Abstract
Hepatocellular carcinoma (HCC) is one of the most common types of primary liver cancer. Despite advancements in the treatment strategies of HCC, there is an urgent requirement to identify and develop novel therapeutic drugs that do not lead to resistance. These novel agents should have the potential to influence the primary mechanisms participating in the pathogenesis of HCC. Heparan sulfate proteoglycans (HSPGs) are major elements of the extracellular matrix that perform structural and signaling functions. HSPGs protect against invasion of tumor cells by preventing cell infiltration and intercellular adhesion. Several enzymes, such as heparanase, matrix metalloproteinase-9 and sulfatase-2, have been reported to affect HSPGs, leading to their degradation and thus enhancing tumor invasion. In addition, some compounds that are produced from the degradation of HSPGs, including glypican-3 and syndecan-1, enhance tumor progression. Thus, the identification of enzymes that affect HSPGs or their degradation products in HCC may lead to the development of novel therapeutic targets. The present review discusses the main enzymes and compounds associated with HSPGs, and their involvement with the pathogenicity of HCC.
Keywords: heparanase, matrix metalloproteinase-9, sulfatase-2, glypican-3, syndecan-1, fascin
1. Introduction
Hepatocellular carcinoma (HCC) accounts for 85–90% of primary liver cancer cases. HCC is an aggressive cancer, which has a marked clinical and epidemiological impact, with 600,000 mortalities and ~1,000,000 new cases of HCC being reported annually, worldwide (1,2). Following a diagnosis of HCC, patients survive <1 year. In addition, the mortality rates are high, with an overall survival rate of <12% (3). HCC arises from the aggregation of normal cells following accumulation of several genetic changes that activates oncogenes and deactivates tumor suppressor genes, nuclear factors, growth factors and cytokines (4). The functions of the liver, as well as the liver reserve, are altered and damaged during the course of disease. Despite advancements in the treatment strategies of HCC, the prognosis of patients remains poor due to metastasis and development of drug resistance. Currently, hepatic resection or transplantation are considered the only curative therapies (5). Even with surgery, ~30% of patients with HCC undergo hepatectomy as they receive a diagnosis at an advanced tumor stage. Furthermore, radiofrequency ablation is used to treat patients with early-stage HCC. Several factors lead to the poor prognosis of patients with HCC, including history of cirrhosis, poverty and limited medical resources. The rapid onset and fast-growing characteristics of HCC results in limited treatment options for patients. In addition, HCC is characterized by a high angioinvasive capacity due to portal vein obstruction (6). Thus, there is an urgent requirement to identify and develop novel therapeutic drugs for the treatment of HCC.
2. Heparan sulfate proteoglycans (HSPGs)
Structure
HSPGs are highly anionic carbohydrate compounds. HSPGs are composed of a limited quantity of a core protein that is covalently linked to ≥1 sugar chain, representing HS side chains. These chains are considered linear polysaccharides, which are built of ≤200 units of a repeated disaccharide formed by N-acetyl glucosamine with uronic acid, glucuronic or iduronic acids (7).
The structure of HSPGs is modified in the Golgi apparatus following the addition of sulfate groups instead of acetyl groups, or by sulfation of the hydroxyl groups at C-6 and C-3 in the N-acetylglucosamine moiety, or by sulfation of the hydroxyl group at C-2 in the uronic acid moiety (8). There are several families of cell surface HSPGs, such as syndecan and glypican. The presence of several carboxyl and sulfate groups in HS is similar to the polyanionic nature of mammalian cells compared with the neighboring cells and extracellular matrix (ECM) (9). Thus, cell surface HSPGs produce multiple structural and signaling functions due to their capability to interact with several protein ligands, including growth factors and their receptors, proteases, cytokines, chemokines, adhesion molecules and ECM proteins, including fibronectin, collagen and fibrin (10).
Function
HSPGs are one of the most important elements of the ECM, and are located on the surface of the cell membrane of most animal cells, such as hepatocytes and leukocytes (11). HSPGs are involved in several interactions between adjacent cells or between cells and the ECM. HSPGs regulate several signaling pathways and receptor trafficking, and control ligand secretion. The variability of HS generated by its modifying enzymes led to the hypothesis of ‘sugar code’, which is characterized by specific HS alterations observed in the embryo to orchestrate development through modification of certain signaling pathways. It depends on the regulation of special areas of HS-modifying enzymes to regulate their activity or even change their functions. The sugar code is considered a dynamic process as HS chains can be hydrolyzed by heparanase or sulfatase enzymes (12). HSPGs participate in an extensive range of biological processes, such as development (13), homoeostasis control (11) and enhancement of inflammatory and malignant diseases (14). In addition, HSPGs control cell adhesion, motility, proliferation, differentiation and apoptosis (8).
HSPGs and cancer
HSPGs act as anchors for the lipoprotein lipase located on the outer surface of capillary endothelial cells. They protect against invasion of tumor cells by preventing both cell infiltration and intercellular adhesion (15). The activities of HSPG-degrading enzymes are elevated in highly invasive cancer cells compared with less invasive cells (16). When the basal membrane is ruptured by hematogenous metastatic cancer cells, HSPGs located inside the tumor microenvironment are attacked by several enzymes, such as heparanase, matrix metalloproteinase-9, sulfatase-2, which are capable of modifying the proteoglycan structure, which alters transportation of inflammatory cells from vessels into the surrounding tissues (17). Consequently, cytokines, proteases, growth factors and angiogenic factors, which bind to HSPGs, are released and promote the infiltration and metastasis of cancer cells (15).
3. Enzymes hydrolyzing HSPGs
Matrix metalloproteinase (MMP)-9
MMPs constitute a family of transmembrane zinc-dependent endopeptidases that have the ability to digest the ECM and basement membrane. The MMP family consists of 25 members in vertebrates and 22 in humans (18). Previously, MMP-9 was called type IV collagenase or gelatinase B. MMP-9 is capable of degrading type IV collagen, a major constituent of the basement membrane (19,20). The active zone of MMP-9 consists of two zinc ions and five calcium ions. The proteolytic activity of MMP-9 is maintained by the two zinc ions and cysteine switch motif of the pro-domain (21). MMP-9 also contains a fibronectin-like domain, which is strongly O-glycosylated and is important for binding to collagen or gelatin (18).
MMPs play an important role in proliferation, invasion and metastasis of tumor cells (22). Deryugina and Quigley (23) demonstrated an association between ECM degradation by MMPs and the invasion of cancer cells. MMP-9 releases fibroblast growth factor (FGF)-1 and FGF-2 from their stores, producing potent angiogenic effects (24). In addition, MMP-9 attacks HSPGs inside the tumor microenvironment to enhance the proteolytic release of syndecan-1 and to potentiate tumor growth and metastasis (17,25). Thus, tumors that express MMP-9 at high levels are more likely to exhibit relapse or metastasis compared with tumors that express low levels of MMP-9.
Previous studies have demonstrated the role of certain MMP-9 inhibitors in the treatment of HCC both in vivo and in vitro (Table I). Although, several synthetic MMP inhibitors have been developed, none of them have reached phase III clinical trials due to either lack of efficacy or serious side effects.
Table I.
Summary of studies that assessed MMP-9 inhibitors in the treatment of HCC.
| Model | Summary | Cell type | (Refs.) |
|---|---|---|---|
| Human | Diosmetin downregulates MMP-9, leading to the inhibition of migration and invasion of HCC cells | SK-HEP-1 | (69) |
| Niclosamide downregulates MMP-9, leading to the suppression of HCC cell migration | HLF and PLC/PRF/5 | (70) | |
| Retinoic acid-induced protein I inhibits the migration and invasion of HCC cells by decreasing MMP-9 levels | Liver cells | (71) | |
| MicroRNA-133a targets MMP-9, and prevents HCC cell proliferation, migration and invasion | HepG2 and SMMC-7721 | (72) | |
| The antibiotic salinomycin blocks the invasion and migration of HCC cells through JNK/JunD-mediated MMP-9 expression | HCCLM3 | (73) | |
| Glabridin inhibits the invasion of human HCC cells, and may act as a chemopreventive agent against liver cancer metastasis | Huh7 and Sk-Hep-1 | (74) | |
| Mice | Tanshinone II-A prevents the invasion and metastasis of HCC cells by blocking the activity of MMP-9 | Liver cells | (75) |
| Rat | Epigallocatechin-gallate blocks MMP-9 activity, leading to hepatoprotective and chemoprotective effects | Liver cells | (76) |
| Doxycycline produces hepatoprotective and antitumor activity by blocking MMP-9 | Liver cells | (77) |
MMP, matrix metalloproteinase; HCC, hepatocellular carcinoma.
Heparanase
Heparanase is an endo-β-glucuronidase that belongs to the glycoside hydrolase 79 family. Heparanase hydrolyses HS at specific intrachain positions with low sulfation and participates in the degradation and remodeling of the ECM (26).
Heparanase is upregulated in several types of human tumor, such as HCC, myeloma and breast cancer, and it strongly enhances the invasiveness of tumors in experimental animals (27). Heparanase releases HS fragments associated with angiogenic factors from the tumor microenvironment to produce an angiogenic response. In addition, heparanase facilitates vascularization, accelerates primary tumor growth and provides a gate for invading metastatic cells, thus leading to cancer progression (28).
Heparanase inhibitors notably decrease the incidence of metastasis in experimental animals (29). Suramin was subsequently assessed in rats with HCC, where it was demonstrated to elevate the percentage of survival rate of rats with HCC, and decrease the level of serum α-fetoprotein. Furthermore, suramin has been demonstrated to ameliorate fibrosis, thus producing an hepatoprotective effect (15). Table II summarizes several studies that assessed heparanase inhibitors in the treatment of HCC.
Table II.
Summary of studies that used heparanase inhibitors for the treatment of HCC.
| Model | Summary | Cell type | (Refs.) |
|---|---|---|---|
| Human | Downregulation of heparanase enhances the suppression of invasion, migration and adhesion in HCC | HepG2, BEL-7402, and HCCLM3 | (77,78) |
| Administering 160 mg/day PI-88 produces significant clinical advantages for patients with HCC | Liver cells | (79,80) | |
| Rats | Suramin exerts antitumor activity in HCC through the deactivation of heparanase | Liver cells | (15) |
HCC, hepatocellular carcinoma.
Sulfatase-2
Sulfatase-2 is an extracellular enzyme that enhances the removal of 6-O-sulfate from HS disaccharides, and controls the interactions between HSPGs and extracellular factors. Sulfatase-1 and −2 are expressed in malignant tumors, including highly invasive brain cancer (30). Sulfatase-1 acts as a tumor suppressor gene, which downregulates the phosphorylation and activation of tyrosine kinase receptors (31). Conversely, sulfatase-2 decreases the affinity of HSPGs for several signaling molecules, such as glypican-3 and syndean-1, detaching them from HSPGs and preparing the transition of different signaling pathways, particularly the insulin-like growth factor (IGF) pathway (32).
Upregulation of sulfatase-2 is considered oncogenic, and is associated with HCC in human, animal and tissue culture models (33,34). Sulfatase-2 enhances the expression of growth factors available to cell surface receptors, thus promoting the proliferation and migration of tumor cells. In addition, it enhances the activity of glypican-3, activates FGF signaling, potentiates the phosphorylation of both Erk and Akt, and induces Wnt/β-catenin signaling (35).
Some studies have focused on the use of sulfatase-2 inhibitors for treating HCC. Adiponectin, a suppressor of the synthesis of sulfatase-2 protein, has been reported to exhibit antitumor activity both in vivo and in vitro (35). In addition, OKN-007, an inhibitor of sulfatase-2, significantly decreases solid tumor growth (36). Table III summarizes the results of previous studies that used sulfatase-2 inhibitors for the treatment of HCC.
Table III.
Summary of studies that assessed sulfatase-2 inhibitors in the treatment of HCC.
| Model | Summary | Cell type | (Refs.) |
|---|---|---|---|
| Human | 2,4-Disulfonylphenyl-tert-butylnitrone (OKN-007) produces antitumor effects against HCC by suppressing TGF-β1/SMAD2 and Hedgehog/GLI1 signaling | Huh7 | (36) |
| Rats | Adiponectin inhibits sulfatase-2 activity, leading to hepatoprotective and chemoprotective effects | Liver cells | (35) |
| Sodium ascorbate produces cytotoxic effects against HCC, which can be explained by the inhibition of sulfatase-2 | Liver cells | (81) | |
| Mice | Silencing sulfatase-2 signaling inhibits angiogenesis and tumor growth by inhibiting TGF-β1/SMAD | Liver cells | (82) |
HCC, hepatocellular carcinoma.
4. Important HSPGs products
Syndecan-1
Syndecan-1 is a transmembrane HSPG that is located on epithelial cells. The syndecan family consists of four members, syndecan-1, syndecan-2, syndecan-3 and syndecan-4. Among these four members, syndecan-1 has been extensively studied. Its name is derived from the Latin syndein, which means binding together, since syndecans are involved in the binding of cells to the ECM (37). Syndecans are composed of three domains forming highly conserved intracellular and transmembrane domains, as well as an extracellular domain, which is uniquely characteristic to each member (38).
Syndecan-1 controls cell-cell and cell-ECM adhesion interactions, as well as their activities through its HS chains. It modulates certain proteolytic enzymes and chemokines in vivo, and controls the recruitment of leukocytes and the remodeling of tissues during inflammation (39). In addition, syndecan-1 modulates proteolytic v, thus leading to the regulation of leucocyte recruitment with subsequent remodeling of tissues (40).
The release of syndecan-1 from its membrane-bound form by MMP-9 (syndecan-1 sheddase) to the soluble molecule inside the circulation represents the transition of the tumor from a proliferative stage to an invasive stage (25). Syndecan-1 binds to both the ECM and FGF family. Overexpression of the MMP-9/syndecan-1/FGF-2 axis potentiates the apoptosis pathway in several tumor models (41,42).
A previous study demonstrated the role of inhibiting syndecan-1 by synstatin, which exhibits promising antitumor activity against rats with HCC (43).
Glypican-3
Glypican-3 is the most commonly studied member of the glypican family of glycosyl-phosphatidylinositol-(GPI) cell-surface HSPGs (44). It consists of six medium-sized HSPGs that are attached to the cell surface via a GPI anchor, with an insertion of 2–4 HS chains. Glypican-3 regulates Wnt, Hedgehog and FGF signaling (38).
Glypican-3 is upregulated in HCC (34); thus, serum glypican-3 may be a promising potential selective marker for HCC (45). Glypican-3 regulates multiple tumor activities through Wnt signaling modulation (46). Glypican-3 enhances both in vitro and in vivo HCC growth, and interacts with growth factors, such as IGF-II and its receptor leading to activation of its signaling pathway (47).
Glypican-3 is considered an attractive therapeutic target in HCC. Antibodies against glypican-3 exhibit strong antitumor activities in several models of HCC (33,34). Recently, several mouse monoclonal antibodies targeting glypican-3 have been produced (48). One of these antibodies is the humanized GC33 (hGC33), which has been assessed in a phase I clinical trial. hGC33 acts against the carboxyl-terminal region of glypican-3 and is effective in HepG2 ×enografts (49). In addition, another human heavy chain variable domain antibody, NH3, inhibits the proliferation of glypican-3-positive cells and blocks HCC xenograft growth in nude mice by modulating the TGF-β/SMAD pathway (50). Zaghloul et al (34) demonstrated that treatment of rats with HCC with monoclonal anti-glypican-3 increased survival rate up to 90% and decreased the level of serum AFP. In addition, anti-glypican-3 was demonstrated to affect the sulafatase-2/IFG-II pathway. Glypican-3 has also been reported to act as a predictive marker of HCC recurrence following radial surgery (51). Table IV represents a summary of studies that have assessed the role of glypican-3 inhibitors in treating HCC.
Table IV.
Summary of studies that assessed glypican-3 inhibitors in the treatment of HCC.
| Model | Summary | Cell type | (Refs.) |
|---|---|---|---|
| Human | Human monoclonal antibody targeting glypican-3 prevents the migration and motility of HCC | Hep3B and HepG2 | (83) |
| Glypican-3-targeted chimeric antigen receptor T cell provides a promising therapeutic target for glypican-3-positive HCC | HepG2, Hep3B, PLC/PRF/5 and SK-Hep-1 | (84) | |
| Silencing the glypican-3 gene protects against HCC | HepG2 | (85–87) | |
| Interfering glypican-3 gene transcription blocks HCC cell apoptosis and prevents metastasis via the Wnt/β-catenin signaling pathways | MHCC-97H and Huh7 | (88) | |
| hGC33 protects patients with HCC | Liver cells | (89–91) | |
| By targeting glypican-3, microRNA-219-5p exerts antitumor effects in HCC | Liver cells | (92) | |
| Rat | Anti-glypican-3 antibody protects against HCC | RH7777 | (93) |
| Anti-glypican-3 antibody exerts antitumor and hepatoprotective effects against HCC | Liver cells | (34) | |
| Mice | Targeted photoimmunotherapy for glypican-3 combined with nanoparticle albumin-bound paclitaxel is a promising method for treating HCC | Liver cells | (94) |
| Glypican-3 cDNA vaccine by using a recombinant plasmid encoding murine glypican-3 cDNA for treatment of HCC produces specific and effective antitumor immunity against HCC | Liver cells | (95) |
HCC, hepatocellular carcinoma.
Fascin
Fascin is an actin-binding protein that controls cell movement under physiological or pathological conditions (52). It regulates cell motility and is considered one of the cytoskeleton-regulatory proteins (53).
Fascin expression has been associated with tumor invasion and metastasis, and its expression is low in normal tissues (52). Overexpression of fascin elevates cell membrane processes, such as broken intercellular junctions, and enforces cell movement associated with changes to the cytoskeleton and ECM, thus facilitating tumor metastasis (54). It has been reported that upregulation of fascin in several tumors, including HCC, is associated with tumor invasion and metastasis (55). In addition, fascin is unable to control cell migration alone, unless it is supported by other factors, such as MMP-9. This can be explained by the fact that the ability of fascin to act as a migration factor is only associated with epithelial-to-mesenchymal transition or MMP-9, which facilitate their invasiveness (56).
5. Products of ECM
Glucosaminoglycans
Glucosaminoglycans are linear polysaccharides composed of repeat units, with areas of glucuronic acid and N-acetyl glucosamine. They contain regions of 2-O-sulfated iduronic acid and N-sulfoglucosamine. Between these regions, there are transition zones with both sulfo-glucosamine and acetyl-glucosamine, which are associated with polypeptide core-forming HSPGs (57).
Glucosamine (2-amino-2-deoxy-α-D-glucose)
Glucosamine is an amino saccharide that is present in almost all tissues, and abundant in liver, kidney and cartilage (58). It is the predominant building unit in the synthesis of glycolipids, glycoproteins, glycosaminoglycans and proteoglycans (59). Glucosamine induces autocrine TGF-β activity (60) and helps in the O-linked glycosylation of proteins. As an alteration of the structure of proteins with O-linked N-acetylglucosamine, glucosamine has evolved as an important regulator of cellular physiology. This alteration is associated with several diseases, such as cancer, neurodegenerative disorders and cardiovascular diseases (61). Notable elevation in the serum levels of glucosamine has been observed in patients and animals with HCC (15,62,63).
Glucuronic acid
Glucuronic acid is synthesized from UDP-glucose inside the liver via UDP-glucosedehydrogenase. It participates in several detoxification pathways, such as xenobiotic and bilirubin (64). Elevated levels of hyaluronic acid in liver diseases are the main cause for increased levels of serum glucosamine and glucuronic acid (65). Degradation of hyaluronic acid, which is initiated by its binding to CD44, notably enhances the activation of cell migration molecules, thus leading to tumor motility (66).
Sialic acid (N-acetyl neuraminic acid)
Sialic acid is part of the plasma membrane of mammalian cells. It binds to N-acetyl galactosamine via an O-glycosidic linkage, which is associated with the proteins that form glycoproteins (58). Sialic acid is a major player in several physiological and pathological processes, such as progression and spread of multiple malignancies, such as neuroblastoma, oral cancer and breast cancer (67). A variation in the sialic acid levels in patients with cirrhosis and HCC is an important diagnostic tool. Elevated sialic acid levels in HCC may be explained by endothelial cell dysfunction or macrovascular disease (68).
6. Conclusions and future directions
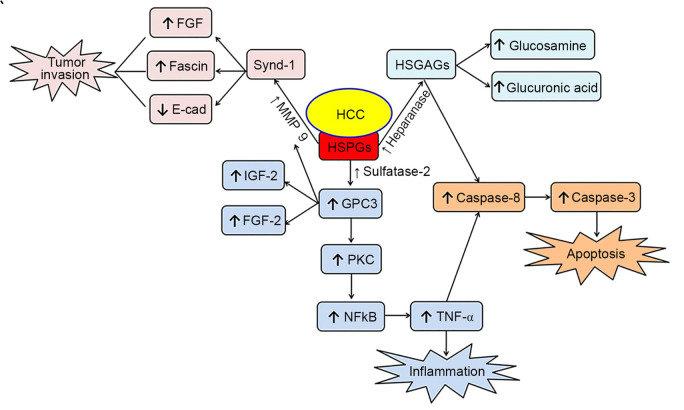
HCC triggers metabolic and dynamic modifications that lead to the activation of certain enzymes, such as MMP-9, sulfatase-2 and heparanase, resulting in the degradation of HSPGs. Increasing evidence suggests that some of the HSPG degradation products, such as syndecan-1 and glypican-3, are associated with the activation, migration and apoptosis of tumor cells (Fig. 1). Thus, an improved understanding of the role of HSPGs and their degradation products will aid the identification of novel effective therapeutic targets and strategies for preventing and treating HCC.
Figure 1.
HSPGs pathway changes in HCC. HCC increases the ability of the enzymes, MMP-9, sulfatase-2 and heparanase to attack HPSGs, leading to the formation of several intermediate compounds that enhance inflammation and tumor invasion. HSPGs, heparan sulfate proteoglycans; HCC, hepatocellular carcinoma; MMP, matrix metalloproteinase; FGF, fibroblast growth factor; E-cad, E-cadherin; Synd-1, syndecan-1; IGF, insulin-like growth factor; GPC3, glypcian-3; PKC, protein kinase C; NFκB, nuclear factor κB; TNF-α, tumor necrosis factor-α.
Cancer treatment has shifted from single target treatment to multiple target therapies. HSPGs represent a goal for a new trend in multiple target therapies, since they comprise several enzymes and important compounds located in the tumor microenvironment that control multiple biological and pathological processes. Prospective studies will focus on the specific post-translational modifications of these compounds in the HSPG pathway, along with further assessment of the inhibitors and modulators of cell signaling.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data and materials are included in the present review.
Authors' contributions
MAA and NNA contributed to the study design. MMA and MAAG acquired the data. MMHAG contributed to the study concept and design. All authors helped draft the initial manuscript, and read and approved the final version.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Balkan E, Bilici M, Gundogdu B, Aksungur N, Kara A, Yasar E, Dogan H, Ozturk G. ERCC2 Lys751Gln rs13181 and XRCC2 Arg188His rs3218536 gene polymorphisms contribute to subsceptibility of colon, gastric, HCC, lung and prostate cancer. J BUON. 2020;25:574–581. [PubMed] [Google Scholar]
- 2.Lv Y, Xu A, Wang N, Mu K, Wang Z, Zhao L, Huang Y, Peng L, Xiang K, Hu D, Qi J. Retrospective study of TACE in the treatment of lobaplatin-induced thrombocytopenia in primary hepatocellular carcinoma. J BUON. 2019;24:2385–2393. [PubMed] [Google Scholar]
- 3.Nazmy EA, El-Khouly OA, Zaki MMA, MM A, Elsherbiny NM, Said E, Al-Gayyar MMH, Salem HA. Targeting p53/TRAIL/caspase-8 signaling by adiponectin reverses thioacetamide-induced hepatocellular carcinoma in rats. Environ Toxicol Pharmacol. 2019;72:103240. doi: 10.1016/j.etap.2019.103240. [DOI] [PubMed] [Google Scholar]
- 4.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): The role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, Zhang J, Ni X. Diagnsosis, treatment and prognosis of hepatocellualr carcinoma with inferior vena cava/right atrium tumor thrombus. Oncol Lett. 2020;20:101. doi: 10.3892/ol.2020.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng Z, Yang B, Liao ZY. Current progress and prospect of immune checkpoint inhipitros in hepatocellualr carcinoma. Oncol Lett. 2020;2:45. doi: 10.3892/ol.2020.11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iozzo RV. Heparan sulfate proteoglycans: Intricate molecules with intriguing functions. J Clin Invest. 2001;108:165–167. doi: 10.1172/JCI200113560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar AV, Katakam SK, Urbanowitz AK, Gotte M. Heparan sulphate as a regulator of leukocyte recruitment in inflammation. Curr Protein Pept Sci. 2015;16:77–86. doi: 10.2174/1573402111666150213165054. [DOI] [PubMed] [Google Scholar]
- 9.Christianson HC, Belting M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014;35:51–55. doi: 10.1016/j.matbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Rosen SD, Lemjabbar-Alaoui H. Sulf-2: An extracellular modulator of cell signaling and a cancer target candidate. Expert Opin Ther Targets. 2010;14:935–949. doi: 10.1517/14728222.2010.504718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 12.Poulain FE, Yost HJ. Heparan sulfate proteoglycans: A sugar code for vertebrate development? Development. 2015;142:3456–3467. doi: 10.1242/dev.098178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: The sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 14.Lindahl U, Kjellén L. Pathophysiology of heparan sulphate: Many diseases, few drugs. J Intern Med. 2013;273:555–571. doi: 10.1111/joim.12061. [DOI] [PubMed] [Google Scholar]
- 15.Tayel A, Abd El Galil KH, Ebrahim MA, Ibrahim AS, El-Gayar AM, Al-Gayyar MM. Suramin inhibits hepatic tissue damage in hepatocellular carcinoma through deactivation of heparanase enzyme. Eur J Pharmacol. 2014;728:151–160. doi: 10.1016/j.ejphar.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Toyoshima M, Nakajima M. Human heparanase. Purification, characterization, cloning, and expression. J Biol Chem. 1999;274:24153–24160. doi: 10.1074/jbc.274.34.24153. [DOI] [PubMed] [Google Scholar]
- 17.Dong S, Wu XZ. Heparanase and hepatocellular carcinoma: Promoter or inhibitor? World J Gastroenterol. 2010;16:306–311. doi: 10.3748/wjg.v16.i3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology (Bethesda) 2013;28:391–403. doi: 10.1152/physiol.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell WC, Matrisian LM. Complex roles of matrix metalloproteinases in tumor progression. Curr Top Microbiol Immunol. 1996;213:1–21. doi: 10.1007/978-3-642-61107-0_1. [DOI] [PubMed] [Google Scholar]
- 20.Gonçalves JL, Roma EH, Gomes-Santos AC, Aguilar EC, Cisalpino D, Fernandes LR, Vieira AT, Oliveira DR, Cardoso VN, Teixeira MM, Alvarez-Leite JI. Pro-inflammatory effects of the mushroom Agaricus blazei and its consequences on atherosclerosis development. Eur J Nutr. 2012;51:927–937. doi: 10.1007/s00394-011-0270-8. [DOI] [PubMed] [Google Scholar]
- 21.Rowsell S, Hawtin P, Minshull CA, Jepson H, Brockbank SM, Barratt DG, Slater AM, McPheat WL, Waterson D, Henney AM, Pauptit RA. Crystal structure of human MMP9 in complex with a reverse hydroxamate inhibitor. J Mol Biol. 2002;319:173–181. doi: 10.1016/S0022-2836(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 22.Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013;34:2041–2051. doi: 10.1007/s13277-013-0842-8. [DOI] [PubMed] [Google Scholar]
- 23.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 24.Tassi E, McDonnell K, Gibby KA, Tilan JU, Kim SE, Kodack DP, Schmidt MO, Sharif GM, Wilcox CS, Welch WJ, et al. Impact of fibroblast growth factor-binding protein-1 expression on angiogenesis and wound healing. Am J Pathol. 2011;179:2220–2232. doi: 10.1016/j.ajpath.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambaerts K, Wilcox-Adelman SA, Zimmermann P. The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr Opin Cell Biol. 2009;21:662–669. doi: 10.1016/j.ceb.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: Structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;13:2057–2073. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- 27.McKenzie EA. Heparanase: A target for drug discovery in cancer and inflammation. Br J Pharmacol. 2007;151:1–14. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38:2018–2039. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Ferro V, Hammond E, Fairweather JK. The development of inhibitors of heparanase, a key enzyme involved in tumour metastasis, angiogenesis and inflammation. Mini Rev Med Chem. 2004;4:693–702. doi: 10.2174/1389557043403729. [DOI] [PubMed] [Google Scholar]
- 30.Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang XP, Liu L, Wang P, Ma SL. Human sulfatase-1 improves the effectiveness of cytosine deaminase suicide gene therapy with 5-fluorocytosine treatment on hepatocellular carcinoma cell line HepG2 in vitro and in vivo. Chin Med J (Engl) 2015;128:1384–1390. doi: 10.4103/0366-6999.156800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bret C, Moreaux J, Schved JF, Hose D, Klein B. SULFs in human neoplasia: Implication as progression and prognosis factors. J Transl Med. 2011;9:72. doi: 10.1186/1479-5876-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaghloul RA, Al-Gayyar MM, El-Shishtawy MM, Ebrahim MA. Cytotoxic effects of antiglypican-3 against HepG2 cell lines. J App Pharm Sci. 2013;3:31–35. [Google Scholar]
- 34.Zaghloul RA, El-Shishtawy MM, El Galil KH, Ebrahim MA, Metwaly AA, Al-Gayyar MM. Evaluation of antiglypican-3 therapy as a promising target for amelioration of hepatic tissue damage in hepatocellular carcinoma. Eur J Pharmacol. 2015;746:353–362. doi: 10.1016/j.ejphar.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Al-Gayyar MM, Abbas A, Hamdan AM. Chemopreventive and hepatoprotective roles of adiponectin (SULF2 inhibitor) in hepatocelluar carcinoma. Biol Chem. 2016;397:257–267. doi: 10.1515/hsz-2015-0265. [DOI] [PubMed] [Google Scholar]
- 36.Zheng X, Gai X, Han S, Moser CD, Hu C, Shire AM, Floyd RA, Roberts LR. The human sulfatase 2 inhibitor 2,4-disulfonylphenyl-tert-butylnitrone (OKN-007) has an antitumor effect in hepatocellular carcinoma mediated via suppression of TGFB1/SMAD2 and Hedgehog/GLI1 signaling. Genes Chromosomes Cancer. 2013;52:225–236. doi: 10.1002/gcc.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stepp MA, Pal-Ghosh S, Tadvalkar G, Pajoohesh-Ganji A. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle) 2015;4:235–249. doi: 10.1089/wound.2014.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baghy K, Tátrai P, Regős E, Kovalszky I. Proteoglycans in liver cancer. World J Gastroenterol. 2016;22:379–393. doi: 10.3748/wjg.v22.i1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Götte M, Kersting C, Radke I, Kiesel L, Wülfing P. An expression signature of syndecan-1 (CD138), E-cadherin and c-met is associated with factors of angiogenesis and lymphangiogenesis in ductal breast carcinoma in situ. Breast Cancer Res. 2007;9:R8. doi: 10.1186/bcr1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metwaly HA, Al-Gayyar MM, Eletreby S, Ebrahim MA, El-Shishtawy MM. Relevance of serum levels of interleukin-6 and syndecan-1 in patients with hepatocellular carcinoma. Sci Pharm. 2012;80:179–188. doi: 10.3797/scipharm.1110-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulze D, Plohmann P, Höbel S, Aigner A. Anti-tumor effects of fibroblast growth factor-binding protein (FGF-BP) knockdown in colon carcinoma. Mol Cancer. 2011;10:144. doi: 10.1186/1476-4598-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao B, Li S, Tan Z, Ma L, Liu J. ACTG1 and TLR3 are biomarkers for alcohol-associated hepatocellular carcinoma. Oncol Lett. 2019;17:1714–1722. doi: 10.3892/ol.2018.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metwaly HA, El-Gayar AM, El-Shishtawy MM. Inhibition of the signaling pathway of syndecan-1 by synstatin: A promising anti-integrin inhibitor of angiogenesis and proliferation in HCC in rats. Arch Biochem Biophys. 2018;652:50–58. doi: 10.1016/j.abb.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Aggio A, Grassi D, Onori E, D'Alessandro A, Masedu F, Valenti M, Ferri C. Endothelium/nitric oxide mechanism mediates vasorelaxation and counteracts vasoconstriction induced by low concentration of flavanols. Eur J Nutr. 2013;52:263–272. doi: 10.1007/s00394-012-0320-x. [DOI] [PubMed] [Google Scholar]
- 45.Jeon Y, Jang ES, Choi YS, Kim JW, Jeong SH. Glypican-3 level assessed by the enzyme-linked immunosorbent assay is inferior to alpha-fetoprotein level for hepatocellular carcinoma diagnosis. Clin Mol Hepatol. 2016;22:359–365. doi: 10.3350/cmh.2016.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stigliano I, Puricelli L, Filmus J, Sogayar MC, Bal de Kier Joffé E, Peters MG. Glypican-3 regulates migration, adhesion and actin cytoskeleton organization in mammary tumor cells through Wnt signaling modulation. Breast Cancer Res Treat. 2009;114:251–262. doi: 10.1007/s10549-008-0009-2. [DOI] [PubMed] [Google Scholar]
- 47.Cheng W, Tseng CJ, Lin TT, Cheng I, Pan HW, Hsu HC, Lee YM. Glypican-3-mediated oncogenesis involves the Insulin-like growth factor-signaling pathway. Carcinogenesis. 2008;29:1319–1326. doi: 10.1093/carcin/bgn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho M. Advances in liver cancer antibody therapies: A focus on glypican-3 and mesothelin. BioDrugs. 2011;25:275–284. doi: 10.2165/11595360-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakano K, Ishiguro T, Konishi H, Tanaka M, Sugimoto M, Sugo I, Igawa T, Tsunoda H, Kinoshita Y, Habu K, et al. Generation of a humanized anti-glypican 3 antibody by CDR grafting and stability optimization. Anticancer Drugs. 2010;21:907–916. doi: 10.1097/CAD.0b013e32833f5d68. [DOI] [PubMed] [Google Scholar]
- 50.Sun CK, Chua MS, He J, So SK. Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-β2. Neoplasia. 2011;13:735–747. doi: 10.1593/neo.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miura M, Fujinami N, Shimizu Y, Mizuno S, Saito K, Suzuki T, Konishi M, Takahashi S, Gotohda N, Suto K, et al. Usefulness of plasma full-length glypican-3 as a predictive marker of hepatocellular carcinoma recurrence after radial surgery. Oncol Lett. 2020;19:2657–2666. doi: 10.3892/ol.2020.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elewa MA, Al-Gayyar MM, Schaalan MF, Abd El Galil KH, Ebrahim MA, El-Shishtawy MM. Hepatoprotective and anti-tumor effects of targeting MMP-9 in hepatocellular carcinoma and its relation to vascular invasion markers. Clin Exp Metastasis. 2015;32:479–493. doi: 10.1007/s10585-015-9721-6. [DOI] [PubMed] [Google Scholar]
- 53.Jayo A, Parsons M. Fascin: A key regulator of cytoskeletal dynamics. Int J Biochem Cell Biol. 2010;42:1614–1617. doi: 10.1016/j.biocel.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 54.Huang X, Ji J, Xue H, Zhang F, Han X, Cai Y, Zhang J, Ji G. Fascin and cortactin expression is correlated with a poor prognosis in hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2012;24:633–639. doi: 10.1097/MEG.0b013e3283515a18. [DOI] [PubMed] [Google Scholar]
- 55.Oh SY, Kim YB, Suh KW, Paek OJ, Moon HY. Prognostic impact of fascin-1 expression is more significant in advanced colorectal cancer. J Surg Res. 2012;172:102–108. doi: 10.1016/j.jss.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Hayashi Y, Osanai M, Lee GH. Fascin-1 expression correlates with repression of E-cadherin expression in hepatocellular carcinoma cells and augments their invasiveness in combination with matrix metalloproteinases. Cancer Sci. 2011;102:1228–1235. doi: 10.1111/j.1349-7006.2011.01938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai JP, Thompson JR, Sandhu DS, Roberts LR. Heparin-degrading sulfatases in hepatocellular carcinoma: Roles in pathogenesis and therapy targets. Future Oncol. 2008;4:803–814. doi: 10.2217/14796694.4.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdel-Hamid NM. Premalignant variations in extracellular matrix composition in chemically induced hepatocellular carcinoma in rats. J Membr Biol. 2009;230:155–162. doi: 10.1007/s00232-009-9196-y. [DOI] [PubMed] [Google Scholar]
- 59.de los Reyes GC, Koda RT, Lien EJ. Glucosamine and chondroitin sulfates in the treatment of osteoarthritis: A survey. Prog Drug Res. 2000;55:81–103. doi: 10.1007/978-3-0348-8385-6_3. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, Liu WS, Han BQ, Peng YF, Wang DF. Antitumor activities of D-glucosamine and its derivatives. J Zhejiang Univ Sci B. 2006;7:608–614. doi: 10.1631/jzus.2006.B0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan HY, Eskandari R, Shen D, Zhu Y, Liu TW, Willems LI, Alteen MG, Madden Z, Vocadlo DJ. Direct one-step fluorescent labeling of O-GlcNAc-modified proteins in live cells using metabolic intermediates. J Am Chem Soc. 2018;140:15300–15308. doi: 10.1021/jacs.8b08260. [DOI] [PubMed] [Google Scholar]
- 62.Tayel A, Ebrahim MA, Ibrahim AS, El-Gayar AM, Al-Gayyar MM. Cytotoxic effects of suramin against HepG2 cells through activation of intrinsic apoptotic pathway. J BUON. 2014;19:1048–1054. [PubMed] [Google Scholar]
- 63.Al-Gayyar MMH, Ebrahim MA, Shams MEE. Measuring serum levels of glycosaminoglycans for prediction and using viscum fraxini-2 for treatment of patients with hepatocellular carcinoma. J Pharm Res. 2013;7:571–575. [Google Scholar]
- 64.Bezabeh T, Ijare OB, Albiin N, Arnelo U, Lindberg B, Smith IC. Detection and quantification of D-glucuronic acid in human bile using 1H NMR spectroscopy: Relevance to the diagnosis of pancreatic cancer. MAGMA. 2009;22:267–275. doi: 10.1007/s10334-009-0171-5. [DOI] [PubMed] [Google Scholar]
- 65.Attallah AM, Toson el-SA, El-Waseef AM, Abo-Seif MA, Omran MM, Shiha GE. Discriminant function based on hyaluronic acid and its degrading enzymes and degradation products for differentiating cirrhotic from non-cirrhotic liver diseased patients in chronic HCV infection. Clin Chim Acta. 2006;369:66–72. doi: 10.1016/j.cca.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-A. [DOI] [PubMed] [Google Scholar]
- 67.Varki NM, Varki A. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab Invest. 2007;87:851–857. doi: 10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arif S, Najeeb-ul-Haq, Hanif R, Khan AS, Jamil-ur-Rehman, Mufti TA. Variations of serum sialic acid level in liver cirrhosis. J Ayub Med Coll Abbottabad. 2005;17:54–57. [PubMed] [Google Scholar]
- 69.Liu J, Wen X, Liu B, Zhang Q, Zhang J, Miao H, Zhu R. Diosmetin inhibits the metastasis of hepatocellular carcinoma cells by downregulating the expression levels of MMP-2 and MMP-9. Mol Med Rep. 2016;13:2401–2408. doi: 10.3892/mmr.2016.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Ishige N. Niclosamide suppresses migration of hepatocellular carcinoma cells and downregulates matrix metalloproteinase-9 expression. Oncol Lett. 2015;10:3515–3518. doi: 10.3892/ol.2015.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Z, Dou C, Jia Y, Li Q, Zheng X, Yao Y, Liu Q, Song T. RIG-I suppresses the migration and invasion of hepatocellular carcinoma cells by regulating MMP9. Int J Oncol. 2015;46:1710–1720. doi: 10.3892/ijo.2015.2853. [DOI] [PubMed] [Google Scholar]
- 72.Chen X, Bo L, Zhao X, Chen Q. MicroRNA-133a inhibits cell proliferation, colony formation ability, migration and invasion by targeting matrix metallopeptidase 9 in hepatocellular carcinoma. Mol Med Rep. 2015;11:3900–3907. doi: 10.3892/mmr.2015.3232. [DOI] [PubMed] [Google Scholar]
- 73.Xu L, Wang T, Meng WY, Wei J, Ma JL, Shi M, Wang YG. Salinomycin inhibits hepatocellular carcinoma cell invasion and migration through JNK/JunD pathway-mediated MMP9 expression. Oncol Rep. 2015;33:1057–1063. doi: 10.3892/or.2014.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsieh MJ, Lin CW, Yang SF, Chen MK, Chiou HL. Glabridin inhibits migration and invasion by transcriptional inhibition of matrix metalloproteinase 9 through modulation of NF-κB and AP-1 activity in human liver cancer cells. Br J Pharmacol. 2014;171:3037–3050. doi: 10.1111/bph.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuxian X, Feng T, Ren L, Zhengcai L. Tanshinone II-A inhibits invasion and metastasis of human hepatocellular carcinoma cells in vitro and in vivo. Tumori. 2009;95:789–795. doi: 10.1177/030089160909500623. [DOI] [PubMed] [Google Scholar]
- 76.Darweish MM, Abbas A, Ebrahim MA, Al-Gayyar MM. Chemopreventive and hepatoprotective effects of Epigallocatechin-gallate against hepatocellular carcinoma: Role of heparan sulfate proteoglycans pathway. J Pharm Pharmacol. 2014;66:1032–1045. doi: 10.1111/jphp.12229. [DOI] [PubMed] [Google Scholar]
- 77.Chen XP, Luo JS, Tian Y, Nie CL, Cui W, Zhang WD. Downregulation of heparanase expression results in suppression of invasion, migration, and adhesion abilities of hepatocellular carcinoma cells. Biomed Res Int. 2015;2015:241983. doi: 10.1155/2015/241983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Z, Zhu L, Li X, Tian H, Fang Y, Liu H, Li S, Li L, Yue W, Li W. Down-regulation of heparanase leads to the inhibition of invasion and proliferation of A549 cells in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai) 2013;45:188–193. doi: 10.1093/abbs/gms109. [DOI] [PubMed] [Google Scholar]
- 79.Liu CJ, Chang J, Lee PH, Lin DY, Wu CC, Jeng LB, Lin YJ, Mok KT, Lee WC, Yeh HZ, et al. Adjuvant heparanase inhibitor PI-88 therapy for hepatocellular carcinoma recurrence. World J Gastroenterol. 2014;20:11384–11393. doi: 10.3748/wjg.v20.i32.11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu CJ, Lee PH, Lin DY, Wu CC, Jeng LB, Lin PW, Mok KT, Lee WC, Yeh HZ, Ho MC, et al. Heparanase inhibitor PI-88 as adjuvant therapy for hepatocellular carcinoma after curative resection: A randomized phase II trial for safety and optimal dosage. J Hepatol. 2009;50:958–968. doi: 10.1016/j.jhep.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 81.Alyoussef A, Al-Gayyar MMH. Cytotoxic and partial hepatoprotective activity of sodium ascorbate against hepatocellular carcinoma through inhibition of sulfatase-2 in vivo and in vitro. Biomed Pharmacother. 2018;103:362–372. doi: 10.1016/j.biopha.2018.04.060. [DOI] [PubMed] [Google Scholar]
- 82.Chen G, Nakamura I, Dhanasekaran R, Iguchi E, Tolosa EJ, Romecin PA, Vera RE, Almada LL, Miamen AG, Chaiteerakij R, et al. Transcriptional induction of periostin by a sulfatase 2-TGFβ1-SMAD signaling axis mediates tumor angiogenesis in hepatocellular carcinoma. Cancer Res. 2017;77:632–645. doi: 10.1158/0008-5472.CAN-15-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao W, Kim H, Ho M. Human monoclonal antibody targeting the heparan sulfate chains of glypican-3 inhibits HGF-mediated migration and motility of hepatocellular carcinoma cells. PLoS One. 2015;10:e0137664. doi: 10.1371/journal.pone.0137664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao H, Li K, Tu H, Pan X, Jiang H, Shi B, Kong J, Wang H, Yang S, Gu J, Li Z. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014;20:6418–6428. doi: 10.1158/1078-0432.CCR-14-1170. [DOI] [PubMed] [Google Scholar]
- 85.Liu S, Li Y, Chen W, Zheng P, Liu T, He W, Zhang J, Zeng X. Silencing glypican-3 expression induces apoptosis in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2012;419:656–661. doi: 10.1016/j.bbrc.2012.02.069. [DOI] [PubMed] [Google Scholar]
- 86.Qi XH, Wu D, Cui HX, Ma N, Su J, Wang YT, Jiang YH. Silencing of the glypican-3 gene affects the biological behavior of human hepatocellular carcinoma cells. Mol Med Rep. 2014;10:3177–3184. doi: 10.3892/mmr.2014.2600. [DOI] [PubMed] [Google Scholar]
- 87.Yu D, Dong Z, Yao M, Wu W, Yan M, Yan X, Qiu L, Chen J, Sai W, Yao D. Targeted glypican-3 gene transcription inhibited the proliferation of human hepatoma cells by specific short hairpin RNA. Tumour Biol. 2013;34:661–668. doi: 10.1007/s13277-012-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yao M, Wang L, Dong Z, Qian Q, Shi Y, Yu D, Wang S, Zheng W, Yao D. Glypican-3 as an emerging molecular target for hepatocellular carcinoma gene therapy. Tumour Biol. 2014;35:5857–5868. doi: 10.1007/s13277-014-1776-5. [DOI] [PubMed] [Google Scholar]
- 89.Ikeda M, Ohkawa S, Okusaka T, Mitsunaga S, Kobayashi S, Morizane C, Suzuki I, Yamamoto S, Furuse J. Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma. Cancer Sci. 2014;105:455–462. doi: 10.1111/cas.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sawada Y, Sakai M, Yoshikawa T, Ofuji K, Nakatsura T. A glypican-3-derived peptide vaccine against hepatocellular carcinoma. Oncoimmunology. 2012;1:1448–1450. doi: 10.4161/onci.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu AX, Gold PJ, El-Khoueiry AB, Abrams TA, Morikawa H, Ohishi N, Ohtomo T, Philip PA. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2013;19:920–928. doi: 10.1158/1078-0432.CCR-12-2616. [DOI] [PubMed] [Google Scholar]
- 92.Huang N, Lin J, Ruan J, Su N, Qing R, Liu F, He B, Lv C, Zheng D, abd Luo R. MiR-219-5p inhibits hepatocellular carcinoma cell proliferation by targeting glypican-3. FEBS Lett. 2012;586:884–891. doi: 10.1016/j.febslet.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 93.Wang K, Kievit FM, Sham JG, Jeon M, Stephen ZR, Bakthavatsalam A, Park JO, Zhang M. Iron-oxide-based nanovector for tumor targeted siRNA delivery in an orthotopic hepatocellular carcinoma xenograft mouse model. Small. 2016;12:477–487. doi: 10.1002/smll.201501985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hanaoka H, Nakajima T, Sato K, Watanabe R, Phung Y, Gao W, Harada T, Kim I, Paik CH, Choyke PL, et al. Photoimmunotherapy of hepatocellular carcinoma-targeting glypican-3 combined with nanosized albumin-bound paclitaxel. Nanomedicine (Lond) 2015;10:1139–1147. doi: 10.2217/nnm.14.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li SQ, Lin J, Qi CY, Fu SJ, Xiao WK, Peng BG, Liang LJ. GPC3 DNA vaccine elicits potent cellular antitumor immunity against HCC in mice. Hepatogastroenterology. 2014;61:278–284. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are included in the present review.