Abstract
The standard care for patients with locally advanced cervical cancer is concurrent chemoradiotherapy. Successful neoadjuvant chemotherapy (NAC) can reduce tumor size and enable patients to be eligible for a hysterectomy, which can improve their prognosis. Selecting the right candidate for NAC is important since NAC failure results in switching to radiation therapy and can lead to a worse prognosis due to a delay in the initiation of the core therapy. Therefore, the identification of biomarkers that can predict the effect of NAC is essential. Previous reports have suggested a relationship between protein arginine methyltransferase (PRMT1) and chemoresistance in several types of cancer. PRMT1 has been demonstrated to methylate apoptosis signal-regulated kinase 1 and to inhibit its activity, thereby contributing to chemoresistance. The present study investigated the association between PRMT1 expression and the efficacy of NAC in locally advanced cervical cancer. Data from 53 patients with locally advanced uterine cervical cancer who were classified into two groups based on effective (n=28) and ineffective (n=25) responses to NAC treatment were evaluated. PRMT1 expression was investigated by immunohistochemistry and scored using a weighted scoring system. Additionally, the present study investigated the effect of RNA interference-mediated downregulation of PRMT1 on the sensitivity of cervical cancer cells to cisplatin in vitro. The results demonstrated that the NAC effective group had significantly lower weighted PRMT1 scores than the NAC ineffective group (P=0.030). In addition, lower tumor expression levels of PRMT1 were significantly associated with increased sensitivity to NAC (P=0.033). Furthermore, downregulation of PRMT1 expression in cervical cancer cells markedly improved their sensitivity to cisplatin in vitro. The present study suggested that PRMT1 expression has potential as a predictive marker of the efficacy of NAC in patients with locally advanced cervical cancer. This finding can contribute to improvements in the prognosis of these patients.
Keywords: uterine cervical cancer, protein arginine methyltransferase 1, neoadjuvant chemotherapy, predictive marker
Introduction
Uterine cervical cancer is the fourth most common cancer and the fourth leading cause of cancer death in women worldwide. As a result, it is considered to be one of the most significant public health concerns (1). Despite advances in screening, prevention, and diagnosis, some patients are diagnosed with a locally advanced stage, including International Federation of Gynecology and Obstetrics (FIGO) cancer stages IIIA, IIIB, and IVA. The established standard of care for locally advanced uterine cervical cancer is concurrent platinum-based chemoradiotherapy (CCRT) (2–4). However, these patients experience a higher rate of recurrence and poorer survival rates compared with patients diagnosed at an early stage of the disease. In addition, the 5-year survival of patients with locally advanced uterine cervical cancer is <60% (5,6). Neoadjuvant chemotherapy (NAC) has been shown to be an effective strategy to reduce tumor size in patients with locally advanced cervical cancer and facilitate a hysterectomy, thereby resulting in a better prognosis (7). However, NAC failure compels physicians to switch to radiation therapy, which can result in a worse prognosis due to a delay in the initiation of the core therapy (8–10). Therefore, there is an urgent need to discover a biomarker that can predict the efficacy of NAC in patients with locally advanced cervical cancer to select the right candidates for NAC (10–14).
The anticancer agent cisplatin exhibits antineoplastic activity by inducing DNA damage, particularly intrastrand DNA crosslinks, leading to apoptosis (15). Therefore, one of the underlying mechanisms of cisplatin resistance is the inactivation of apoptotic pathways (16). Protein arginine methyltransferase (PRMT) is an enzyme that catalyzes the methylation of histone and non-histone proteins and transfers methyl groups from S-adenosylmethionine to arginine (17). PRMT1 has been shown to methylate apoptosis signal-regulated kinase 1 (ASK1) and inhibit its activity resulting in chemotherapy resistance (18,19). In other carcinomas (esophageal cancer and colorectal cancer), previous reports suggest a relationship between PRMT1 and chemotherapy resistance (20,21). However, the effects of PRMT1 on the response to cisplatin in cervical cancer cell lines and patients with cervical cancer are unknown.
In the present study, we aimed to examine the association between the tumor expression of PRMT1 and the efficacy of NAC in locally advanced cervical cancer and determine the use of PRMT1 expression as a predictive biomarker.
Materials and methods
Patients and tissue samples
Fifty-three patients with locally advanced cervical cancer (FIGO stages IIIB) were evaluated. All patients were under 70 years of age and first cared for at Osaka City University Hospital (Osaka, Japan) between April 1995 and March 2010. Tumor tissue specimens were acquired by a punch biopsy prior to NAC. Based on the effect on NAC, patients were divided into two groups: Patients who responded to NAC underwent a hysterectomy and received radiation therapy (NAC + OP + R group; n=28), and patients who were not successfully treated with NAC and received radiation therapy only (NAC + R group; n=25). Balloon-occlusive arterial infusion chemotherapy for NAC was given to all patients. Cisplatin (Bristol-Myers Squibb) was injected into the artery through a catheter for more than 30 min. Cisplatin was given three times at doses of 50, 75, or 100 mg/m2, depending on the patient's age and renal function (22). All patients were treated with cisplatin every 4 weeks. After the completion of three courses of NAC with cisplatin, the effect of NAC was evaluated. If NAC was effective, the patient underwent surgery 1 month after the administration of NAC. If NAC was ineffective, radiotherapy was initiated soon after the evaluation. We evaluated the effect of NAC by pelvic examination and computed tomography or magnetic resonance imaging. We considered NAC successful if the stage was downgraded to stage I or II, and surgery was able to be performed. Informed consent was acquired from all patients prior to the tumor biopsy. This study was approved by the Institutional Review Board of Osaka City University Hospital (IRB no. 4282).
Immunohistochemical staining
PRMT1 protein expression was evaluated using 4-µm sections generated from paraffin-embedded tissue samples using a rabbit polyclonal antibody against PRMT1 (cat. no. ab-70724; Abcam) and Dako LSAB2 Peroxidase kit (cat. no. K0675; Agilent Technologies). Following routine deparaffinization and rehydration, sections were immersed in 3% hydrogen peroxide for 10 min at room temperature to inhibit endogenous peroxidase activity. For heat-mediated antigen retrieval, sections were incubated in 10 mM citrate buffer (pH 6.0) in an autoclave at 110°C for 20 min. After washing with phosphate-buffered saline (PBS), tumor tissue sections were incubated at 4°C overnight in a 1:500 dilution of the anti-PRMT1 antibody. Next, the sections were washed in PBS for 15 min, incubated with biotinylated goat anti-mouse and anti-rabbit immunoglobulin G secondary antibodies included in the Dako LSAB2 Peroxidase kit (cat. no. K0675; Agilent Technologies) for 10 min, and then incubated with a streptavidin-peroxidase complex solution and 3,3′-diaminobenzidine as a colorimetric agent for color development. Lastly, the tissue sections were counterstained using hematoxylin. The primary antibodies were excluded, and the specificity control was prepared in the same way.
PRMT1 expression was quantitatively analyzed using the weighted score method described by Sinicrope et al (23). The average percent of stained tumor cells was scored on a scale of 0 to 4 as follows: 0, ≤5%; 1, 5–25%; 2, 25–50%; 3, 50–75%; and 4, >75%. The staining intensity was categorized into three classes: 1+, weak; 2+, moderate; and 3+, intense. The score for the percentage of stained tumor cells was multiplied by the score for staining intensity, and the weighted scores were calculated for each tissue sample.
Cell culture
The human cervical cancer cell line Ca Ski (cat. no. IFO50007; Japanese Collection of Research Biosources Cell Bank) was cultured in RMPI medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin and maintained at 37°C in a moist atmosphere containing 5% CO2.
RNA interference
A small interfering RNA (siRNA) targeting PRMT1 (5′-GCAACUCCAUGUUUCAUAAtt, 5′-UUAUGAAACAUGGAGUUGCgg) and negative control sequence (cat. no. sc-37007) were obtained from Santa Cruz Biotechnology. Cells seeded into 6-well plates were transfected with siRNA (Invitrogen; Thermo Fisher Scientific, Inc.) overnight using lipofectamine RNAiMax following the description of the manufacturer. The medium was changed, and the cells were taken to the laboratory 24 h after transfection.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from Ca Ski cells using the RNeasy Mini kit (Qiagen GmbH) following the instructions of the manufacturer. High capacity cDNA reverse transcription kits (Applied Biosystems; Thermo Fisher Scientific, Inc.) were used to reverse transcribe the RNA. A TaqMan Gene expression assay (Applied Biosystems; Thermo Fisher Scientific, Inc.) and an Applied Biosystems 7500 Fast Real-Time PCR system were used to perform PCR. PRMT1 mRNA levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA in the same sample. The TaqMan probes were Hs04193290_g1 for PRMT1 and Hs99999905_m1 for GAPDH. The 2(-Delta Delta C(T)) method was used to analyze the relative changes in gene expression from real-time quantitative PCR experiments (24).
Chemosensitivity assay
The sensitivity of Ca Ski cells to cisplatin was evaluated with a Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies). In the first step, cells were transfected with negative control or PRMT1-specific siRNA as described above and seeded at a density of 2×103 cells/well in 96-well tissue culture plates. After 24 h, the medium was removed, and vehicle or cisplatin (0 to 7.5 µM) was added to the cells for 48 h. Then, 10 µl/well CCK-8 was added, and the plates were incubated for 2 h. The absorbance at 450 nm was then measured with a microplate reader (Corona Electric, Co., Ltd.). Dose-response curves for the percentage of viable cells relative to untreated cells were generated.
Statistical analysis
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan). Data are shown as the mean ± standard deviation in the Tables and as the mean ± standard error in the Figures. A prognostic analysis was performed using Kaplan-Meier plots and the log-rank test. The Mann-Whitney U test was used to compare the weighted scores. Significant differences between group means were compared using Student's t-test, and the χ2 test was used to identify relationships between group classification variables. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
Fifty-three patients diagnosed with locally advanced cervical cancer were classified into the two following groups based on the treatment effect: The NAC effective group (NAC + OP + R group; n=28) and the NAC ineffective group (NAC + R group; n=25). Table I shows the patient clinicopathological characteristics. We found no statistically significant difference between the two groups.
Table I.
Characteristics of patients in the NAC+OP+R and NAC+R groups.
| Variables | NAC+OP+R | NAC+R | P-value |
|---|---|---|---|
| No. of patients | 28 | 25 | |
| Age, years | 0.269a | ||
| Mean ± SD | 48.5±13.4 | 52.3±11.5 | |
| Range | 24–69 | 36–68 | |
| Histology, n | 0.555b | ||
| SCC | 24 | 21 | |
| A | 4 | 3 | |
| AS | 0 | 1 | |
| Tumor size, mm | 0.456a | ||
| Mean ± SD | 48.4±17.2 | 51.8±12.3 |
Student's t-test
χ2 test. NAC+OP+R, neoadjuvant chemotherapy + surgery + radiotherapy; NAC+R, neoadjuvant chemotherapy + radiotherapy; SCC, squamous cell carcinoma; A, adenocarcinoma; AS, adenosquamous carcinoma.
PRMT1 expression in uterine cervical cancer tissues
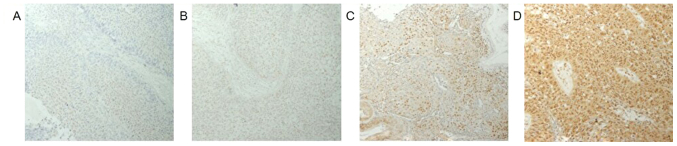
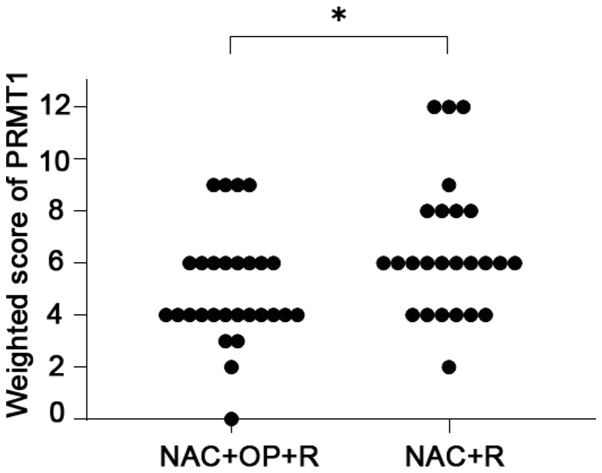
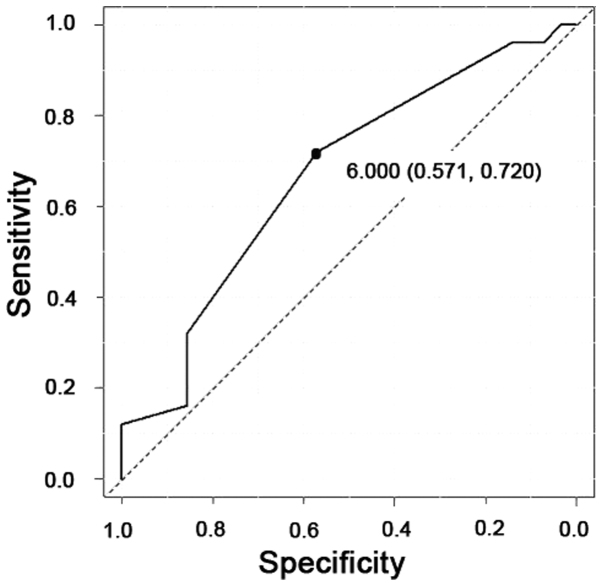
PRMT1 expression was found in both the nuclei and cytoplasm of the tumor cells (Fig. 1). The weighted scores of PRMT1 tissue staining are shown in Table II. The weighted score of the NAC ineffective group was significantly higher than that of the NAC effective group (P=0.030) (Fig. 2). To predict the efficacy of NAC, receiver operator characteristic (ROC) curves were generated, and the cut-off value of the PRMT1 score was examined. When a score of 6 was used as the cut-off value, the specificity was 57%, and the sensitivity was 72% (Fig. 3). Therefore, we classified the cases into two different groups. One was the low expression group with a score of 0 to 4, and the other was the high expression group with a score of 6–12. We found no statistically significant difference between the two groups (Table II).
Figure 1.
Immunohistochemical staining of PRMT1 in locally advanced cervical cancer. (A) Negative control staining performed without the primary antibody. Representative sections stained with a primary antibody against PRMT1 showing scores of (B) 4, (C) 6 and (D) 12. Sections were counterstained with hematoxylin. Magnification, ×400. PRMT1, protein arginine methyltransferase 1.
Table II.
Characteristics of the patients in the low and high PRMT1 expression groups.
| PRMT1 expression | |||
|---|---|---|---|
| Variables | Score ≤4 | Score ≥6 | P-value |
| No. of patients | 23 | 30 | |
| Age, years | 0.820a | ||
| Mean ± SD | 50.7±14.0 | 49.9±11.6 | |
| Range | 24–69 | 37–68 | |
| Histology, n | 0.514b | ||
| SCC | 19 | 26 | |
| A | 4 | 3 | |
| AS | 0 | 1 | |
| Tumor size, mm | 0.133a | ||
| Mean ± SD | 45.9±14.5 | 52.8±15.2 | |
Student's t-test
χ2 test. NAC+OP+R, neoadjuvant chemotherapy + surgery + radiotherapy; NAC+R, neoadjuvant chemotherapy + radiotherapy; SCC, squamous cell carcinoma; A, adenocarcinoma; AS, adenosquamous carcinoma; PRMT1, protein arginine methyltransferase 1.
Figure 2.
Weighted scores for PRMT1 expression in tumor samples from patients with locally advanced cervical cancer. *P=0.030 (Mann-Whitney U test). NAC + OP + R, neoadjuvant chemotherapy + surgery + radiotherapy; NAC + R, neoadjuvant chemotherapy + radiotherapy; PRMT1, protein arginine methyltransferase 1.
Figure 3.
Receiver operating characteristic curve analysis of the cut-off value of protein arginine methyltransferase 1 scores. When a score of 6 was used as the cut-off value, the specificity was 57% and the sensitivity was 72%. Area under the curve, 0.668. 95% confidence interval, 0.526–0.809.
NAC effectiveness correlates with PRMT1 expression
In a group of 23 patients with low PRMT1 expression, 16 (69.6%) were in the NAC effective group, and 7 (30.4%) were in the NAC ineffective group. In the high PRMT1 expression group, 12 patients (40.0%) were in the NAC effective group, and 18 (60.0%) were in the NAC ineffective group. In this way, we found that the low PRMT1 expression group was more responsive to NAC than the high PRMT1 expression group (P=0.033, Table III).
Table III.
Numbers of patients with low and high PRMT1 expression in the NAC+OP+R and NAC+R groups.
| Expression | NAC+OP+R, n (%) | NAC+R, n (%) | P-value |
|---|---|---|---|
| Low (score ≤4) | 16 (69.6) | 7 (30.4) | 0.033a |
| High (score ≥6) | 12 (40.0) | 18 (60.0) |
χ2 test. NAC+OP+R, neoadjuvant chemotherapy + surgery + radiotherapy; NAC+R, neoadjuvant chemotherapy + radiotherapy; PRMT1, protein arginine methyltransferase 1.
Survival
Overall survival was significantly longer in the NAC effective group than the NAC ineffective group (P<0.001) (Fig. 4) and in the low PRMT1 expression group than the high PRMT1 expression group (P=0.012) (Fig. 5).
Figure 4.
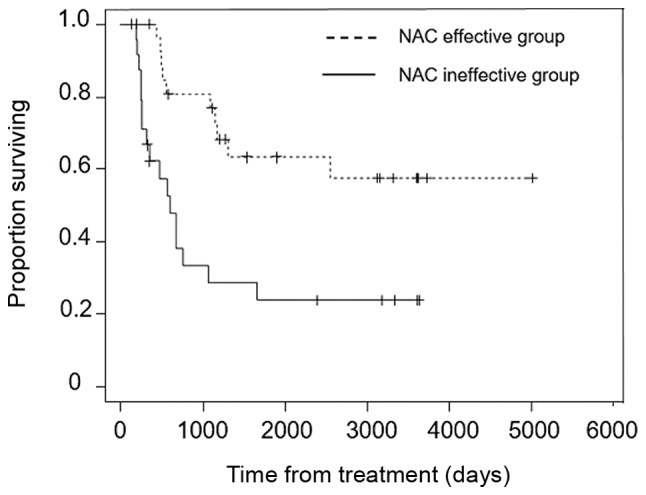
Overall survival rate in the NAC effective group (dashed line; n=28) and NAC ineffective group (solid line; n=25). P<0.001 (Kaplan-Meier analysis and log-rank test). NAC, neoadjuvant chemotherapy.
Figure 5.
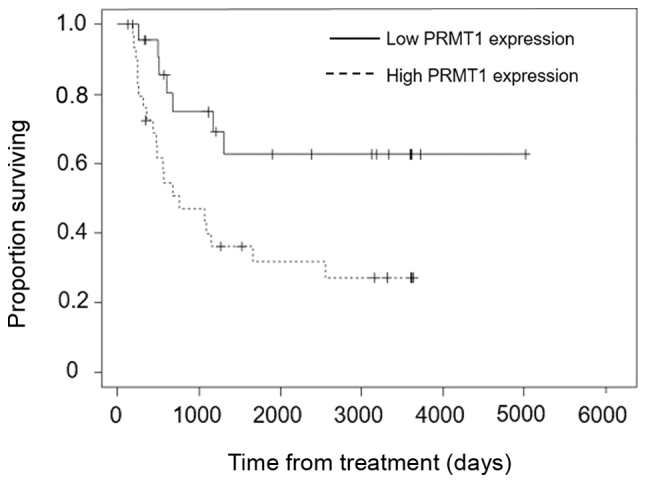
Overall survival in the low PRMT1 expression (solid line; n=23) and high PRMT1 expression (dashed line; n=30) groups. P=0.012 (Kaplan-Meier analysis and log-rank test). PRMT1, protein arginine methyltransferase 1.
PRMT1 knockdown enhances the sensitivity of a uterine cervical cancer cell line to cisplatin treatment
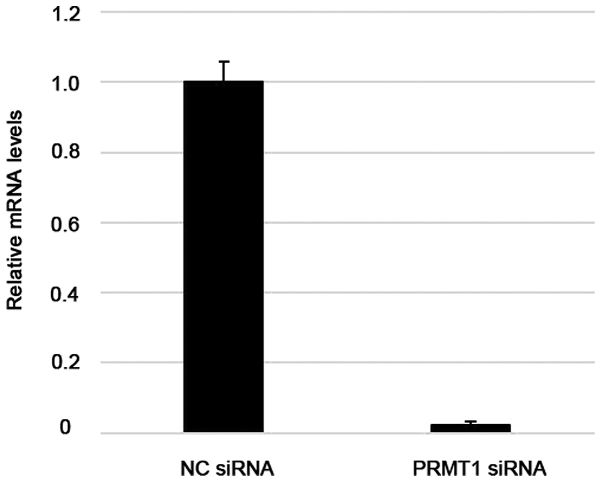
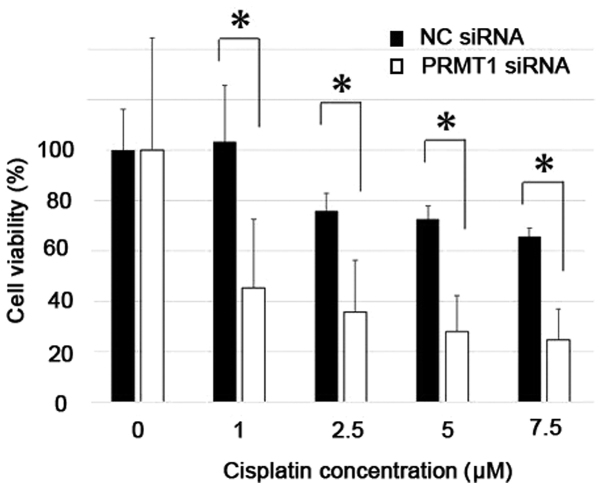
RT-qPCR analysis of Ca Ski cells showed that transfection with specifically targeted siRNAs efficiently suppressed PRMT1 expression, whereas non-targeted control siRNAs did not (Fig. 6). Differences in the sensitivity of cells transfected with PRMT1-specific siRNA to cisplatin were significantly greater than those of control cells (P<0.05) (Fig. 7).
Figure 6.
Reverse transcription-quantitative PCR analysis of PRMT1 mRNA expression in the uterine cervical cancer Ca Ski cell line after transfection with control or PRMT1-targeted siRNAs. mRNA levels were normalized to GAPDH. The values are presented as the mean ± standard error. NC, negative control; PRMT1, protein arginine methyltransferase 1; siRNA, small interfering RNA.
Figure 7.
Cell viability of Ca Ski cells transiently transfected with control or PRMT1-targeting siRNAs and incubated with the indicated concentrations of cisplatin for 24 h. *P<0.05 (Student's t-test). The values are presented as the mean ± standard error. NC, negative control; PRMT1, protein arginine methyltransferase 1; siRNA, small interfering RNA.
Discussion
The standard of care for patients with locally advanced cervical cancer is CCRT. However, the prognosis of these patients remains poor. Successful NAC can shrink the tumor and enable patients to undergo a hysterectomy, which can improve their prognosis (7). It is important to select the right candidate for NAC because NAC failure compels the physician to switch to radiation therapy, which can delay the initiation of the core therapy and result in a worse prognosis (8–10). Thus, the identification of biomarkers that can predict the effect of NAC in patients with locally advanced cervical cancer is essential for the effective treatment of these patients.
The methylation of various protein substrates is catalyzed by PRMTs, many of which are linked to the development, progression, and aggressiveness of various cancers (17). PRMTs are categorized as Type I or Type II according to the characteristics of the modification of their substrate. Both types catalyze the production of monomethylarginine as an intermediate, but type I enzymes mediate the production of asymmetric dimethylarginine, whereas type II enzymes produce symmetric dimethylarginine. PRMT1 is a Type I arginine methyltransferase. PRMT1 catalyzes the methylation of ASK1 and leads to the negative regulation of downstream signaling events, resulting in stress-induced stimulation of ASK1 and apoptotic cell death (25).
Anticancer agents, such as cisplatin, exhibit their antineoplastic activity by introducing DNA damage, particularly intrastrand DNA crosslinks, leading to apoptosis (15). Therefore, the inactivation of apoptotic pathways is associated with cisplatin resistance. The PRMT1-induced inactivation of apoptotic pathways via ASK1 inhibition can contribute to the development of cisplatin resistance.
This study found a significant association between PRMT1 expression and the efficacy of NAC in patients with locally advanced cervical cancer; patients with low PRMT1 expression were more likely to benefit from NAC and undergo surgery after NAC. Consistent with this, the low PRMT1 expression and NAC effective groups had longer overall survival periods than the high PRMT1 expression and NAC ineffective groups, respectively. We also observed that downregulating PRMT1 expression increased the cisplatin sensitivity of cultured cervical cancer cells, indicating that PRMT1 is a cisplatin-resistance factor. To the best of our knowledge, this study is the first to report a clearly defined relationship between PRMT1 expression and the effectiveness of NAC in locally advanced cervical cancer. However, the efficacy of NAC cannot be determined based on PRMT1 expression alone yet. As far as the standard treatment for locally advanced cervical cancer patients is CRT, we should choose NAC in just clinical trial settings. Moreover, one limitation of this study was that it included only 53 patients. Therefore, further investigation with more cases is needed to confirm our findings.
In summary, PRMT1 expression has the potential to be a predictive marker of the efficacy of NAC in patients with locally advanced cervical cancer. This finding can contribute to improvements in the prognosis of these patients.
Acknowledgements
The authors would like to thank Dr Melissa Crawford for editing a draft of this manuscript.
Funding
The present study was supported by The Osaka Medical Research Foundation for Intractable Diseases (grant no. 26-2-47).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
TF and TS designed the current study. MS, HM, YI, SN, YA and MY performed the experiment and collected the data. MS, TF, TY and TS analyzed the data. MS and TF wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of Osaka City University Hospital (IRB no. 4282) prior to initiation of the study. Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2011;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Japan Society of Gynecologic Oncology (eds), corp-author Kanehara & Co.; Tokyo: 2011. Formulation Committee of the Treatment Guidelines for Cervical Cancer, 2011. (In Japanese) [Google Scholar]
- 4.National Comprehensive Cancer Network, corp-author. NCCN Clinical Practice Guidelines in Oncology. Cervical Cancer Version II. https://www2.tri-kobe.org/nccn/guideline/gynecological/english/cervical.pdf 2013 [Google Scholar]
- 5.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 6.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, Rotman M, Gershenson D, Mutch DG. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 7.Ishiko O, Sumi T, Yasui T, Matsumoto Y, Kawamura N, Ogita S, Kamino T, Nakamura K, Yamada R. Balloon-occluded arterial infusion chemotherapy, simple total hysterectomy and radiotherapy as a useful combination-therapy for advanced cancer of the uterine cervix. Oncol Rep. 2000;7:141–144. [PubMed] [Google Scholar]
- 8.Souhami L, Gil RA, Allan SE, Canary PC, Araújo CM, Pinto LH, Silveira TR. A randomized trial of chemotherapy followed by pelvic radiation therapy in stage IIIB carcinoma of the cervix. J Clin Oncol. 1991;9:970–977. doi: 10.1200/JCO.1991.9.6.970. [DOI] [PubMed] [Google Scholar]
- 9.Tattersall MH, Lorvidhaya V, Vootiprux V, Cheirsilpa A, Wong F, Azhar T, Lee HP, Kang SB, Manalo A, Yen MS, et al. Randomized trial of epirubicin and cisplatin chemotherapy followed by pelvic radiation in locally advanced cervical cancer. Cervical cancer study group of the asian oceanian clinical oncology association. J Clin Oncol. 1995;13:444–451. doi: 10.1200/JCO.1995.13.2.444. [DOI] [PubMed] [Google Scholar]
- 10.Ishiko O, Sumi T, Yasui T, Matsumoto Y, Ogita S, Kaminou T, Nakamura K, Yamada R. Tumor marker and MR imaging criteria for evaluating the efficacy of cyclic balloon-occluded arterial infusion for advanced cancer of the uterine cervix. Oncol Rep. 2000;7:827–830. doi: 10.3892/or.7.4.827. [DOI] [PubMed] [Google Scholar]
- 11.Ishiko O, Sumi T, Yoshida H, Ogita S, Yamada R. Expression of apoptosis regulatory proteins in advanced cancer of the uterine cervix after cyclic balloon-occluded arterial infusion chemotherapy. Int J Oncol. 2001;18:1151–1155. doi: 10.3892/ijo.18.6.1151. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto E, Sumi T, Misugi F, Nobeyama H, Hattori K, Yoshida H, Matsumoto Y, Yasui T, Honda K, Ishiko O. Expression of apoptosis-related proteins in advanced uterine cervical cancer after balloon-occluded arterial infusion chemotherapy as an indicator of the efficiency of this therapy. Int J Mol Med. 2005;15:41–47. [PubMed] [Google Scholar]
- 13.Nobeyama H, Sumi T, Misugi F, Okamoto E, Hattori K, Matsumoto Y, Yasui T, Honda K, Iwai K, Ishiko O. Association of HPV infection with prognosis after neoadjuvant chemotherapy in advanced uterine cervical cancer. Int J Mol Med. 2004;14:101–105. [PubMed] [Google Scholar]
- 14.Benedetti Panici P, Bellati F, Manci N, Pernice M, Plotti F, Di Donato V, Calcagno M, Zullo MA, Muzii L, Angioli R. Neoadjuvant chemotherapy followed by radical surgery in patients affected by FIGO stage IVA cervical cancer. Ann Surg Oncol. 2007;14:2643–2648. doi: 10.1245/s10434-007-9408-6. [DOI] [PubMed] [Google Scholar]
- 15.Siddik ZH. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Shi S, He W, Padilla MT, Zhang L, Wang X, Zhang B, Lin Y. Retaining MKP1 expression and attenuating JNK-mediated apoptosis by RIP1 for cisplatin resistance through miR-940 inhibition. Oncotarget. 2014;5:1304–1314. doi: 10.18632/oncotarget.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell. 2005;18:263–72. doi: 10.1016/j.molcel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Baldwin RM, Morettin A, Côté J. Role of PRMTs in cancer: Could minor isoforms be leaving a mark? World J Biol Chem. 2014;5:115–129. doi: 10.4331/wjbc.v5.i2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirata Y, Katagiri K, Nagaoka K, Morishita T, Kudoh Y, Hatta T, Naguro I, Kano K, Udagawa T, Natsume T, et al. TRIM48 promotes ASK1 activation and cell death through ubiquitination-dependent degradation of the ASK1-negative regulator PRMT1. Cell Rep. 2017;21:2447–2457. doi: 10.1016/j.celrep.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Liao HW, Hsu JM, Xia W, Wang HL, Wang YN, Chang WC, Arold ST, Chou CK, Tsou PH, Yamaguchi H, et al. PRMT1-mediated methylation of the EGF receptor regulates signaling and cetuximab response. J Clin Invest. 2015;125:4529–4543. doi: 10.1172/JCI82826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Lu Q, Li C, Wang X, Jiang L, Huang L, Wang C, Chen H. PRMT1 regulates the tumour-initiating properties of esophageal squamous cell carcinoma through histone H4 arginine methylation coupled with transcriptional activation. Cell Death Dis. 2019;10:359. doi: 10.1038/s41419-019-1595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuji K, Yamada R, Kawabata M, Mitsuzane K, Sato M, Iwahashi M, Kitayama S, Nakano R. Effect of balloon occluded arterial infusion of anticancer drugs on the prognosis of cervical cancer treated with radiation therapy. Int J Radiat Oncol Biol Phys. 1995;32:1337–1345. doi: 10.1016/0360-3016(94)00651-Z. [DOI] [PubMed] [Google Scholar]
- 23.Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. Bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237–241. [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Cho JH, Lee MK, Yoon KW, Lee J, Cho SG, Choi EJ. Arginine methylation-dependent regulation of ASK1 signaling by PRMT1. Cell Death Differ. 2012;19:859–870. doi: 10.1038/cdd.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.