Abstract
Myeloid-derived suppressor cells (MDSCs) are one of the major components of the tumor microenvironment (TME), and are the main mediators of tumor-induced immunosuppression. Recent studies have reported that the survival, differentiation and immunosuppressive activity of MDSCs are affected by the Toll-like receptor (TLR) signaling pathway. However, the regulatory effect of TLR signaling on MDSCs remains controversial. TLR-induced MDSC can acquire different immunosuppressive activities to influence the immune response that can be either beneficial or detrimental to cancer immunotherapy. The present review summarizes the effects of TLR signals on the number, phenotype and inhibitory activity of MDSCs, and their role in cancer immunotherapy, which cannot be ignored if effective cancer immunotherapies are to be developed for the immunosuppression of the TME.
Keywords: MDSC, TLR signals, antitumor, protumor
1. Introduction
Myeloid-derived suppressor cells (MDSCs) are heterogeneous cell populations that are precursors of dendritic cells (DCs), macrophages and/or granulocytes (1,2). Under physiological conditions, MDSCs mature and differentiate into DCs, macrophages and granulocytes, while their differentiation is inhibited in the context of inflammation and tumors, leading to their accumulation in tumors and lymphoid organs as a negative balance mechanism to prevent excessive T cell activation (3). Tumor cells use this mechanism for immune escape, and MDSCs are directly involved in several processes that promote tumor development, damaging the immune response of T cells and natural killer (NK) cells, particularly CD8+ T cell activation and effector function (4). High levels of circulating MDSCs in patients with tumor are associated with a worse prognosis and disease progression (5). Thus, one of the directions of development in cancer immunotherapy is to target either the MDSC populations and/or the signals involved in their recruitment and function (6–8).
MDSCs express several TLR family members, including TLR2 (9), TLR3 (10), TLR4 (11), TLR5 (12) and TLR7/8/9 (13) in mice, and TLR2 (14) and TLR7/8 (15) in humans. MDSCs accumulate in the tumor microenvironment (TME) and are an important target for TLR signaling regulation (16–18). However, the effects of TLR signaling on MDSCs and its effect on tumor growth are not yet fully understood. Previous studies have demonstrated that TLR ligands are inducers of MDSCs, and have emphasized that myeloid differentiation primary response 88 (MyD88) is essential for acquiring the direct suppressive activity of MDSCs and the ability to promote tumor growth, whereas these abilities are inhibited by blocking MyD88-mediated signaling (19–21). The accumulation of MDSCs mediated by MyD88-nuclear factor kappa-B (NF-κB) signaling increases the production of IL-10, which inhibits the function of DCs in liver cancer (22). In addition, it has been demonstrated that increased expression of interferon regulatory factor (IRF)4, a negative feedback regulator of TLR signaling (23), decreases the MDSC population, particularly the G-MDSC population (24).
Furthermore, immune-checkpoint protein V-domain immunoglobulin suppressor of T-cell activation (VISTA) is a chief myeloid cell-intrinsic immune-checkpoint protein that can control antitumor immunity (25). VISTA modulates the polyubiquitination and protein expression of TNF receptor associated factor 6 to inhibit TLR-mediated activation of the mitogen-activated protein kinase (MAPK)/Activator protein-1 (AP-1) and IKK/NF-κB signaling cascades, which decreases the ability of MDSCs to produce proinflammatory mediators and enhance their T cell-suppressive functions, thereby protumor progression (26).
However, other groups have disputed these findings, reporting that TLR signaling activation can decrease the immunosuppressive activity of MDSCs (13,27,28). Loss of MyD88 results in an increase in prostate intraepithelial tumors and highly differentiated adenocarcinoma areas in TRAMP transgenic mice, accompanied by an increase in the frequency of MDSCs and the production of inducible nitric oxide synthase (iNOS), prostate arginase 1 (Arg1), and cytokine Interleukin (IL)-10 (27). Furthermore, other studies have reported that TLR stimulation decreases the MDSC population and enhances their differentiation into tumoricidal macrophages (13,28). Thus, the regulation of TLR signaling on MDSCs is diverse. The present review summarizes the effects of TLR signals on the number, phenotype and inhibitory activity of MDSCs, and their role in cancer immunotherapy. It remains essential to address this in the present and future cancer immunotherapies for the immunosuppression of the TME.
2. General aspects of MDSCs and TLR signaling
MDSCs
MDSCs can be divided into two categories, including polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs or G-MDSCs) that are phenotypically and morphologically similar to neutrophils, and monocyte-myeloid-derived suppressor cells (M-MDSCs), which are similar to monocytes. In mice, the phenotype of G-MDSCs is CD11b+Ly6G+Ly6Clo, and M-MDSCs is CD11b+Ly6G−Ly6Chi (29). In humans, the phenotype of G-MDSCs is CD11b+CD14−CD66b+ or CD11b+CD14−CD15+, and the phenotype of M-MDSCs is CD11b+CD14+HLA−DR−/loCD15− (29). Increasing evidence suggests that MDSCs promote tumor progression and metastasis through their immunosuppressive activity, the mechanism of which can be summarized as i) Arg1 and iNOS produced by MDSCs consume arginine and cysteine, which are nutrients required by lymphocytes, leading to the downregulation of the ζ chain in the T cell receptor (TCR) complex and inhibiting the proliferation of antigen-activated T cells (30); ii) reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated by MDSCs induce the formation of oxidative stress, leading to the loss of T cell ζ chain expression and interfering with IL-2 receptor signaling cascades (31,32); iii) MDSCs interfere with the transportation and survival of lymphocytes, affecting the migration of CD8+ T cells to the TME and restricting T cell recycling to the lymph nodes (33,34) and iv) MDSCs improve naïve CD4+ T cell differentiation into regulatory T cells, thereby inhibiting T cell function (35). Thus, it remains essential to develop cancer immunotherapies that aim to decrease the negative impact of MDSCs on effector immune cells.
Previous studies have demonstrated that targeting the MDSC population and the signals involved in their function can delay tumor progression (36,37). Thus, understanding the regulators and signaling pathways involved in the survival, development, differentiation and activation of MDSCs is essential for cancer immunotherapy targeting MDSCs.
Currently, two-signal models are used to describe the differentiation of MDSCs. The model includes two stages: The first stage is the expansion of immature bone marrow cells and the inhibition of terminal differentiation; the second stage is the activation stage, which transforms immature bone marrow cells into MDSCs (38). Notably, the TLR signaling pathways are involved in both stages of MDSC differentiation, and TLR receptors are expressed positively on MDSCs. TLR signals are considered important regulators of the differentiation and acquisition of the immunosuppressive function of MDSCs (39).
TLR signaling
TLRs are type I transmembrane proteins, with 10 existing in humans (TLR1-10) and 12 in mice (TLR1-9, TLR11-13) (40). TLR1, TLR2, TLR4, TLR5 and TLR6 are located on the cell surface and are mainly involved in the detection of extracellular bacterial products, while TLR3, TLR7, TLR8 and TLR9 are located in intracellular compartments that are involved in the detection of nucleic acids from viral and bacterial sources (40). These TLRs recognize pathogen-associated molecular patterns to initiate the appropriate host immune response. Binding of the TLR to the ligand results in the activation of two major signaling pathways, the MyD88-dependent and Toll-IL-1 receptor-domain containing adaptor-inducing interferon-β (TRIF)-dependent signaling pathways. Excluding TLR3, all TLRs activate MyD88-dependent signaling pathways, and both TLR3 and TLR4 activate TRIF-dependent signaling pathways. The MyD88-dependent pathway activates nuclear factor NF-κB and the MAPK pathway, and induces the development of inflammatory responses. However, the TRIF-dependent pathway activates the interferon IRF pathway and induces antiviral type 1 interferon, which is involved in the antiviral response (40).
Currently, Bacillus Calmette-Guerin (BCG, TLR2/TLR4 agonist), Monophosphoryl lipid A (MPL, TLR4 agonist), and Imiquimod (Imiq) (TLR7 agonist) have been approved by the Food and Drug Administration (FDA) for clinical treatment of patients with cancer (41). Furthermore, TLR agonists are extensively used as adjuvants for cancer vaccines to enhance their antitumor effects. The antitumor effect of TLR agonists is attributable to the activation of TLR signals that enhance antigen-specific humoral and cellular immune responses. However, due to the existence of the immune tolerant microenvironment, TLR agonists alone or as cancer vaccine adjuvants can only produce moderate clinical benefits in cancer treatment (42,43). Thus, it remains critical to identify and develop therapeutic approaches to overcome the immunosuppressive TME and to enhance the efficiency of current tumor immunotherapies. Understanding the effect of TLR signaling on MDSCs will help optimize the role of TLR agonists in antitumor therapy and provide novel insight into the development of cancer immunotherapies.
3. Pro-tumorigenic effects of MDSCs induced by TLR signaling
Increasing evidence suggests that the accumulation and activation of MDSCs is associated with tumor progression, recurrence and a negative clinical outcome (44). Recently, a number of studies have demonstrated that TLR signaling induces MDSC accumulation and enhances the ability to inhibit tumor-specific T cell responses, resulting in tumor progression (45,46). Related studies and potential TLR signaling pathways are summarized in Table I and Fig. 1.
Table I.
Pro-tumorigenic effects of MDSCs induced by TLR signaling in cancer.
| TLR | Stimulus | Species | Cancer | Number and phenotype | Function and mediator(s) | (Refs.) |
|---|---|---|---|---|---|---|
| TLR2 | Pam2CSK | M | Lymphoma | Accumulation in tumor sites supported survival | – | (9) |
| H | Colon, prostate, pancreatic, liver cancer | M2-like (25F9+/CD200R+) | Inhibited T cell proliferation. Mediator: IL-6, IL-10 | (14) | ||
| M | Lung cancer and lymphoma | Prolonged survival | iNOS, NO | (45) | ||
| Hsp72/Hsp70 | M, H | Breast cancer melanoma lymphoma and RCC | Expansion | Arg1, iNOS | (47–50) | |
| TRF2 | M | OSCC | Accumulation and activation | Triggered NK and T cell suppression. Mediator: Arg1, IL-10, TGF-β, | (46) | |
| SAA3 | M | Breast and CRC | Prolonged survival inhibited MDSCs differentiation into M1 | NOS2, Arg1, Nox2, | (51) | |
| TLR4 | MPL | M | – | Accumulation | Suppressed T cell proliferation. | (57) |
| Mediator: IL-10, NO | ||||||
| S100A9 | M | CRC | – | Inhibited CD8+T cell activity. | (53) | |
| Mediator: Arg1 and iNOS | ||||||
| M | MM | – | TNF-α, IL-6, and IL-10 | (54) | ||
| S100A4 | M | Melanoma lung cancer | Prolonged survival | – | (58) | |
| PUAF | M, H | Pancreatic cancer | Arg1, NO, and ROS | (65) | ||
| HMGB1 | M | – | Suppressed T cell proliferation | (66) | ||
| sCRT/39-272 | M | Melanoma | Prolonged survival inhibited MDSCs differentiation into DC | S100A8 and S100A9 | (52) | |
| EV | M, H | Melanoma | Upregulated PD-L1 expression | (69) | ||
| TLR9 | CpG | M | Pancreatic carcinoma | Accumulation in tumor sites | (59) | |
| TLR7 | CL264 | M | Lung adenocarcinoma | Accumulation in tumor site | (60) |
M-MDSCs, monocyte-myeloid-derived suppressor cells; TLRs, Toll-like receptors; M, mouse; H, human; PMN, polymorphonuclear; OSCC, oral squamous cell cancer; RCC, renal cell carcinoma; CRC, colorectal cancer; MM, multiple myeloma; TNFα, tumor necrosis factor-α; NO, nitric oxide; DC, dendritic cell; iNOS, inducible nitric oxide synthase; Arg1, arginase 1; IFN-γ, interferon-γ; IL, interleukin; TGF-β, transforming growth factor-β.
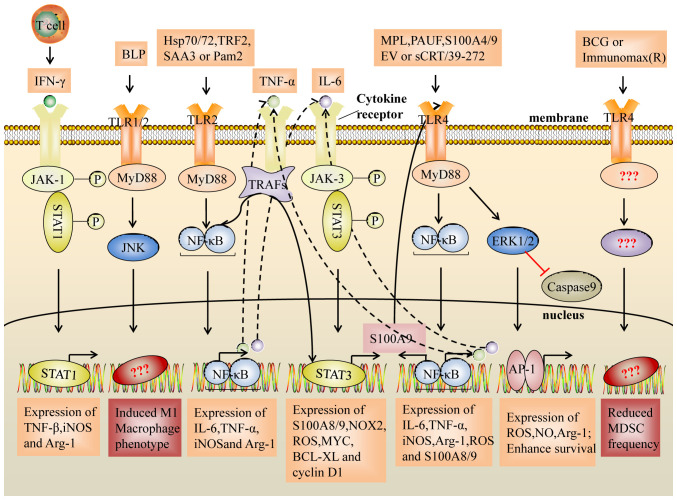
Figure 1.
Suppressive activity of MDSCs induced by the TLR2 and TLR4 signaling pathways. The NF-κB pathway activated by TLR2/4 induces the expression of inflammatory factors (IL-6 and TNF-α). In turn, IL-6 and TNF-α activate the STAT3 signaling pathway and the NF-κB signaling pathway. Notably, STAT3 regulates the expression of the inflammatory factors, S100A8 and S100A9, which act as TLR4 ligands to activate the NF-κB pathway, resulting in upregulation of IL-6 and TNF-α expression, and they form a loop that enhances the expansion and activation of MDSCs. In addition, TLR2/JUK signals induce an M1-like macrophage phenotype and decrease the immunosuppressive activity of MDSCs, whereas transcription factors for the differentiation to M1 macrophages or decreasing frequency on MDSC are unclear. TLR, Toll-like receptor; BLP, bacterial lipoprotein; sCRT/39-272, Recombinant CRT fragment 39–272; PAUF, protein pancreatic adenocarcinoma upregulated factor; SAA3, serum amyloid A3; TRF2, telomeric repeat-binding factor 2; EV, extracellular vesicles; Hsp, heat shock protein; MPL, monophosphoryl Lipid A; BCG, bacillus Calmette-Guerin; Pam2, Pam2CSK4; TNFα, tumor necrosis factor-α; IL-6, interleukin-6; IFNγ, interferon-γ; MyD88, myeloid differentiation primary response 88; TRAF, TNF receptor associated factor; ERK, extracellular regulated protein kinases; JNK, c-Jun kinase; JAK, Janus kinase; NF-κB, nuclear factor kappa-B; AP-1, activator protein-1; ROS, reactive oxygen species; iNOS, inducible nitric oxide synthase; Arg1, arginase 1; BCl-XL, B-cell lymphoma XL.
The accumulation and survival of MDSCs induced by TLR signaling
TLR2 and MDSCs
In EG7 tumor-bearing mice, it has been demonstrated that Pam2CSK4, a TLR2 agonist, induces the accumulation of MDSCs and prolongs the survival of MDSCs, leading to the suppression of the antitumor immune response (9,45). In addition, signal transducer and activator of transcription (STAT3) is an important transcription factor for MDSC expansion, which is attributed to the abnormal and continuous activation of STAT3 in myeloid progenitor cells that prevents them from differentiating into mature myeloid cells (4). Recently, some studies have confirmed this effect (46–50). It has been demonstrated that heat shock protein (Hsp72/Hsp70) in tumor cell-derived exosomes and telomeric repeat-binding factor 2 promote the recruitment and expansion of MDSCs through the activation of STAT3, which is induced in a TLR2/MyD88/IL-6-dependent manner (46–50). Furthermore, serum amyloid A3 can activate STAT3 by TLR2/MyD88/tumor necrosis factor (TNF)α signaling, leading to the enhanced survival of MDSCs (51). Notably, STAT3 also regulates the expression of the inflammatory factors S100A8 and S100A9, which act as TLR4 ligands to activate the immunosuppressive activity of MDSCs (52–54).
TLR4 and MDSCs
The anticonvulsant drug valproic acid, which decreases the frequency of MDSCs, is accompanied by the downregulation of TLR4 mRNA expression (55). In addition, both tumor volume and pulmonary recruitment of MDSCs decrease with a TLR4/MD-2 complex antagonist (56). Previous studies have suggested that the TLR4 signaling pathway may also be involved in the accumulation and survival of MDSCs (55,56). Recently, MPL, a TLR2 and TLR4 agonist, has been confirmed to have this effect (57). MPL induces the accumulation of MDSCs both in vitro and in vivo by inhibiting DC development from myeloid cells (57). In addition, in a melanoma mouse model, soluble calreticulin (sCRT39-272) was demonstrated to promote the migration and survival of tumor-derived MDSCs via interactions with TLR4 (52). Notably, Li et al (58) demonstrated that exogenous S100A4 upregulates TLR4 receptor expression on MSC2 cells and protects MDSCs from apoptosis via the TLR4/extracellular regulated protein kinases (ERK)1/2 signaling axis, both in vitro and in vivo.
TLR7/9 and MDSCs
Notably, some intracellular TLR7/9 signaling pathways also promote accumulation of MDSCs (59,60). CpG ODN (CpG, TLR9 agonist) administration induces the accumulation of tumor-infiltrating MDSCs in pancreatic ductal adenocarcinoma (59). It has also been demonstrated that CL264 (TLR7 agonist) directly interacts with the TLR7 receptor on murine lung adenocarcinoma LLC-Luc cells, which promotes the accumulation of G-MDSCs by increasing the secretion of granulocyte/macrophage CSF and chemokine (C-C motif) ligand 2 (CCL2) in the TME, resulting in an increased number of lung metastases and the promotion of tumor progression (60,61). However, some studies have reported that TLR7 and TLR9 signals weaken MDSC immune activity (16,17).
Differentiation and activation of MDSCs induced by TLR signaling
TLR2 and MDSCs
It has been demonstrated that Pam2CSK4 inhibits TCR-stimulated syngeneic T cell proliferation by inducing M-MDSCs to differentiate into the M2-like (25F9+/CD200R+) phenotype, and produce IL-6 and IL-10 (14). Another study revealed a novel mechanism of inducing the immunosuppressive activity of M-MDSCs: Pam2CSK4 promotes the differentiation of M-MDSCs into CD11b+F4/80+ macrophages, which inhibits DC-induced T cell proliferation through nitric oxide (NO) produced by iNOS (45). In addition, TLR2 signaling activates MDSCs by increasing the expression levels of NOS2, Arg1, iNOS, IL-10 and transforming growth factor (TGF)-β, thereby triggering NK and T cell suppression (46).
TLR agonists induce CD8+ T cells to produce interferon-γ (IFN-γ), which is beneficial for killing tumor cells (62). However, studies have demonstrated that the IFN-γ-STAT1-IRF1 axis is essential for the inhibitory activity obtained by M-MDSCs, and may upregulate the expression levels of iNOS and Arg-1 (39,63). This phenomenon has also been confirmed by Shime et al (45), who demonstrated that the TLR agonist, Pam2CSK4, induces IFN-γ production by CD8+ T cells, accompanied by interferon gamma receptor 1 (IFNgR1) expression on M-MDSCs. Thus, IFN-γ interacts with IFNgR1 on M-MDSCs, induces iNOS expression and inhibits the proliferation of T cells. These results suggest the rationality of targeting the IFN-γ-STAT1-IRF1 axis in MDSCs while targeting MDSC inhibition.
TLR4 and MDSCs
SA100A8/A9 are important pro-inflammatory cytokines that increase MSC accumulation and immunosuppressive activity in the TME (64). De Veirman et al (54) demonstrated that S100A9 acts as a chemokine for multiple myeloma (MM) cells and induces MDSCs to express and secrete inflammatory and promyeloma cytokines, including TNF-α, IL-6 and IL-10. In addition, He et al (52) demonstrated that TLR4 signaling inhibits MDSC differentiation into DCs and promotes their functional maturation, and the chemotactic migration of MDSCs by initiating the expression of S100A8 and S100A9. Recent studies on colorectal cancer (53) and MM (54) have demonstrated that S100A9 promotes the expression levels of Arg1, iNOS and IL-10, and ROS production in MDSCs via TLR4-NF-κB signaling cascades, thereby inhibiting CD8+ T cell activity and promoting tumor progression (53). It has also been demonstrated that the TLR4/ERK/AP-1 and TLR4-IRF axis signaling pathways enhance the immunosuppressive function of MDSCs (65,66). Furthermore, TLR4 signaling can increase the production of IL-10 and attenuate the production of IL-12 in MDSCs, thereby enhancing the interaction between MDSCs and macrophages, and promoting a shift from the tumoricidal Th1 response to the pro-tumorigenic Th2 response (67,68).
Notably, Fleming et al (69) demonstrated that ret mouse melanoma cell-derived extracellular vesicles can induce the upregulation of PD-L1 on bone marrow (BM)-derived murine immature myeloid cells, the immortalized myeloid suppressor cell line, MSC-2, and normal human CD14+ monocytes in a TLR4-MyD88/TRIF-NF-κB signaling-dependent manner, thereby strongly suppressing CD8+ T cell activation through PD-1/PD-L1 signaling cascades (70,71). Similarly, Ki-67 expression in MDSCs is upregulated by the TLR4 mAb, accompanied by increased PD-L1 and iNOS expression on MDSCs, particularly on M-MDSCs (11). These results suggest the possibility of MDSC inhibition and PD-1/PD-L1 signal inhibition, synergistically breaking the immune tolerance microenvironment.
4. Antitumor effects of MDSCs induced by TLR signaling
Recent studies have demonstrated that activation of TLR signaling decreases the ability of MDSCs to inhibit T cell proliferation, thereby inhibiting tumor growth. This effect is mainly manifested in the decreased number of MDSCs, differentiation of MDSCs into antigen-presenting cells and decreased production of inhibitory mediators (72,73). Related studies and potential TLR signaling pathways are summarized in Table II and Fig. 2.
Table II.
Antitumor effects of MDSCs induced by TLR signaling in cancer.
| TLR | Stimulus | Species | Cancer | Number and phenotype | Function and mediator(s) | (Refs.) |
|---|---|---|---|---|---|---|
| TLR3 | Poly(I:C) | M | Breast cancer | Decreased MDSC frequency, and upregulated MHC II, I-Ad, CD80 and CD86 | Decreased ROS production | (76) |
| M | CC | Decreased the number of MDSCs | Attenuated the immunosuppressive activity | (28) | ||
| M | Lymphoma | Abrogated the immunosuppressive activity | (88) | |||
| OR | M | Melanoma lymphoma | Decreased M-MDSC frequencies | Abrogated the immunosuppressive activity | (10) | |
| TLR7 | Imiq | M | Lung cancer | Decreased the number of MDSCs | – | (77,78) |
| M | CC | Phenotype: Ly6C−F4/80+ macrophage phenotype | – | (13) | ||
| SC1 | M | CC | Decreased the number of G-MDSCs | (18) | ||
| s-P-sh | M | Melanoma | Decreased MDSC proportion | – | (79) | |
| TLR7/8 | R848 | M | CC | Decreased MDSC frequency, and upregulated CD11c, F4/80, MHC-I and MHC-II | Abrogated the immunosuppressive activity | (16) |
| M | CRC | Phenotype: F4/80+iNOS+ M1 macrophages | TNF-α and IL-1β | (89) | ||
| H | CC, prostate, pancreatic, liver cancer | Phenotype: M1-like (25F9+/CD200R2) | Increased the ability to kill tumor cells and lost immunosuppressive activity. Mediator: IL-6 and IL-12 | (14) | ||
| TLR8 | Resiq | M | Lymphoma | Phenotype: F4/80+ macrophages and CD11c+/I-Ad+ DCs | Enhanced the proliferation of T cells | (90) |
| Moto | H | Melanoma, CC and prostate | Decreased M-MDSC frequency | (15) | ||
| TLR9 | CpG | M | CC | Decreased M-MDSC frequency, and upregulated F4/80 and downregulated Ly6c and Gr-1 | Abrogated immunosuppressive activity. Mediator: IL-6, TNF-α, IL-12 | (13) |
| M | CC and melanoma | Decreased G-MDSC frequency, and upregulated Sca1(Ly6A/E), F4/80, MHC II and CD11c | Abrogated immunosuppressive activity | (17) | ||
| M | Hepatoma | Attenuated the immunosuppressive activity. Mediator: IFN-γ | (83) | |||
| Ad-CpG/ CpG | M | Melanoma and RCC | Decreased MDSC frequency | – | (80,81) | |
| TLR4 | BCG | M | Bladder cancer | Decreased MDSC frequency | – | (84) |
| I(R) | M | Metastatic breast cancer | Decreased MDSC frequency | – | (85) | |
| TLR1/2 | BLP | M | Lung cancer | Upregulated CD80, CD86, MHCII, F4/80. Phenotype: M1-like macrophage | High levels of NOS2, IL1β, IL-6 and TNF-α, and low levels of Arg1 and CD206 | (73) |
| M | Glioma | Decreased MDSC frequency in TME | – | (87) | ||
| TLR2 | pAbM | M | Mammary carcinoma | Upregulated CD86 and MHCII. Phenotype: M1 macrophage | IL-6, IL-12, TNF-α and iNOS | (86) |
M-MDSC, monocyte-myeloid-derived suppressor cells; TLR, Toll-like receptor; M, mouse; H, human; PMN, polymorphonuclear; OR, oncolytic reovirus; Imiq, imiquimod; s-P-sh, ssRNA-Pim3-shRNA; Resiq, resiquimod; Moto, motolimod; I(R), Immunomax®; RCC, renal cell carcinoma; TNFα, tumor necrosis factor-α; NO, nitric oxide; DCs, dendritic cells; iNOS, inducible nitric oxide synthase; Arg1, arginase 1; IFN-γ, interferon-γ; IL, interleukin; ROS, reactive oxygen species; TME, tumor microenvironment; CC, colon cancer.
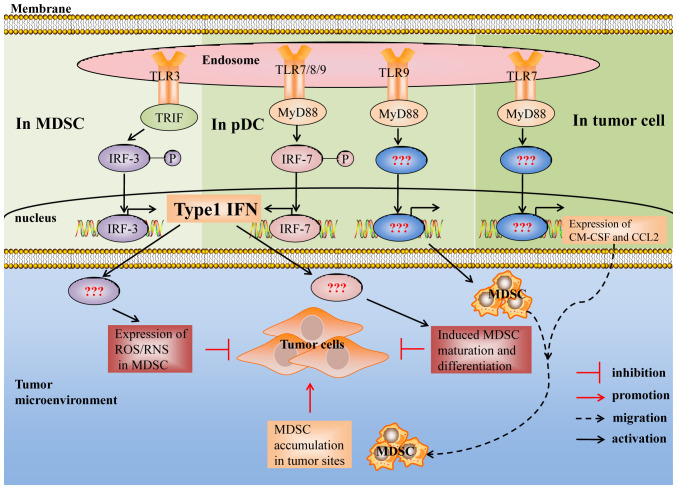
Figure 2.
Suppressive activity of MDSCs induced by the TLR3, TLR7, TLR8 and TLR9 signaling pathways. Type I interferon is essential for TLR3/7/8/9 signal induced MDSCs to inhibit the growth of tumors, whereas the mechanism remains unclear. In addition, the downstream signals and transcription factors of TLR7/9 signal induced MDSCs that promote tumor growth remain unclear. TLR, Toll-like receptor; MyD88, myeloid differentiation primary response 88; TRIF, TIR adaptor-inducing interferon-β; IRF3/7, interferon regulatory factor 3/7; ROS, reactive oxygen species; RNS, reactive nitrogen species; GM, granulocyte/macrophage; CCL2, chemokine (C-C motif) ligand 2.
TLR signaling decreases the number of MDSCs
Intracellular TLRs, including TLR3, TLR7, TLR8 and TLR9, are intrinsically capable of detecting nucleic acids, in which TLR7, TLR8 and TLR9 receptors are similar in terms of expressed cells, recognized ligands, localization on cells and activated pathways, and all intracellular TLRs can induce the production of type I IFN (74,75). Some studies have demonstrated that the TLR3/7/8/9 signaling pathway plays a similar role in regulating MDSCs. It has been reported that TLR3/7/8/9 agonists decrease the MDSC frequency by activating TLR3, TLR7, TLR8 and TLR9 signaling pathways in vivo, thereby enhancing their antitumor effects (16,17,76) (Table II).
TLR3 and MDSCs
Poly (I:C) treatment decreased the MDSC frequency in BM, blood, spleen and tumors (28,76). In addition, oncolytic reovirus, which activates the TLR3 signaling pathway, mainly decreased the M-MDSC frequency and failed to change the G-MDSC frequency (10). Imiquimod (77,78), SC1 (a novel synthetic agonist with exquisite specificity for TLR7) (18) and ssRNA-Pim-3-shRNA, a synthetic dual-function vector that triggers TLR7 receptors via ssRNA fragments) (79) all decreased MDSCs in the TME, thereby inhibiting the growth of tumors in mice. It has been demonstrated that TLR7 signals decrease the number of MDSCs in a type I IFN-dependent manner (18,79). Similarly, TLR7/TLR9 signal-dependent type I IFN production in plasmacytoid DCs (pDCs) is imperative for decreasing MDSC suppressive activity, as well as promoting antitumor immunity (17,18,79). Thus, it was hypothesized that endosome TLR-induced MDSC inhibitory activity is associated with TLR signaling pathway-induced type I IFN production. However, prospective studies are required to confirm this hypothesis and determine how type I IFN regulates MDSC suppressive activity.
TLR7/8 and MDSCs
Systemic application of R848 (a TLR7/8 agonist) significantly decreases the frequency of M-MDSCs in tumors, blood and spleen instead of bone marrow, as well as the frequency of MDSCs in a mouse subcutaneous CT26 colon cancer model and the mouse 4T1 breast cancer model; however, this decrease is not as obvious as in the CT26 model (16). In addition, motolimod (Moto) treatment significantly increases the cell death of M-MDSCs in vitro and in patients with cancer (15). Moto significantly increases the mean fluorescence of FAS on M-MDSCs and upregulates CD69 and FAS-L expression on the T-cell surface; therefore, Moto induces apoptosis of M-MDSCs, in part, through the link between FAS and FAS-L (15).
TLR9 and MDSCs
The effect of TLR9 signals on MDSCs in vivo may be associated with the injection methods used to deliver the TLR9 agonist; intratumoral injection of CpG decreases the proportion of M-MDSCs in tumor-bearing mice (13), whereas subcutaneous injection of CpG significantly decreases the amount of G-MDSCs in the spleen of mice in vivo (17). In addition, Ad5D24 CpG (Ad-CpG), an adenovirus targeting the TLR9 receptor, enhanced the antitumor efficacy in a lung cancer model and significantly decreased the total number and immunosuppressive activation of MDSCs in tumors instead of the spleen (80). Similarly, in a mouse renal cell carcinoma model, CpG treatment decreased the amount and frequency of a large number of MDSCs in tumor-bearing kidney tissues instead of renal blood vessels in vivo (81). However, CpG failed to decrease MDSCs in patients with cancer (15,82), which may be due to the negative expression of the TLR9 receptor on human MDSCs (14).
Notably, Lin et al (83) demonstrated that CpG significantly increases the M-MDSC frequency in nontumor parts of the liver and suppresses murine hepatic tumor growth. This phenomenon was named ‘intrahepatic myeloid aggregation for T cell expansion’, which was attributed to CpG promoting the mRNA expression of IFN-γ in both M-MDSCs and G-MDSCs in the TME. Although these CpG-induced MDSCs still express high levels of IL-10 and Arg-1 mRNA, presenting a suppressive phenotype, their suppressive ability is attenuated (83).
TLR4 and MDSCs
The TLR4 signaling pathway induces the activation and accumulation of MDSCs; however, TLR4 signal activated by BCG (84) and Immunomax® (IR) (85) decreases the frequency of MDSCs. Notably, BCG and PD-L1 blockade synergistically inhibit the growth of bladder cancer and decrease the proportion of MDSCs (84). BCG and PD-L1 have been approved for individual use by the FDA for the treatment of cancer. Their combined application synergistically decreases the proportion of MDSCs (84). This suggests the possibility of a combined application of TLR agonists and PD-1/PD-L1 inhibitors to synergistically break the immune tolerance microenvironment.
TLR signaling decreases the immunosuppressive activity of MDSCs
TLR1/2 and MDSCs
TLR2 signaling promotes the accumulation and activation of MDSCs (9,48). However, some studies have demonstrated that TLR2 signaling also weakens the inhibitory activity of MDSCs (73,86).
It has been demonstrated that TLR1/2 agonists decrease the immunosuppressive activity of MDSCs by inducing M1-type macrophage characteristics in MDSCs (73,86). Notably, Deng et al (73) reported that TLR1/TLR2/c-Jun kinase signaling promotes M-MDSC differentiation into M1-type macrophages, thereby preventing M-MDSC inhibition. Furthermore, the CCL2-CCR2 signaling pathway was implicated in the attraction of M-MDSCs to the tumor site. The disruption of CCL2-CCR2 signaling notably decreases the monocyte influx into the tumor, decreases the number of TAMs, and generally delays tumor growth (72). Similarly, Zhang et al (87) demonstrated that the combination of adoptively transferred antigen-specific T cells and bacterial lipoprotein decreased the MDSC frequency in the TME, which may be associated with low CCL2 expression.
TLR5 and MDSCs
CXCL5 is the main chemokine involved in the migration of MDSCs into tissues, including tumors. It has been demonstrated that intratumoral injection of TLR5 ligand-secreting T cells, engineered tumor-reactive T cells that secrete bacterial flagellin (TLR5 ligand), resulted in a decrease in the number of MDSCs in the spleen and tumor, particularly M-MDSCs, and upregulated the expression levels of CD80, CD86, MHCI and MHCII on MDSCs (12). In addition, the decrease in MDSCs was associated with a striking reduction in CXCL5 levels (12).
However, bacterial flagellin, a TLR5 ligand, failed to influence the ability of MDSCs to inhibit T cell proliferation and slightly affected CD80, MHCI or MHCII expression in MDSCs. It has been suggested that the modification of TLR agonists can change their regulatory effect on MDSCs, which provides new ideas and a theoretical basis for improving the antitumor effect of TLR agonists (12).
TLR3 and MDSCs
In addition to decreasing the number of MDSCs, TLR signals also induce MDSCs to differentiate into antigen-presenting cells and weaken their ability to suppress T cell responses (76). It has been reported that TLR3 signaling activated by PolyI:C decreases the immunosuppressive activity of MDSCs by upregulating MHC II, I-Ad, CD80 and CD86, and decreasing the secretion of ROS in breast cancer models (76). In addition, TLR3 signaling also abrogates the capacity of MDSCs to suppress T cell proliferation in B16 and EL4 tumor models (10). In addition, Shime et al (88) demonstrated that G-MDSCs that had been activated with PolyI:C exhibit cytotoxicity and inhibit tumor growth through the production of ROS/RNS in a TLR3/TRIF/type I IFN-dependent manner.
TLR7/8 and MDSCs
Increasing evidence suggests that TLR7/8 signaling activated by Imiq (13), R848 (89) and resiquimod (16,90) induces MDSCs to differentiate into tumoricidal M1 macrophages in mice. Furthermore, R848 can induce M-MDSCs to differentiate into an M1-like (25F9+/CD200R2) phenotype in patients with cancer, induce the production of IL-6 and IL-12 in M-MDSCs, increase their ability to kill A549 tumor cells, and lose their ability to inhibit T cell proliferation (14). However, R848 is a topical immune response modifier. When it was administered systemically, undesirable side effects were observed. Thus, novel TLR7/8 (3M-055 and CL-075) agonists were designed and found to be safe when administered to mice (91,92). Studies have demonstrated that each of these agonists duplicates the ability of R848 to induce human M-MDSCs to mature into M1-like macrophages, and that they are safe when administered to mice (14,93). These results are exciting, and they also provide new ideas for the development of TLR agonists that decrease side effects and disrupt the inhibition of MDSCs.
TLR9 and MDSCs
Recently, an increasing number of preclinical and clinical trials have used CpG as a vaccine adjuvant to improve the antitumor effect of cancer vaccines. It has been demonstrated that CpG binding with TLR9 on MDSCs directly induces M-MDSC differentiation into Ly6C−F4/80+ macrophages and upregulates CD40, CD80 and CD86 expression on MDSCs in vitro (13,81). However, another study has reported that CpG indirectly upregulates the expression levels of CD11c, MHCII, CD80 and F4/80 on MDSCs through type I IFN produced by pDCs mediated by CpG (17), which may be attributed to CpG administration. Preclinical trials in our laboratory indicated that the recombinant mucin1-maltose-binding protein vaccine, including recombinant mucin1-maltose-binding protein and CpG 2006, significantly downregulated the ratio of MDSCs in the spleen and tumor microenvironment (94). Taken together, these studies provide a rationality for the application of CpG as a cancer vaccine adjuvant.
TLR-TLR crosstalk and MDSCs
Previous studies have proven that a combination of TLR agonists synergistically enhances the activity of cancer vaccines (95,96). Thus, studying the impact of TLR-TLR crosstalk on MDSCs is essential for understanding the synergistic mechanism of TLR agonists and the rational combined application of TLR agonists.
Notably, TLR7 and TLR9 signals have synergistic effects in regulating MDSCs. 3M-052 and CpG can synergistically decrease the frequency of tumor infiltrating M-MDSCs by nearly 90%, and a synergistic reduction of Arg1 and Nos2 mRNA expression, particularly Nos2 mRNA, resulting in a nearly 90% reduction (97). Furthermore, the combination of CpG plus 3M-052 was more successful against both CT26 colon cancer and B16-F10 melanomas compared with CpG or 3M-052 alone, and cure rates around 80–90% can be achieved via combination therapy (97).
However, Triozzi et al (98) demonstrated that Imiq or CpG given individually as an adjuvant both enhance the antitumor effect of tumor vaccines and decrease the MDSC frequency, whereas the combination of Imiq and CpG as adjuvants increases the frequency of MDSCs in the spleen, and the secretion of Arg1 in MDSCs and the production of M2-type macrophages in tumors, accompanied by a reduction in the M1 polarized marker CXCL10, suggesting that TLR7 and TLR9 signals play an antagonistic role in the regulation of MDSCs.
Chang et al (99) demonstrated that TLR2 and TLR9 have synergistic effects in regulating MDSCs. It was reported that Rlipo-E7 m, a recombinant lipoprotein that has intrinsic TLR2 agonist activity, significantly decreases MDSC frequency in the circulation and the tumor microenvironment, and this ability to inhibit MDSCs is enhanced when Rlipo-E7 m is combined with CpG ODN.
5. Conclusions and perspectives
The regulation of MDSCs by TLR signals is a double-edged sword in cancer. TLR signaling can activate the immunosuppressive activity of MDSCs to promote tumor progression, and also abrogate the immunosuppressive activity of MDSCs and inhibit tumor growth. Although several compounds have been investigated for the therapeutic targeting of MDSCs, finding TLR agonists that are able to modulate the suppressive function of tumor-expanded MDSCs could be a better choice, which represents a desirable tipping of the balance toward an increase in immunostimulatory activity with the concomitant loss of immunosuppressive MDSCs. In addition, a combination of TLR agonists and immunotherapy targeting MDSC suppression to decrease the activation effect of MDSCs induced by TLR signaling appears to be feasible in cancer treatment. Furthermore, targeted MDSC cancer immunotherapy through modifying TLR ligands may be an attractive direction, enabling enhanced immune activity, accompanying the loss of MDSC immunosuppressive activity and reversing the immunosuppressive microenvironment, which may be expected to cause tumors to regress further. Studies on MDSCs and their subsets (G-MDSC and M-MDSC) regulation by TLR is still relatively limited. G-MDSC and M-MDSC utilize different molecular mechanisms to suppress the immune response in the TME. Thus, understanding the effect of TLR signaling on MDSCs subsets is beneficial to provide new ideas for the development of cancer immunotherapies.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AP-1
activator protein 1
- Arg1
arginase 1
- BCG
Bacillus Calmette-Guerin
- BLP
bacterial lipoprotein
- BM
bone marrow
- CCL2
chemokine (C-C motif) ligand 2
- DCs
dendritic cells
- ERK
extracellular regulated protein kinases
- Hsp
heat shock protein
- IFNgR1
interferon gamma receptor 1
- IFN-γ
interferon-γ
- Imiq
imiquimod
- iNOS
inducible nitric oxide synthase
- IRF
interferon regulatory factor
- MAPK
mitogen-activated protein kinase
- M-MDSCs
monocyte-myeloid-derived suppressor cells
- MM
multiple myeloma
- Moto
motolimod
- MPL
monophosphoryl lipid A
- MyD88
myeloid differentiation primary response 88
- NF-κB
nuclear factor kappa-B
- NK
natural killer
- pDCs
plasmacytoid DCs
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TCR
T cell receptor
- TLR
Toll-like receptor
- TME
tumor microenvironment
- TRIF
Toll-IL-1 receptor-domain containing adaptor-inducing interferon-β
- VISTA
V-domain immunoglobulin suppressor of T-cell activation
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HZ drafted the initial manuscript, edited and critically revised the manuscript. MJ, HY and WN contributed substantially in drafting the manuscript, editing and critically revising the manuscript for intellectual content. GT put forward the concept, critically revised the article for intellectual content, and was responsible for the organization, revision and submission of the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orillion A, Hashimoto A, Damayanti N, Shen L, Adelaiye-Ogala R, Arisa S, Chintala S, Ordentlich P, Kao C, Elzey B, et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res. 2017;23:5187–5201. doi: 10.1158/1078-0432.CCR-17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA, Jr, Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci USA. 2014;111:11774–11779. doi: 10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Hossain DM, Duttagupta P, Moreira D, Zhao X, Won H, Buettner R, Nechaev S, Majka M, Zhang B, et al. Serum-resistant CpG-STAT3 decoy for targeting survival and immune checkpoint signaling in acute myeloid leukemia. Blood. 2016;127:1687–1700. doi: 10.1182/blood-2015-08-665604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruyama A, Shime H, Takeda Y, Azuma M, Matsumoto M, Seya T. Pam2 lipopeptides systemically increase myeloid-derived suppressor cells through TLR2 signaling. Biochem Biophys Res Commun. 2015;457:445–450. doi: 10.1016/j.bbrc.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Katayama Y, Tachibana M, Kurisu N, Oya Y, Terasawa Y, Goda H, Kobiyama K, Ishii KJ, Akira S, Mizuguchi H, Sakurai F. Oncolytic reovirus inhibits immunosuppressive activity of myeloid-derived suppressor cells in a TLR3-dependent manner. J Immunol. 2018;200:2987–2999. doi: 10.4049/jimmunol.1700435. [DOI] [PubMed] [Google Scholar]
- 11.Tsukamoto H, Kozakai S, Kobayashi Y, Takanashi R, Aoyagi T, Numasaki M, Ohta S, Tomioka Y. Impaired antigen-specific lymphocyte priming in mice after Toll-like receptor 4 activation via induction of monocytic myeloid-derived suppressor cells. Eur J Immunol. 2019;49:546–563. doi: 10.1002/eji.201847805. [DOI] [PubMed] [Google Scholar]
- 12.Geng D, Kaczanowska S, Tsai A, Younger K, Ochoa A, Rapoport AP, Ostrand-Rosenberg S, Davila E. TLR5 ligand-secreting T cells reshape the tumor microenvironment and enhance antitumor activity. Cancer Res. 2015;75:1959–1971. doi: 10.1158/0008-5472.CAN-14-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592–1599. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Shirota Y, Bayik D, Shirota H, Tross D, Gulley JL, Wood LV, Berzofsky JA, Klinman DM. Effect of TLR agonists on the differentiation and function of human monocytic myeloid-derived suppressor cells. J Immunol. 2015;194:4215–4221. doi: 10.4049/jimmunol.1402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang Y, Rutnam ZJ, Dietsch G, Lu H, Yang Y, Hershberg R, Disis ML. TLR8 ligation induces apoptosis of monocytic myeloid-derived suppressor cells. J Leukoc Biol. 2018;103:157–164. doi: 10.1002/JLB.5AB0217-070R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spinetti T, Spagnuolo L, Mottas I, Secondini C, Treinies M, Rüegg C, Hotz C, Bourquin C. TLR7-based cancer immunotherapy decreases intratumoral myeloid-derived suppressor cells and blocks their immunosuppressive function. Oncoimmunology. 2016;5:e1230578. doi: 10.1080/2162402X.2016.1230578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoglmeier C, Bauer H, Noerenberg D, Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S, Bourquin C. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17:1765–1775. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- 18.Vascotto F, Petschenka J, Walzer KC, Vormehr M, Brkic M, Strobl S, Rösemann R, Diken M, Kreiter S, Türeci Ö, Sahin U. Intravenous delivery of the toll-like receptor 7 agonist SC1 confers tumor control by inducing a CD8+ T cell response. Oncoimmunology. 2019;8:1601480. doi: 10.1080/2162402X.2019.1601480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong EH, Chang SY, Lee BR, Kim YS, Lee JM, Kang CY, Kweon MN, Ko HJ. Blockade of Myd88 signaling induces antitumor effects by skewing the immunosuppressive function of myeloid-derived suppressor cells. Int J Cancer. 2013;132:2839–2848. doi: 10.1002/ijc.27974. [DOI] [PubMed] [Google Scholar]
- 20.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llitjos JF, Auffray C, Alby-Laurent F, Rousseau C, Merdji H, Bonilla N, Toubiana J, Belaïdouni N, Mira JP, Lucas B, et al. Sepsis-induced expansion of granulocytic myeloid-derived suppressor cells promotes tumour growth through Toll-like receptor 4. J Pathol. 2016;239:473–483. doi: 10.1002/path.4744. [DOI] [PubMed] [Google Scholar]
- 22.Hu CE, Gan J, Zhang RD, Cheng YR, Huang GJ. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol. 2011;46:156–164. doi: 10.3109/00365521.2010.516450. [DOI] [PubMed] [Google Scholar]
- 23.Savitsky D, Tamura T, Yanai H, Taniguchi T. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol Immunother. 2010;59:489–510. doi: 10.1007/s00262-009-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam S, Kang K, Cha JS, Kim JW, Lee HG, Kim Y, Yang Y, Lee MS, Lim JS. Interferon regulatory factor 4 (IRF4) controls myeloid-derived suppressor cell (MDSC) differentiation and function. J Leukoc Biol. 2016;100:1273–1284. doi: 10.1189/jlb.1A0215-068RR. [DOI] [PubMed] [Google Scholar]
- 25.Xu W, Hiếu T, Malarkannan S, Wang L. The structure, expression, and multifaceted role of immune-checkpoint protein VISTA as a critical regulator of anti-tumor immunity, autoimmunity, and inflammation. Cell Mol Immunol. 2018;15:438–446. doi: 10.1038/cmi.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu W, Dong J, Zheng Y, Zhou J, Yuan Y, Ta HM, Miller HE, Olson M, Rajasekaran K, Ernstoff MS, et al. Immune-checkpoint protein VISTA regulates antitumor immunity by controlling myeloid cell-mediated inflammation and immunosuppression. Cancer Immunol Res. 2019;7:1497–1510. doi: 10.1158/2326-6066.CIR-18-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peek EM, Song W, Zhang H, Huang J, Chin AI. Loss of MyD88 leads to more aggressive TRAMP prostate cancer and influences tumor infiltrating lymphocytes. Prostate. 2015;75:463–473. doi: 10.1002/pros.22932. [DOI] [PubMed] [Google Scholar]
- 28.Di S, Zhou M, Pan Z, Sun R, Chen M, Jiang H, Shi B, Luo H, Li Z. Combined adjuvant of poly I:C improves antitumor effects of CAR-T cells. Front Oncol. 2019;9:241. doi: 10.3389/fonc.2019.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 31.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 32.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 33.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 36.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: A clinical perspective. J Immunother. 2012;35:107–115. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 38.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 2015;98:913–922. doi: 10.1189/jlb.4RI0515-204R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dowling JK, Mansell A. Toll-like receptors: The swiss army knife of immunity and vaccine development. Clin Transl Immunology. 2016;5:e85. doi: 10.1038/cti.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urban-Wojciuk Z, Khan MM, Oyler BL, Fåhraeus R, Marek-Trzonkowska N, Nita-Lazar A, Hupp TR, Goodlett DR. The role of TLRs in anti-cancer immunity and tumor rejection. Front Immunol. 2019;10:2388. doi: 10.3389/fimmu.2019.02388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carpentier A, Metellus P, Ursu R, Zohar S, Lafitte F, Barrié M, Meng Y, Richard M, Parizot C, Laigle-Donadey F, et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: A phase II study. Neuro Oncol. 2010;12:401–408. doi: 10.1093/neuonc/nop047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carpentier A, Laigle-Donadey F, Zohar S, Capelle L, Behin A, Tibi A, Martin-Duverneuil N, Sanson M, Lacomblez L, Taillibert S, et al. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro Oncol. 2006;8:60–66. doi: 10.1215/S1522851705000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleming V, Hu X, Weber R, Nagibin V, Groth C, Altevogt P, Utikal J, Umansky V. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front Immunol. 2018;9:398. doi: 10.3389/fimmu.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shime H, Maruyama A, Yoshida S, Takeda Y, Matsumoto M, Seya T. Toll-like receptor 2 ligand and interferon-γ suppress anti-tumor T cell responses by enhancing the immunosuppressive activity of monocytic myeloid-derived suppressor cells. Oncoimmunology. 2017;7:e1373231. doi: 10.1080/2162402X.2017.1373231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cherfils-Vicini J, Iltis C, Cervera L, Pisano S, Croce O, Sadouni N, Győrffy B, Collet R, Renault VM, Rey-Millet M, et al. Cancer cells induce immune escape via glycocalyx changes controlled by the telomeric protein TRF2. EMBO J. 2019;38:e100012. doi: 10.15252/embj.2018100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gobbo J, Marcion G, Cordonnier M, Dias AMM, Pernet N, Hammann A, Richaud S, Mjahed H, Isambert N, Clausse V, et al. Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J Natl Cancer Inst. 2015;108 doi: 10.1093/jnci/djv330. [DOI] [PubMed] [Google Scholar]
- 48.Xiang X, Liu Y, Zhuang X, Zhang S, Michalek S, Taylor DD, Grizzle W, Zhang HG. TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am J Pathol. 2010;177:1606–1610. doi: 10.2353/ajpath.2010.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diao J, Yang X, Song X, Chen S, He Y, Wang Q, Chen G, Luo C, Wu X, Zhang Y. Exosomal Hsp70 mediates immunosuppressive activity of the myeloid-derived suppressor cells via phosphorylation of Stat3. Med Oncol. 2015;32:453. doi: 10.1007/s12032-014-0453-2. [DOI] [PubMed] [Google Scholar]
- 51.Lee JM, Kim EK, Seo H, Jeon I, Chae MJ, Park YJ, Song B, Kim YS, Kim YJ, Ko HJ, Kang CY. Serum amyloid A3 exacerbates cancer by enhancing the suppressive capacity of myeloid-derived suppressor cells via TLR2-dependent STAT3 activation. Eur J Immunol. 2014;44:1672–1684. doi: 10.1002/eji.201343867. [DOI] [PubMed] [Google Scholar]
- 52.He XY, Gong FY, Chen Y, Zhou Z, Gong Z, Gao XM. Calreticulin fragment 39–272 promotes B16 melanoma malignancy through myeloid-derived suppressor cells in vivo. Front Immunol. 2017;8:1306. doi: 10.3389/fimmu.2017.01306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang M, Wu R, Chen L, Peng Q, Li S, Zhang Y, Zhou L, Duan L. S100A9 regulates MDSCs-mediated immune suppression via the RAGE and TLR4 signaling pathways in colorectal carcinoma. Front Immunol. 2019;10:2243. doi: 10.3389/fimmu.2019.02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Veirman K, De Beule N, Maes K, Menu E, De Bruyne E, De Raeve H, Fostier K, Moreaux J, Kassambara A, Hose D, et al. Extracellular S100A9 protein in bone marrow supports multiple myeloma survival by stimulating angiogenesis and cytokine secretion. Cancer Immunol Res. 2017;5:839–846. doi: 10.1158/2326-6066.CIR-17-0192. [DOI] [PubMed] [Google Scholar]
- 55.Xie Z, Ago Y, Okada N, Tachibana M. Valproic acid attenuates immunosuppressive function of myeloid-derived suppressor cells. J Pharmacol Sci. 2018;137:359–365. doi: 10.1016/j.jphs.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 56.Deguchi A, Tomita T, Ohto U, Takemura K, Kitao A, Akashi-Takamura S, Miyake K, Maru Y. Eritoran inhibits S100A8-mediated TLR4/MD-2 activation and tumor growth by changing the immune microenvironment. Oncogene. 2016;35:1445–1456. doi: 10.1038/onc.2015.211. [DOI] [PubMed] [Google Scholar]
- 57.Chen J, Sun B, Zhao X, Liang D, Liu J, Huang Y, Lei W, Chen M, Sun W. Monophosphoryl lipid A induces bone marrow precursor cells to differentiate into myeloid-derived suppressor cells. Mol Med Rep. 2013;8:1074–1078. doi: 10.3892/mmr.2013.1653. [DOI] [PubMed] [Google Scholar]
- 58.Li Q, Dai C, Xue R, Wang P, Chen L, Han Y, Erben U, Qin Z. S100A4 protects myeloid-derived suppressor cells from intrinsic apoptosis via TLR4-ERK1/2 signaling. Front Immunol. 2018;9:388. doi: 10.3389/fimmu.2018.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, Seifert L, Daley D, Greco SH, Deutsch M, et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med. 2015;212:2077–2094. doi: 10.1084/jem.20142162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dajon M, Iribarren K, Petitprez F, Marmier S, Lupo A, Gillard M, Ouakrim H, Victor N, Vincenzo DB, Joubert PE, et al. Toll like receptor 7 expressed by malignant cells promotes tumor progression and metastasis through the recruitment of myeloid derived suppressor cells. Oncoimmunology. 2018;8:e1505174. doi: 10.1080/2162402X.2018.1505174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dajon M, Iribarren K, Cremer I. Dual roles of TLR7 in the lung cancer microenvironment. Oncoimmunology. 2015;4:e991615. doi: 10.4161/2162402X.2014.991615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jie J, Zhang Y, Zhou H, Zhai X, Zhang N, Yuan H, Ni W, Tai G. CpG ODN1826 as a promising mucin1-maltose-binding protein vaccine adjuvant induced DC maturation and enhanced antitumor immunity. Int J Mol Sci. 2018;19:920. doi: 10.3390/ijms19030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schouppe E, Mommer C, Movahedi K, Laoui D, Morias Y, Gysemans C, Luyckx A, De Baetselier P, Van Ginderachter JA. Tumor-induced myeloid-derived suppressor cell subsets exert either inhibitory or stimulatory effects on distinct CD8+ T-cell activation events. Eur J Immunol. 2013;43:2930–2942. doi: 10.1002/eji.201343349. [DOI] [PubMed] [Google Scholar]
- 64.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song J, Lee J, Kim J, Jo S, Kim YJ, Baek JE, Kwon ES, Lee KP, Yang S, Kwon KS, et al. Pancreatic adenocarcinoma up-regulated factor (PAUF) enhances the accumulation and functional activity of myeloid-derived suppressor cells (MDSCs) in pancreatic cancer. Oncotarget. 2016;7:51840–51853. doi: 10.18632/oncotarget.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tachibana M. The immunosuppressive function of myeloid-derived suppressor cells is regulated by the HMGB1-TLR4 axis. Yakugaku Zasshi. 2018;138:143–148. doi: 10.1248/yakushi.17-00158. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 67.Li J, Yang F, Wei F, Ren X. The role of toll-like receptor 4 in tumor microenvironment. Oncotarget. 2017;8:66656–66667. doi: 10.18632/oncotarget.19105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85:996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fleming V, Hu X, Weller C, Weber R, Groth C, Riester Z, Hüser L, Sun Q, Nagibin V, Kirschning C, et al. Melanoma extracellular vesicles generate immunosuppressive myeloid cells by upregulating PD-L1 via TLR4 signaling. Cancer Res. 2019;79:4715–4728. doi: 10.1158/0008-5472.CAN-19-0053. [DOI] [PubMed] [Google Scholar]
- 70.Karwacz K, Bricogne C, MacDonald D, Arce F, Bennett CL, Collins M, Escors D. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol Med. 2011;3:581–592. doi: 10.1002/emmm.201100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 blockade: Have we found the key to unleash the antitumor immune response? Front Immunol. 2017;8:1597. doi: 10.3389/fimmu.2017.01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tcyganov E, Mastio J, Chen E, Gabrilovich DI. Plasticity of myeloid-derived suppressor cells in cancer. Curr Opin Immunol. 2018;51:76–82. doi: 10.1016/j.coi.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng Y, Yang J, Qian J, Liu R, Huang E, Wang Y, Luo F, Chu Y. TLR1/TLR2 signaling blocks the suppression of monocytic myeloid-derived suppressor cell by promoting its differentiation into M1-type macrophage. Mol Immunol. 2019;112:266–273. doi: 10.1016/j.molimm.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Boozari M, Butler AE, Sahebkar A. Impact of curcumin on toll-like receptors. J Cell Physiol. 2019;234:12471–12482. doi: 10.1002/jcp.28103. [DOI] [PubMed] [Google Scholar]
- 76.Forghani P, Waller EK. Poly (I: C) modulates the immunosuppressive activity of myeloid-derived suppressor cells in a murine model of breast cancer. Breast Cancer Res Treat. 2015;153:21–30. doi: 10.1007/s10549-015-3508-y. [DOI] [PubMed] [Google Scholar]
- 77.Chuang CM, Monie A, Hung CF, Wu TC. Treatment with imiquimod enhances antitumor immunity induced by therapeutic HPV DNA vaccination. J Biomed Sci. 2010;17:32. doi: 10.1186/1423-0127-17-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho JH, Lee HJ, Ko HJ, Yoon BI, Choe J, Kim KC, Hahn TW, Han JA, Choi SS, Jung YM, et al. The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma. Oncotarget. 2017;8:24932–24948. doi: 10.18632/oncotarget.15326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu J, Hu Y, Guo Q, Yu X, Shao L, Zhang C. Enhanced anti-melanoma efficacy of a Pim-3-targeting bifunctional small hairpin RNA via single-stranded RNA-mediated activation of plasmacytoid dendritic cells. Front Immunol. 2019;10:2721. doi: 10.3389/fimmu.2019.02721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cerullo V, Diaconu I, Romano V, Hirvinen M, Ugolini M, Escutenaire S, Holm SL, Kipar A, Kanerva A, Hemminki A. An oncolytic adenovirus enhanced for toll-like receptor 9 stimulation increases antitumor immune responses and tumor clearance. Mol Ther. 2012;20:2076–2086. doi: 10.1038/mt.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.James BR, Anderson KG, Brincks EL, Kucaba TA, Norian LA, Masopust D, Griffith TS. CpG-mediated modulation of MDSC contributes to the efficacy of Ad5-TRAIL therapy against renal cell carcinoma. Cancer Immunol Immunother. 2014;63:1213–1227. doi: 10.1007/s00262-014-1598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tarhini AA, Butterfield LH, Shuai Y, Gooding WE, Kalinski P, Kirkwood JM. Differing patterns of circulating regulatory T cells and myeloid-derived suppressor cells in metastatic melanoma patients receiving anti-CTLA4 antibody and interferon-alpha or TLR-9 agonist and GM-CSF with peptide vaccination. J Immunother. 2012;35:702–710. doi: 10.1097/CJI.0b013e318272569b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin YC, Hsu CY, Huang SK, Fan YH, Huang CH, Yang CK, Su WT, Chang PC, Dutta A, Liu YJ, et al. Induction of liver-specific intrahepatic myeloid cells aggregation expands CD8 T cell and inhibits growth of murine hepatoma. Oncoimmunology. 2018;7:e1502129. doi: 10.1080/2162402X.2018.1502129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Liu J, Yang X, Liu Y, Liu Y, Li Y, Sun L, Yang X, Niu H. Bacillus Calmette-Guérin and anti-PD-L1 combination therapy boosts immune response against bladder cancer. Onco Targets Ther. 2018;11:2891–2899. doi: 10.2147/OTT.S165840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghochikyan A, Pichugin A, Bagaev A, Davtyan A, Hovakimyan A, Tukhvatulin A, Davtyan H, Shcheblyakov D, Logunov D, Chulkina M, et al. Targeting TLR-4 with a novel pharmaceutical grade plant derived agonist, Immunomax®, as a therapeutic strategy for metastatic breast cancer. J Transl Med. 2014;12:322. doi: 10.1186/s12967-014-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y, Zhang L, Zhu X, Wang Y, Liu W, Gong W. Polysaccharide Agaricus blazei Murill stimulates myeloid derived suppressor cell differentiation from M2 to M1 type, which mediates inhibition of tumour immune-evasion via the Toll-like receptor 2 pathway. Immunology. 2015;146:379–391. doi: 10.1111/imm.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y, Luo F, Li A, Qian J, Yao Z, Feng X, Chu Y. Systemic injection of TLR1/2 agonist improves adoptive antigen-specific T cell therapy in glioma-bearing mice. Clin Immunol. 2014;154:26–36. doi: 10.1016/j.clim.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Shime H, Matsumoto M, Seya T. Double-stranded RNA promotes CTL-independent tumor cytolysis mediated by CD11b+Ly6G+ intratumor myeloid cells through the TICAM-1 signaling pathway. Cell Death Differ. 2017;24:385–396. doi: 10.1038/cdd.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu Z, Mao H, Yu M, Wang X. TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett. 2020;469:173–185. doi: 10.1016/j.canlet.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 90.Lee M, Park CS, Lee YR, Im SA, Song S, Lee CK. Resiquimod, a TLR7/8 agonist, promotes differentiation of myeloid-derived suppressor cells into macrophages and dendritic cells. Arch Pharm Res. 2014;37:1234–1240. doi: 10.1007/s12272-014-0379-4. [DOI] [PubMed] [Google Scholar]
- 91.Butchi NB, Pourciau S, Du M, Morgan TW, Peterson KE. Analysis of the neuroinflammatory response to TLR7 stimulation in the brain: Comparison of multiple TLR7 and/or TLR8 agonists. J Immunol. 2008;180:7604–7612. doi: 10.4049/jimmunol.180.11.7604. [DOI] [PubMed] [Google Scholar]
- 92.Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 93.Le Mercier I, Poujol D, Sanlaville A, Sisirak V, Gobert M, Durand I, Dubois B, Treilleux I, Marvel J, Vlach J, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 2013;73:4629–4640. doi: 10.1158/0008-5472.CAN-12-3058. [DOI] [PubMed] [Google Scholar]
- 94.Zhou H, Zhang Z, Liu G, Jiang M, Wang J, Liu Y, Tai G. The effect of different immunization cycles of a recombinant mucin1-maltose-binding protein vaccine on T cell responses to B16-MUC1 melanoma in mice. Int J Mol Sci. 2020;21:5810. doi: 10.3390/ijms21165810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 96.Tan RS, Ho B, Leung BP, Ding JL. TLR cross-talk confers specificity to innate immunity. Int Rev Immunol. 2014;33:443–453. doi: 10.3109/08830185.2014.921164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao BG, Vasilakos JP, Tross D, Smirnov D, Klinman DM. Combination therapy targeting toll like receptors 7, 8 and 9 eliminates large established tumors. J Immunother Cancer. 2014;2:12. doi: 10.1186/2051-1426-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Triozzi PL, Aldrich W, Ponnazhagan S. Regulation of the activity of an adeno-associated virus vector cancer vaccine administered with synthetic Toll-like receptor agonists. Vaccine. 2010;28:7837–7843. doi: 10.1016/j.vaccine.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 99.Chang LS, Leng CH, Yeh YC, Wu CC, Chen HW, Huang HM, Liu SJ. Toll-like receptor 9 agonist enhances anti-tumor immunity and inhibits tumor-associated immunosuppressive cells numbers in a mouse cervical cancer model following recombinant lipoprotein therapy. Mol Cancer. 2014;13:60. doi: 10.1186/1476-4598-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.