Abstract
The present study aimed to investigate the prognostic value of baseline 18F-FDG PET/CT quantitative parameters and interim treatment response, and to assess whether the combination of these could improve the predictive efficacy in patients with diffuse large B-cell lymphoma (DLBCL) receiving R-CHOP chemotherapy. PET/CT images and clinical data of 64 patients with DLBCL who had undergone 18F-FDG PET/CT scan before and after 3 or 4 cycles of R-CHOP chemotherapy were retrospectively reviewed. The quantitative parameters including standardized uptake value (SUV), metabolic tumor volume (MTV), total lesion glycolysis (TLG), and maximum diameter of the maximum lesion (Dmax) were measured on baseline PET/CT images. Cox proportional hazards model was used to evaluate the influence of baseline PET/CT parameters, clinical indicators and interim treatment response on prognosis. Survival analysis was performed using Kaplan-Meier method. Receiver operating characteristic (ROC) curve analysis was performed to estimate the predictive efficacy of the combination of baseline PET/CT parameters and interim treatment response. Ann Arbor stage, International Prognostic Index (IPI), lactate dehydrogenase (LDH), necrosis, MTVmax, TLGmax, Dmax and interim treatment response showed association with 2-year progression-free survival (PFS, P<0.05). LDH, necrosis, MTVmax, MTVsum, TLGmax, TLGsum, Dmax and interim treatment response showed association with 2-year overall survival (OS, P<0.05). Ann Arbor stage, Dmax and interim treatment response were found to be independent predictors of 2-year PFS (P<0.05), while Dmax and interim treatment response were found to be independent predictors of 2-year OS (P<0.05). The PFS and OS curves of Dmax <5.7 cm group and Dmax ≥5.7 cm group, complete response (CR) group and non-CR group were significantly different, respectively (P<0.05). The baseline 18F-FDG PET/CT parameters and interim treatment response have important prognostic values in DLBCL patients who received R-CHOP chemotherapy. Combined application of Dmax and interim treatment response improved the predictive efficacy of 2-year PFS. It may be helpful to identify patients who are at high-risk of relapse and to guide early clinical intervention of these patients.
Keywords: diffuse large B-cell lymphoma, PET/CT imaging, interim treatment response, disease prognosis, R-CHOP chemotherapy
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a type of lymphoma with high heterogeneity in regards to immunophenotype, gene expression, morphology, clinical symptoms and prognosis (1). The International Prognostic Index (IPI) is the most commonly used prognostic index for predicting the outcome in clinics for patients with DLBCL. Prognostic evaluation and risk stratification are made by IPI based on 5 aspects, including age, Ann Arbor stage, lactate dehydrogenase (LDH) levels, physical condition score, and the number of extranodal organ involvement. However, DLBCL patients with the same IPI score might still have different outcomes after undergoing similar chemotherapy due to tumor heterogeneity. Furthermore, the prognostic value of an intermediate IPI score still remains to be unclear (2,3). With the introduction of rituximab into the first-line chemotherapy regimens (R-CHOP), the prognostic value of IPI still faced great challenges. Thus, more reliable prognostic indicators or evaluation models are urgently needed to identify patients who are more likely to relapse in clinical practice (4,5). Some scholars have carried out relevant basic research and proposed gene predictors such as cell origin, MYC and BCL2/BCL6 double expression, but their application value still requires further confirmation (6,7).
According to previous studies, baseline 18F-FDG PET/CT parameters, such as metabolism of tumor volume (MTV) and total lesion glycolysis (TLG), could provide personalized information on metabolic activity and metabolic volume of the tumor, and have important prognostic value in patients with DLBCL (8,9). However, the results of these studies are not completely consistent, and this may be due to the distribution bias of enrolled cases, different threshold selection methods and standards for measurement of PET/CT parameters. Assessment of interim treatment response based on PET/CT has great prognostic value in DLBCL patients and it has been included in criteria for response assessment (10,11). Interim treatment response has attracted much clinical attention, but some patients with a good interim treatment response and negative interim PET/CT may still have recurrence and progression. Currently, there are relatively few studies that have discussed the prognostic value of the combination of baseline PET/CT quantitative parameters and interim treatment response in DLBCL patients (12,13).
Hence, in the present study, the relationship between baseline PET/CT quantitative parameters, interim treatment response and prognostic survival of 64 patients with DLBCL receiving R-CHOP chemotherapy was analyzed, and the predictive efficacy of the combination of baseline PET/CT parameters and interim treatment response for survival was evaluated with the aim to guide the implementation of appropriate treatment and follow-up strategies for high-risk patients and to improve their long-term survival.
Patients and methods
Patient selection
The inclusion criteria were as follows: i) Patients with pathologically and immunohistochemically confirmed DLBCL after surgery or biopsy, ii) patients who received first-line R-CHOP (rituximab, cyclophosphamide, hydroxydaunomycin, oncovin and prednisone) chemotherapy, iii) patients who underwent 18F-FDG PET/CT scans before and after 3 or 4 cycles of R-CHOP chemotherapy, respectively, iv) patients no less than 18 years of age, and v) patients with complete clinical records. The exclusion criteria were as follows: i) Patients with primary central nervous system lymphoma, ii) patients with a history of malignancy or with other malignancies at present, iii) patients who received chemotherapy, radiotherapy or surgical resection before PET/CT scan prior to the study enrollment, iv) patients who dropped out during the treatment due to any reason, and v) patients with incomplete clinical records. Between July 2014 and December 2018, a total of 358 patients with DLBCL were admitted to our institution, and 294 patients of these were excluded, including those who did not receive rituximab treatment (n=67), did not undergo baseline and interim PET/CT scan (n=65), with primary central nervous system lymphoma (n=17), with a history of malignancy or with other malignancies at present (n=9), received other therapies before PET/CT scan (n=76), dropped out during the course of treatment or follow-up (n=29), or with incomplete clinical records (n=31). Finally, a total of 64 patients were enrolled in this study. Clinical data such as sex, age, B symptoms, Ann Arbor staging, IPI, LDH, β2-MG and immunohistochemical results were obtained according to the medical records. This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2016-KL002-01), and patient informed consent was waived due to the retrospective nature of the present study.
PET/CT imaging
18F-FDG PET/CT image acquisition was performed with Discovery PET/CT Elite scanner (GE Healthcare). After fasting for at least 6 h, patients were intravenously injected with 18F-FDG (3.5 to 4.0 MBq/kg). The weight of the patients was obtained and the fasting blood glucose levels were controlled to less than 150 mg/dl before injection. Patients after injection were advised to rest for 1 h before initiating the PET/CT scan. Patients were placed in a supine position with quiet breathing. CT images were acquired from the skull vertex to proximal thigh initially and then the corresponding PET data were collected. CT data were used for attenuation correction and the standard protocol settings were as follows: 120 KV, 180 mA, slice thickness of 3.75 mm. PET scanning images were acquired in 7 to 8 bed positions and the acquisition time was 3 min per bed position. Image fusion was performed after reconstruction by iterative method.
PET/CT parameters
All PET/CT images were reconstructed and reviewed using Volume Viewer software on Workstation AW 4.5 (GE Healthcare) by two experienced radiologists and a nuclear medicine physician who were blinded to the clinical information. Visual assessment and semi-quantitative analysis were used for image analysis. Tumor contours covering the entire lesion volume in axial, coronal and sagittal images were delineated automatically or manually as and when necessary, and then the quantitative parameters such as maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), metabolic tumor volume of the maximum lesion (MTVmax), sum of the metabolic tumor volume (MTVsum), total lesion glycolysis of the maximum lesion (TLGmax), sum of total lesion glycolysis (TLGsum), maximum diameter of the maximum lesion (Dmax) were measured or calculated. MTV was measured with a threshold of 40% SUVmax. TLG was the product of MTV and SUVmean.
Interim treatment response evaluation
Response to 3 or 4 cycles of R-CHOP chemotherapy was assessed according to the Lugano criteria (11) and patients were categorized into four types: Complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD) as described here. CR: PET/CT-based response: Score 1, 2, or 3 with or without a residual mass on 5PS (1, no uptake above background; 2, uptake ≤ mediastinum; 3, uptake > mediastinum but ≤ liver; 4, uptake moderately > liver; 5, uptake markedly higher than liver and/or no new lesions; X, new areas of uptake unlikely to be related to lymphoma), no new lesions and no evidence of FDG-avid disease in marrow. PR: Score 4 or 5 with reduced uptake compared with baseline and residual mass(es) of any size, no new lesions, residual uptake higher than uptake in normal marrow but reduced compared with baseline. SD: Score 4 or 5 with no significant change in FDG uptake from baseline at interim or end of treatment, no new lesions, no change in marrow uptake from baseline. PD: Score 4 or 5 with an increase in intensity of uptake from baseline, new FDG-avid foci consistent with lymphoma rather than another etiology (eg. infection, inflammation), new or recurrent FDG-avid foci in marrow. In the present study, all patients were divided into two groups including CR group and non-CR group. Patients with CR were included in the CR group and patients with PR, SD or PD were included in the non-CR group.
Follow-up assessment
Follow-up was performed by conducting phone interview or reviewing of hospital records. Progression-free survival (PFS) was defined as the time from initial diagnosis until the first occurrence of disease recurrence, progression, death due to any cause or at the end of the follow-up period. Overall survival (OS) was defined as the time from initial diagnosis until death due to any cause or the end of follow-up period.
Statistical analysis
Non-normally distributed data are expressed as median (Q1 and Q3). Intraclass correlation coefficient (ICC) was used to assess interobserver consistency of PET/CT parameters. Cox proportional hazard models were used in the univariate and multivariate analyses. Survival curves were constructed using Kaplan-Meier method. Receiver operating characteristic (ROC) analysis was performed to evaluate the predictive efficacy of the indicators. A P-value of less than 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS software (version 21.0) (IBM Corp.).
Results
Patient characteristics
A total of 64 patients, including 33 men (51.6%) and 31 women (48.4%), were enrolled in this study. The median age at diagnosis was 57 years (range, 25–80 years). The clinical characteristics of the 64 patients with DLBCL are listed in Table I. Of the entire cohort, 39 (60.9%) patients achieved CR and 25 (39.1%) patients had non-CR after 3 or 4 cycles of R-CHOP chemotherapy. Follow-up time ranged from 6 to 62 months, and the median follow-up time was 25 months. Relapse and progression occurred in 23 patients, while 17 patients died within two years. The 2-year PFS rate and 2-year OS rate were 64.1 and 73.4%, respectively.
Table I.
Clinical characteristics of the 64 patients with DLBCL.
| Characteristics | No. of patients | Percentage (%) |
|---|---|---|
| Sex | ||
| Male | 33 | 51.6 |
| Female | 31 | 48.4 |
| Age (years) | ||
| ≤60 | 34 | 53.1 |
| >60 | 30 | 46.9 |
| B symptoms | ||
| Yes | 17 | 26.6 |
| No | 47 | 73.4 |
| Ann Arbor stage | ||
| I+II | 22 | 34.4 |
| III+IV | 42 | 65.6 |
| IPI | ||
| ≤2 | 40 | 62.5 |
| >2 | 24 | 37.5 |
| LDH | ||
| Normal | 34 | 53.1 |
| Abnormal | 30 | 46.9 |
| β2-MG | ||
| Normal | 37 | 57.8 |
| Abnormal | 27 | 42.2 |
| Nln | ||
| <2 | 14 | 21.9 |
| ≥2 | 50 | 78.1 |
| Neo | ||
| <2 | 49 | 76.6 |
| ≥2 | 15 | 23.4 |
| BMI | ||
| No | 52 | 81.2 |
| Yes | 12 | 18.8 |
| Necrosis | ||
| No | 49 | 76.6 |
| Yes | 15 | 23.4 |
DLBCL, diffuse large B-cell lymphoma; IPI, International Prognostic Index, LDH, lactate dehydrogenase; β2-MG, β2 microglobulin; Nln, number of lymph node area involvement; Neo, number of extranodal organ involvement; BMI, bone marrow involvement.
Interobserver agreement
Baseline PET/CT parameters were measured by two observers. Consistency test showed intraclass correlation coefficient (ICC) that ranged between 0.663 and 0.991, showing good agreement. ICC values are shown in Table SI and baseline PET/CT parameters of the patients are listed in Table SII.
Univariate analysis
The median SUVmax, SUVmean, MTVmax, MTVsum, TLGmax, TLGsum and Dmax of the entire population were 17.6, 10.6, 63.5 cm3, 132.6 cm3, 628.7 g, 1135.9 g and 5.7 cm, respectively.
Of all the clinical indicators, baseline PET/CT parameters and interim treatment response evaluated, Ann Arbor stage, IPI, LDH, necrosis, MTVmax, TLGmax, Dmax and interim treatment response showed association with 2-year PFS (P<0.05). LDH, necrosis, MTVmax, MTVsum, TLGmax, TLGsum, Dmax and interim treatment response showed association with 2-year OS (P<0.05) (Tables II and III).
Table II.
Univariate analyses of the clinical characteristics for PFS and OS.
| 2-year PFS | 2-year OS | |||||
|---|---|---|---|---|---|---|
| Characteristics | RR | 95% CI | P-value | RR | 95% CI | P-value |
| Sex | ||||||
| Male | 1 | 1 | ||||
| Female | 0.418 | 0.170–1.028 | 0.057 | 0.274 | 0.090–0.835 | 0.061 |
| Age (years) | ||||||
| ≤60 | 1 | 1 | ||||
| >60 | 0.544 | 0.228–1.300 | 0.171 | 0.439 | 0.163–1.180 | 0.103 |
| B symptoms | ||||||
| Yes | 1 | 1 | ||||
| No | 0.513 | 0.214–1.227 | 0.134 | 0.410 | 0.159–1.062 | 0.066 |
| Ann Arbor stage | ||||||
| I+II | 1 | 1 | ||||
| III+IV | 2.754 | 1.013–7.485 | 0.047a | 1.927 | 0.686–5.414 | 0.213 |
| IPI score | ||||||
| ≤2 | 1 | 1 | ||||
| >2 | 2.501 | 1.076–5.816 | 0.033a | 2.381 | 0.943–6.012 | 0.066 |
| LDH level | ||||||
| Normal | 1 | 1 | ||||
| Abnormal | 5.926 | 1.986–17.680 | 0.001a | 4.495 | 1.469–13.758 | 0.008a |
| β2-MG | ||||||
| Normal | 1 | 1 | ||||
| Abnormal | 1.252 | 0.535–2.930 | 0.605 | 1.469 | 0.579–3.726 | 0.418 |
| Nln | ||||||
| <2 | 1 | 1 | ||||
| ≥2 | 2.139 | 0.723–6.327 | 0.170 | 2.381 | 0.686–8.256 | 0.172 |
| Neo | ||||||
| <2 | 1 | 1 | ||||
| ≥2 | 2.124 | 0.886–5.092 | 0.091 | 2.202 | 0.850–5.703 | 0.104 |
| BMI | ||||||
| No | 1 | 1 | ||||
| Yes | 1.307 | 0.442–3.868 | 0.629 | 0.977 | 0.280–3.401 | 0.970 |
| Necrosis | ||||||
| No | 1 | 1 | ||||
| Yes | 3.594 | 1.526–8.465 | 0.003a | 4.085 | 1.612–10.356 | 0.003a |
Statistically significant. PFS, progression-free survival; OS, overall survival; RR, relative risk; CI, confidence interval; IPI, International Prognostic Index; LDH, lactate dehydrogenase; β2-MG, β2 microglobulin; Nln, number of lymph node area involvement; Neo, number of extranodal organ involvement; BMI, bone marrow involvement.
Table III.
Univariate analyses of baseline PET/CT parameters and interim treatment response for PFS and OS.
| 2-year PFS | 2-year OS | |||||
|---|---|---|---|---|---|---|
| Variables | RR | 95% CI | P-value | RR | 95% CI | P-value |
| SUVmax | ||||||
| <17.6 | 1 | 1 | ||||
| ≥17.6 | 1.575 | 0.673–3.690 | 0.295 | 1.531 | 0.591–3.969 | 0.381 |
| SUVmean | ||||||
| <10.6 | 1 | 1 | ||||
| ≥10.6 | 1.208 | 0.522–2.798 | 0.659 | 1.531 | 0.591–3.969 | 0.381 |
| MTVmax (cm3) | ||||||
| <63.5 | 1 | 1 | ||||
| ≥63.5 | 4.716 | 1.722–12.916 | 0.003a | 6.683 | 1.924–23.209 | 0.003a |
| MTVsum (cm3) | ||||||
| <132.6 | 1 | 1 | ||||
| ≥132.6 | 2.267 | 0.946–5.434 | 0.067 | 3.564 | 1.265–10.043 | 0.016a |
| TLGmax (g) | ||||||
| <628.7 | 1 | 1 | ||||
| ≥628.7 | 4.716 | 1.722–12.916 | 0.003a | 6.433 | 1.852–22.350 | 0.003a |
| TLGsum (g) | ||||||
| <1135.9 | 1 | 1 | ||||
| ≥1135.9 | 2.076 | 0.868–4.968 | 0.101 | 3.267 | 1.159–9.211 | 0.025a |
| Dmax (cm) | ||||||
| <5.7 | 1 | 1 | ||||
| ≥5.7 | 4.716 | 1.722–12.916 | 0.003a | 6.895 | 1.982–23.984 | 0.002a |
| Interim treatment response | ||||||
| CR | 1 | 1 | ||||
| Non-CR | 4.642 | 1.699–11.685 | 0.003a | 6.496 | 1.855–21.176 | 0.002a |
Statistically significant. PFS, progression-free survival; OS, overall survival; RR, relative risk; CI, confidence interval; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; MTVmax, metabolic tumor volume of the maximum lesion; MTVsum, sum of metabolic tumor volume; TLGmax, total lesion glycolysis of the maximum lesion; TLGsum, sum of total lesion glycolysis; Dmax, maximum diameter of the maximum lesion; CR, complete remission.
Multivariate analysis
The statistical significant indicators in univariate analysis were included in the multivariate analysis. Due to the close relationship between MTVmax and TLGmax, MTVsum and TLGsum, only TLGmax and TLGsum were included in multivariate analysis.
Ann Arbor stage, Dmax and interim treatment response were considered as independent prognostic factors for 2-year PFS (P<0.05). Dmax and interim treatment response were shown to be independent prognostic factors for 2-year OS (P<0.05) (Table IV).
Table IV.
Multivariate analyses of clinical characteristics, baseline PET/CT parameters and interim treatment response for PFS and OS.
| 2-year PFS | 2-year OS | |||||
|---|---|---|---|---|---|---|
| Variables | RR | 95% CI | P-value | RR | 95% CI | P-value |
| Ann Arbor stage | 2.415 | 0.836–6.976 | 0.043 | – | ||
| Dmax | 2.854 | 0.946–8.609 | 0.036 | 4.016 | 1.103–14.629 | 0.035 |
| Interim treatment response | 11.437 | 3.594–36.397 | <0.001 | 7.619 | 2.092–27.742 | 0.002 |
PFS, progression-free survival; OS, overall survival; RR, relative risk; CI, confidence interval; Dmax, maximum diameter of the maximum lesion.
Survival curves
Kaplan-Meier survival curves showed that PFS and OS curves of the Dmax ≥5.7 cm group were shown to be significantly lower than that of the Dmax <5.7 cm group, respectively. The 2-year PFS rate of the Dmax <5.7 cm group and Dmax ≥5.7 cm group were 88.8 and 49.5%, respectively (P<0.001). The 2-year OS rate of the Dmax <5.7 cm group and Dmax ≥5.7 cm group were 89.5 and 51.1%, respectively (P<0.001) (Figs. 1 and 2).
Figure 1.
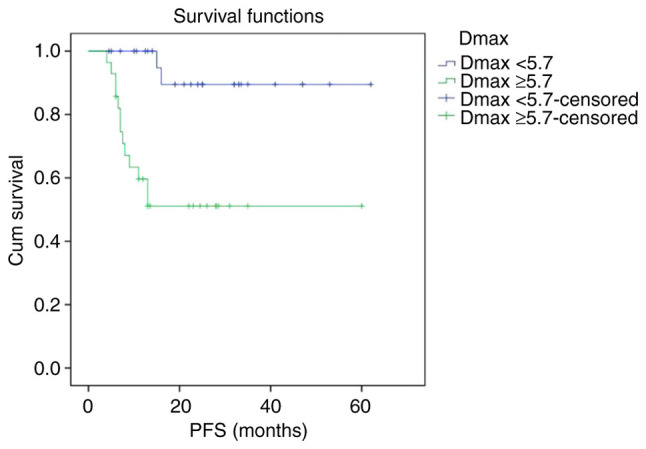
Kaplan-Meier survival analysis of PFS according to Dmax. PFS, progression-free survival; Dmax, maximum diameter of the maximum lesion.
Figure 2.
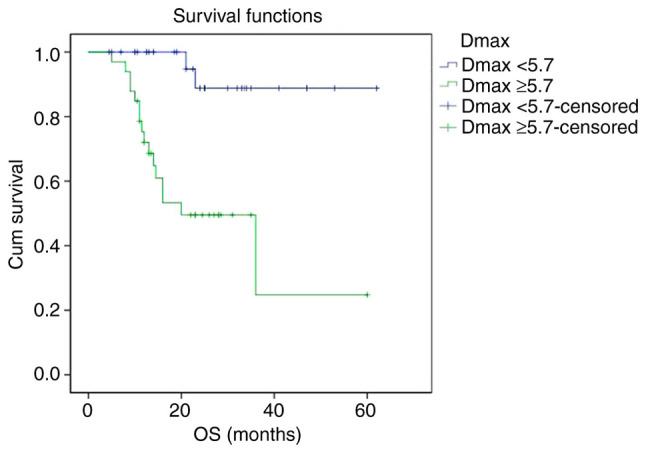
Kaplan-Meier survival analysis of OS according to Dmax. OS, overall survival; Dmax, maximum diameter of the maximum lesion.
The PFS and OS curves of the non-CR group were significantly lower than that of CR group, respectively. The 2-year PFS rate of the CR group and non-CR group were 87.6 and 23.7%, respectively (P<0.001). The 2-year OS rate of the CR group and non-CR group were 89.9 and 31.7%, respectively (P<0.001) (Figs. 3 and 4).
Figure 3.
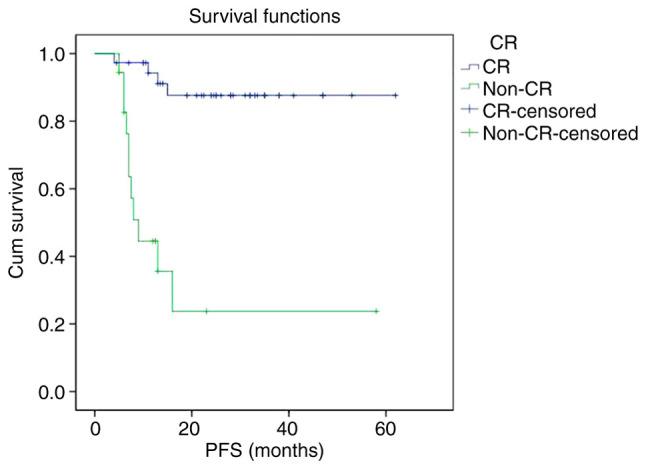
Kaplan-Meier survival analysis of PFS according to interim treatment response. PFS, progression-free survival; CR, complete response.
Figure 4.
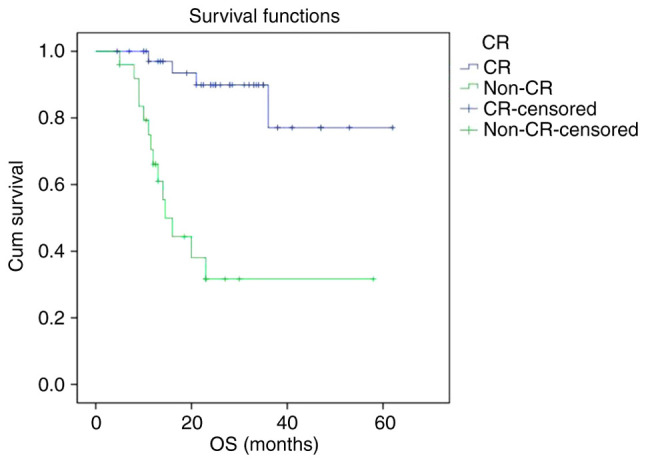
Kaplan-Meier survival analysis of OS according to interim treatment response. OS, overall survival; CR, complete response.
Prognostic value of the combination of two factors
The AUC, sensitivity and specificity of the combination of Dmax and interim treatment response for predicting the 2-year PFS were 0.801, 73.9 and 92.7%, respectively. Compared with single index Dmax, the predictive performance of the combination was found to be slightly improved, the specificity was significantly increased, while the sensitivity was slightly decreased (Fig. 5). The AUC, sensitivity and specificity of the combination of Dmax and interim treatment response for predicting the 2-year OS were found to be 0.689, 76.5 and 76.6%, respectively. Compared with single index Dmax, the predictive performance of the combination was decreased, the specificity was slightly improved, and the sensitivity was decreased (Fig. 6).
Figure 5.
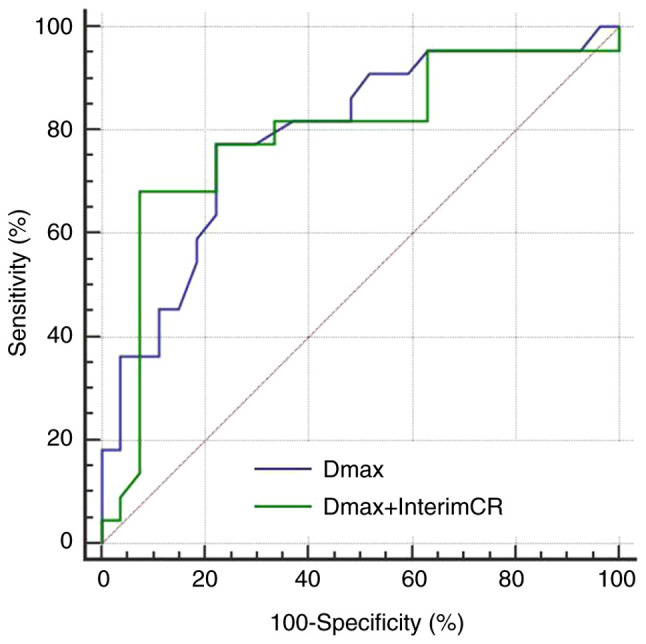
ROC curves of baseline Dmax and the combination of baseline Dmax and interim treatment response for prediction of 2-year PFS. ROC, receiver operating characteristic; Dmax, maximum diameter of the maximum lesion; PFS, progression-free survival; CR, complete response.
Figure 6.
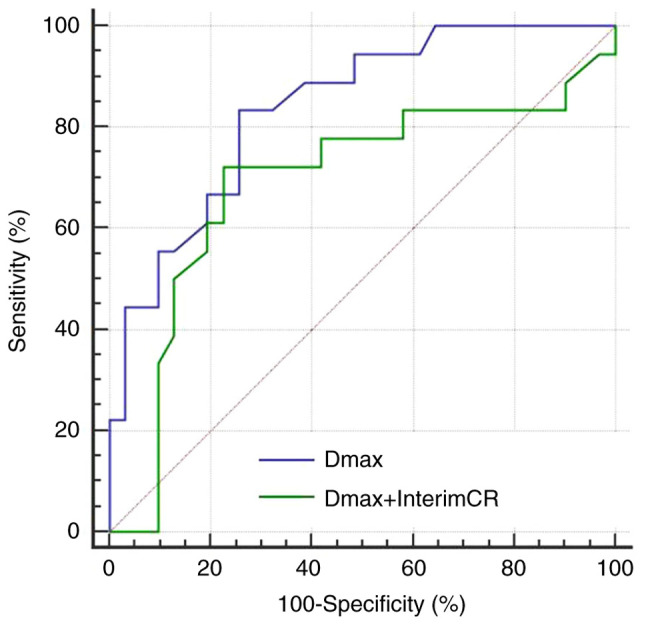
ROC curves of baseline Dmax and the combination of baseline Dmax and interim treatment response for prediction 2-year OS. ROC, receiver operating characteristic; Dmax, maximum diameter of the maximum lesion; CR, complete response; OS, overall survival.
Discussion
The prognostic factors associated with diffuse large B-cell lymphoma (DLBCL) patients receiving R-CHOP chemotherapy have been the main research focus of both domestic as well as foreign scholars. It is crucial to identify the patients who are at high-risk of relapse and to select proper treatment strategies for them. The International Prognostic Index (IPI), Revised R-IPI and an Enhanced International Prognostic Index (NCCN-IPI) are currently the internationally recognized prognostic indicators (14,15), and are widely used in risk stratification before treatment, but their prognostic value is challenged to some extent in the rituximab treatment era. The present study confirmed the correlation between IPI and 2-year progression-free survival (PFS) and overall survival (OS) in DLBCL patients receiving R-CHOP chemotherapy, while multivariate analysis showed that IPI is not an independent predictor. This is similar to the results obtained by Kwon et al (16). Clinical studies have also revealed that although IPI can accurately evaluate the prognosis in most of the patients with DLBCL, a part of patients with similar IPI score still have different rates of long-term survival (3,14). Therefore, individual characteristics and response to chemotherapy of each patient are regarded as the best indicators of prognosis.
Compared with IPI, baseline PET/CT parameters can assist in quantifying the invasion and burden of tumors of individuals, which may in turn be more advantageous in predicting the prognosis and guiding personalized treatment plans. Of all the baseline PET/CT parameters, Dmax was the only independent predictor of 2-year PFS and OS in this study. This result suggests that the tumor burden of the largest lesion acts as a more important prognostic factor than the gross tumor burden. This is similar to the result put forwarded by the previous clinical study by Parvez et al (17). This study further confirmed that patients with large masses usually have a poor prognosis. Compared with other PET/CT parameters such as MTV and TLG, Dmax can be easily obtained, and measurement of the largest lesion might be the simplest and most feasible method for predicting patient prognosis. In the present study, although MTV and TLG were not found to be independent predictors of 2-year PFS and OS, they were shown to be significantly associated with 2-year PFS and OS. This further confirmed the prognostic value of baseline PET/CT quantitative parameters. Kim et al (18) found TLG to be a better prognostic indicator than IPI in DLBCL patients. A study conducted by Esfahani et al (19) demonstrated that TLG of the baseline PET/CT is the only independent risk factor for PFS. Parvez et al (17) studied 82 patients with invasive B-cell lymphoma and found that MTV with SUV=3 or 6 as the threshold showed an association with OS. Song et al (20) also suggest that MTV is a prognostic factor for DLBCL. Although it is not completely consistent with the results of our study, all the findings discussed above indicate that baseline PET/CT quantitative parameters are valuable for prognostic prediction and can assist clinicians in identifying patients who are at high risk for recurrence before treatment initiation. Yet, a few scholars have come to a negative conclusion (21). Gallicchio et al (22) demonstrated that SUVmax is the most influential factor of event-free survival (EFS) in DLBCL patients, while MTV and TLG are not related with EFS. Adams et al (23) studied 73 DLBCL patients and found that MTV and TLG were not associated with disease prognosis. The main reason for this inconsistency might include distribution bias of the enrolled patients, different methods and standards of threshold selection for measurement and calculation of MTV and TLG, different chemotherapy regimens and different predictive cut-off time of survival. In the present study, relative threshold method of 40% SUVmax was adopted as the threshold to measure MTV. For patients with higher SUVmax, the absolute value of the threshold remained relatively high, which may in turn lead to underestimation of the actual tumor burden. Currently, there is no clear consensus as to which threshold selection method is the most appropriate and there are few literature data available on this (24). In this study, Dmax was found to act as an independent predictor of 2-year PFS and OS, while MTV and TLG did not. We speculated that this might be due to necrosis in the large masses in some patients. In these patients, no uptake of 18F-FDG was observed in necrosis, and MTV and TLG might underestimate the true tumor burden. In addition, there was no significant correlation between SUVmax, SUVmean and PFS, OS in our study, which is consistent with the results obtained by Manohar et al (25). This indicates that the tumor metabolism level is not the key factor that affects prognosis. According to the results of this study, DLBCL patients with high Dmax, MTV and TLG, even if the IPI score was low and might have poor survival prospects, intensive treatment was considered to improve their prognosis.
Previous literature has reported that interim treatment response to first-line chemotherapy acts as an important prognostic factor in DLBCL patients. Patients with poor interim treatment response and positive interim PET are more likely to have recurrence and progression, and the prognosis generally remains worsened (26–29). Poor interim treatment response is an indication for early clinical intervention, including salvage treatment, intensive treatment or autologous stem cell transplantation (30). The present study showed that the risk of recurrence, progression and death within 2 years in the non-CR patients were significantly higher than that in CR patients, and interim treatment response acted as an independent predictor of 2-year PFS and OS. This result is similar to that obtained by previous studies. Huntington et al (31) also believed that patients with interim negative PET/CT or those who reached interim CR had longer PFS and OS. Pregno et al (32) found that patients with interim negative PET generally had a better prognosis, while interim positive PET indicated no greater risk of recurrence.
Interim treatment response evaluation based on PET/CT is regarded as an important prognostic factor in DLBCL patients. The prognosis of patients who fail to respond to mid-term chemotherapy was found to remain poor, but there is no clear evidence that the prognostic value is better than IPI (33). In clinical practice, even patients with a good interim treatment response and interim negative PET may still have the potential to recur or progress to a later stage. Therefore, it is not sufficient to judge prognosis based solely on interim response to chemotherapy. In the present study, we combined the two risk factors, baseline PET/CT parameters and interim treatment response, in order to identify high-risk patients with poor prognosis, aiming to provide valuable information for early intervention. At present, there are few scholars who have evaluated the prognosis of DLBCL patients with the combination of baseline PET/CT metabolic parameters and interim treatment response, and relevant reports are rare and the indicators adopted are different (12,13,34). Mikhaeel et al (12) demonstrated improvement in the prognostic value of interim PET and screened out the population with poor prognosis by combining the baseline MTV and interim PET results. Zhang et al combined baseline TLG >1036.61 g and ΔSUVmax <86.02% to predict the recurrence or progression, showing good screening ability (13). Recently, Islam et al (8) found that baseline and interim PET/CT parameters of MTV show important predictive value for PFS, and could be helpful for guiding further treatment strategies in DLBCL patients. In the present study, baseline PET/CT parameter Dmax was screened through univariate and multivariate analyses. Compared with single indicator Dmax, the combination of Dmax and interim treatment response showed improved predictive efficiency for 2-year PFS, but showed no improvement in the predictive efficiency of 2-year OS.
The limitations of this study mainly include four aspects. Firstly, the sample size of this study is relatively small and the results of this study require external verification in the future. Secondly, we directly selected the median PET/CT parameters as the cutoff value for classification of patients in this study. Thus, the correlation of other cutoff points of variables with survival need to be discussed. Thirdly, DLBCL subgroup analysis was not performed in this study. The survival outcomes of DLBCL patients in different molecular subtypes and gene expression warrant further investigation. Finally, the follow-up time of some cases was relatively short. Most of the positive events such as recurrence, progression or death occur within 2 years after diagnosis. Therefore, this study only conducted univariate and multivariate analysis of 2-year PFS and OS. In future, follow-up of these patients will be continued and 3- or 5-year survival analysis will be conducted to further explore the prognostic value of baseline PET/CT parameters.
In conclusion, baseline 18F-FDG PET/CT parameters and interim treatment response have important prognostic value in DLBCL patients receiving R-CHOP chemotherapy. Combined application of Dmax and interim treatment response assists in improving the predictive efficacy of 2-year PFS. It may be helpful to identify patients who are at high risk of relapse and to guide early clinical intervention for these patients.
Supplementary Material
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors contributions
LZ and YM designed the study and drafted the manuscript. LG, HZ, YS and SL were responsible for the collection and analysis of the experimental data. AW, XZ, JS, JZ and KX revised the manuscript critically for important intellectual content evaluating literature data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Affiliated Hospital of Xuzhou Medical University (XYFY2016-KL002-01). Patient informed consent was waived due to the retrospective nature of the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Crombie JL, Armand P. Diffuse large B-cell lymphoma and high-grade B-cell lymphoma: Genetic classification and its implications for prognosis and treatment. Hematol Oncol Clin North Am. 2019;33:575–585. doi: 10.1016/j.hoc.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 2.International Non-Hodgkins Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkins lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 3.Vaidya R, Witzig TE. Prognostic factors for diffuse large B-cell lymphoma in the R(X)CHOP era. Ann Oncol. 2014;25:2124–2133. doi: 10.1093/annonc/mdu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampedro F, Domenech A, Escalera S. Obtaining quantitative global tumoral state indicators based on whole-body PET/CT scans: A breast cancer case study. Nucl Med Commun. 2014;35:362–371. doi: 10.1097/MNM.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 5.Park S, Moon SH, Park LC, Hwang DW, Ji JH, Maeng CH, Cho SH, Ahn HK, Lee JY, Kim SJ, et al. The impact of baseline and interim PET/CT parameters on clinical outcome in patients with diffuse large B cell lymphoma. Am J Hematol. 2012;87:937–940. doi: 10.1002/ajh.23267. [DOI] [PubMed] [Google Scholar]
- 6.Koff JL, Flowers CR. Prognostic modeling in diffuse large B-cell lymphoma in the era of immunochemotherapy: Where do we go from here? Cancer. 2017;123:3222–3225. doi: 10.1002/cncr.30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy A, Zhang J, Davis NS, Moffitt AB, Love CL, Waldrop A, Leppa S, Pasanen A, Meriranta L, Karjalainen-Lindsberg ML, et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell. 2017;171:481–494.e15. doi: 10.1016/j.cell.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islam P, Goldstein J, Flowers CR. PET-derived tumor metrics predict DLBCL response and progression-free survival. Leuk Lymphoma. 2019;60:1965–1971. doi: 10.1080/10428194.2018.1562181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottereau AS, Lanic H, Mareschal S, Meignan M, Vera P, Tilly H, Jardin F, Becker S. Molecular profile and FDG-PET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2016;22:3801–3809. doi: 10.1158/1078-0432.CCR-15-2825. [DOI] [PubMed] [Google Scholar]
- 10.Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32:3048–3058. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance, Australasian Leukaemia and Lymphoma Group and Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium et al. Recommendations for initial evaluation, staging, and response assessment Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikhaeel NG, Smith D, Dunn JT, Phillips M, Møller H, Fields PA, Wrench D, Barrington SF. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging. 2016;43:1209–1219. doi: 10.1007/s00259-016-3315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang YY, Song L, Zhao MX, Hu K. A better prediction of progression-free survival in diffuse large B-cell lymphoma by a prognostic model consisting of baseline TLG and %ΔSUVmax. Cancer Med. 2019;5:5137–5147. doi: 10.1002/cam4.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J, et al. The revised international prognostic index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA, Rodriguez MA, et al. An enhanced international prognostic index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837–842. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon SH, Kang DR, Kim J, Yoon JK, Lee SJ, Jeong SH, Lee HW, An YS. Prognostic value of negative interim 2-[18F]-fluoro-2-deoxy-d-glucose PET/CT in diffuse large B-cell lymphoma. Clin Radiol. 2016;71:280–286. doi: 10.1016/j.crad.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Parvez A, Tau N, Hussey D, Maganti M, Metser U. 18F-FDG PET/CT metabolic tumor parameters and radiomics features in aggressive non-Hodgkins lymphoma as predictors of treatment outcome and survival. Ann Nucl Med. 2018;32:410–416. doi: 10.1007/s12149-018-1271-y. [DOI] [PubMed] [Google Scholar]
- 18.Kim TM, Paeng JC, Chun IK, Keam B, Jeon YK, Lee SH, Kim DW, Lee DS, Kim CW, Chung JK, et al. Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the international prognostic index for patients with diffuse large B cell lymphoma. Cancer. 2013;119:1195–1202. doi: 10.1002/cncr.27855. [DOI] [PubMed] [Google Scholar]
- 19.Esfahani SA, Heidari P, Halpern EF, Hochberg EP, Palmer EL, Mahmood U. Baseline total lesion glycolysis measured with 18F-FDG PET/CT as a predictor of progression-free survival in diffuse large B-cell lymphoma: A pilot study. Am J Nucl Med Mol Imaging. 2013;3:272–281. [PMC free article] [PubMed] [Google Scholar]
- 20.Song MK, Chung JS, Shin HJ, Lee SM, Lee SE, Lee HS, Lee GW, Kim SJ, Lee SM, Chung DS. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol. 2012;91:697–703. doi: 10.1007/s00277-011-1357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatsumi M, Isohashi K, Matsunaga K, Watabe T, Kato H, Kanakura Y, Hatazawa J. Volumetric and texture analysis on FDG PET in evaluating and predicting treatment response and recurrence after chemotherapy in follicular lymphoma. Int J Clin Oncol. 2019;24:1292–1300. doi: 10.1007/s10147-019-01482-2. [DOI] [PubMed] [Google Scholar]
- 22.Gallicchio R, Mansueto G, Simeon V, Nardelli A, Guariglia R, Capacchione D, Soscia E, Pedicini P, Gattozzi D, Musto P, Storto G. F-18 FDG PET/CT quantization parameters as predictors of outcome in patients with diffuse large B-cell lymphoma. Eur J Haematol. 2014;92:382–389. doi: 10.1111/ejh.12268. [DOI] [PubMed] [Google Scholar]
- 23.Adams HJ, de Klerk JM, Fijnheer R, Heggelman BG, Dubois SV, Nievelstein RA, Kwee TC. Prognostic superiority of the national comprehensive cancer network international prognostic index over pretreatment whole-body volumetric-metabolic FDG PET/CT metrics in diffuse large B-cell lymphoma. Eur J Haematol. 2015;94:532–539. doi: 10.1111/ejh.12467. [DOI] [PubMed] [Google Scholar]
- 24.Xie M, Zhai W, Cheng S, Zhang H, Xie Y, He W. Predictive value of F-18 FDG PET/CT quantization parameters for progression-free survival in patients with diffuse large B-cell lymphoma. Hematology. 2016;21:99–105. doi: 10.1179/1607845415Y.0000000033. [DOI] [PubMed] [Google Scholar]
- 25.Manohar K, Mittal BR, Bhattacharya A, Malhotra P, Varma S. Prognostic value of quantitative parameters derived on initial staging 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with high-grade non-Hodgkins lymphoma. Nucl Med Commun. 2012;33:974–981. doi: 10.1097/MNM.0b013e32835673ec. [DOI] [PubMed] [Google Scholar]
- 26.Fuertes S, Setoain X, Lopez-Guillermo A, Carrasco JL, Rodríguez S, Rovira J, Pons F. Interim FDG PET/CT as a prognostic factor in diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2013;40:496–504. doi: 10.1007/s00259-012-2320-8. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Pertovaara H, Korkola P, Vornanen M, Järvenpää R, Dastidar P, Eskola H, Kellokumpu-Lehtinen PL. Early interim PET/CT predicts post-treatment response in diffuse large B-cell lymphoma. Acta Oncol. 2014;53:1093–1099. doi: 10.3109/0284186X.2014.927074. [DOI] [PubMed] [Google Scholar]
- 28.Oñate-Ocaña LF, Cortés V, Castillo-Llanos R, Terrazas A, Garcia-Perez O, Pitalúa-Cortes Q, Ponce M, Dueñas-Gonzalez A, Candelaria M. Metabolic tumor volume changes assessed by interval 18fluorodeoxyglucose positron emission tomography-computed tomography for the prediction of complete response and survival in patients with diffuse large B-cell lymphoma. Oncol Lett. 2018;16:1411–1418. doi: 10.3892/ol.2018.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tateishi U, Tatsumi M, Terauchi T, Ando K, Niitsu N, Kim WS, Suh C, Ogura M, Tobinai K. Prognostic significance of metabolic tumor burden by positron emission tomography/computed tomography in patients with relapsed/refractory diffuse large B-cell lymphoma. Cancer Sci. 2015;106:186–193. doi: 10.1111/cas.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrich AM, Gandhi M, Jovanovic B, Castillo JJ, Rajguru S, Yang DT, Shah KA, Whyman JD, Lansigan F, Hernandez-Ilizaliturri FJ, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: A multicenter retrospective analysis. Blood. 2014;124:2354–2361. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- 31.Huntington SF, Nasta SD, Schuster SJ, Doshi JA, Svoboda J. Utility of interim and end-of-treatment [18F]-fluorodeoxyglucose positron emission tomography-computed tomography in front line therapy of patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2015;56:2579–2584. doi: 10.3109/10428194.2015.1007506. [DOI] [PubMed] [Google Scholar]
- 32.Pregno P, Chiappela A, Bellò M, Botto B, Ferrero S, Franceschetti S, Giunta F, Ladetto M, Limerutti G, Menga M, et al. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood. 2012;119:2066–2073. doi: 10.1182/blood-2011-06-359943. [DOI] [PubMed] [Google Scholar]
- 33.Adams HJ, Kwee TC. Prognostic value of interim FDG-PET in R-CHOP-treated diffuse large B-cell lymphoma: Systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;106:55–63. doi: 10.1016/j.critrevonc.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Jiang SY, Qin Y, Liu P, Yang J, Yang S, He X, Zhou S, Gui L, Zhang C, Zhou L, et al. A prognostic nomogram constructed for relapsed or refractory diffuse large B-cell lymphoma patients. https://doi.org/10.1111/ajco.13222. Asia Pac J Clin Oncol. 2019 Jul 1; doi: 10.1111/ajco.13222. (Online ahead of print) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


