keurlings PAJ, Mummery CJ, Bloem BR. Reversible myoclonus-ataxia as a postinfectious manifestation of COVID-19. Mov Disord Clin Pract. 2020;7(8):977–979. doi: 10.1002/mdc3.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steardo L, Steardo L, Zorec R, Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol (Oxf) 2020;229(3):e13473. doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei H, Yin H, Huang M, Guo Z. The 2019 novel cornoavirus pneumonia with onset of oculomotor nerve palsy: a case study. J Neurol. 2020;267(5):1550–1553. doi: 10.1007/s00415-020-09773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zito A, Alfonsi E, Franciotta D, Todisco M, Gastaldi M, Cotta Ramusino M et al (2020) COVID-19 and Guillain–Barré syndrome: a case report and review of literature. Front Neurol [Internet] 11(August):1–7 [DOI] [PMC free article] [PubMed]
- 82.Suwanwongse K, Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus. 2020;12(4):10–13. doi: 10.7759/cureus.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chan KH, Slim J. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26(10):2535–2535. doi: 10.3201/eid2610.202225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, et al. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Medicherla CB, Pauley RA, de Havenon A, Yaghi S, Ishida K, Torres JL. Cerebral venous sinus thrombosis in the coronavirus disease 2019 pandemic. J Neuroophthalmol. 2020;Publish Ah:457–462. doi: 10.1097/WNO.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 86.Gigli GL, Vogrig A, Nilo A, Fabris M, Biasotto A, Curcio F, Miotti V, Tascini C, Valente M. HLA and immunological features of SARS-CoV-2-induced Guillain-Barré syndrome. Neurol Sci. 2020;41(12):3391–3394. doi: 10.1007/s10072-020-04787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pilato MS, Urban A, Alkawadri R, Barot N V., Castellano JF, Rajasekaran V et al (2020) EEG findings in coronavirus disease. J Clin Neurophysiol [Internet] Publish Ah(00):1–7 [DOI] [PubMed]
- 88.Kassardjian CD, Desai U, Narayanaswami P. Practical guidance for managing EMG requests and testing during the COVID-19 pandemic. Muscle Nerve. 2020;62:30–33. doi: 10.1002/mus.26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 90.Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;8(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manji H, Carr AS, Brownlee WJ, Lunn MP. Neurology in the time of COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(6):568–570. doi: 10.1136/jnnp-2020-323414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.