Abstract
Cancer cachexia is a life-threatening syndrome characterized by muscle atrophy. Cancer cachectic muscle atrophy (CCMA) is associated with mitochondrial injury. Mitochondrial calpains have been reported to induce mitochondrial injury in mouse cardiomyocytes and pulmonary smooth muscle. In the present study, the presence of calpain in the mitochondria of skeletal muscle and its potential role in CCMA were investigated. Transwell plates were used to develop a myotube-carcinoma cell co-culture model to simulate the cancer cachexia environment in vitro. The calpain inhibitors, calpastatin (CAST) and calpeptin (CAPT), were used to inhibit calpain activity in myotubes during co-culture. Calpain-1, calpain-2 and CAST were found to be present in mouse myotube mitochondria. Co-culture activated calpain in both cytoplasm and mitochondria, which caused myotube atrophy. CAST and CAPT treatment prevented calpain activation in both cytoplasm and mitochondria, which inhibited myotube atrophy during co-culture. Additionally, CAST and CAPT treatment increased mitochondrial complex I activity, decreased mitochondrial permeability transition pore opening and improved mitochondrial membrane potential in myotubes during co-culture. In addition, CAST and CAPT treatment increased AKT/mTOR activity, inhibited FoxO3a activity and decreased atrogin-1 content in myotubes during co-culture. The present findings provide new insights to understand the mechanism of CCMA and further help the development of focused approaches to treat CCMA by manipulating the mitochondrial and cytosolic calpain activity.
Keywords: cancer cachexia, calpain, mitochondria, muscle atrophy, co-culture
Introduction
Cancer cachectic muscle atrophy (CCMA) is a multifactorial metabolic syndrome characterized by muscle atrophy and a progressive loss of muscle function (1). It occurs in ~85% of terminal patients with upper gastrointestinal cancer and was responsible for ~20% of all cancer deaths worldwide in 2010 (2). Conventional nutritional support cannot fully reverse CCMA, and no effective treatment has been reported (3). Although the pathogenesis of CCMA has been constantly researched in the past decade, it remains not well established.
CCMA is associated with increased muscle proteolysis and decreased protein synthesis, which involve the calpain system (4), the ubiquitin-proteasome system (5), the autophagy-lysosome system (6) and the PI3K/AKT signaling pathway (7). Since only the calpain system can degrade intact myofilaments, calpain-dependent cleavage of myofilaments is considered as the initial step in muscle proteolysis, serving a critical role in CCMA (8).
Calpains are a family of 15 calcium-activated cysteine proteases, including two ubiquitously expressed members, calpain-1 and calpain-2, and one muscle-specific member, calpain-3 (9,10). Calpastatin (CAST) is the only known ubiquitously expressed endogenous calpain inhibitor (9). Calpains and CAST are generally considered to be localized in the cytoplasm and involved in numerous physiological and disease processes, including muscular dystrophy, diabetes, neurological disorders and hematonosis (11,12). Calpain-1, calpain-2 and CAST have been found in the mitochondria of cardiomyocytes and pulmonary smooth muscle cells; in these cells, activation of mitochondrial calpain induces mitochondrial injury and cell damage (13,14).
It has been reported that CCMA is also associated with mitochondrial injury (15). To the best of our knowledge, the role of mitochondrial calpain in CCMA has not yet been investigated. In the present study, a Transwell-plate system was used to develop a myotube-carcinoma cell co-culture model to simulate the cancer cachexia environment in vitro. The presence of calpains in the mitochondria of skeletal muscle and their potential role in CCMA were investigated.
Materials and methods
Cell culture
Mouse C2C12 myoblasts and CT26 colon carcinoma cells were obtained from the American Type Culture Collection and maintained in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin. The cells were cultured in an atmosphere of 5% CO2 at 37°C.
To establish the co-culture system, C2C12 myoblasts were seeded in 6-well plates at a density of 30,000 cells/cm2 and cultured in growth medium for 24–48 h at 37°C to reach 90–100% confluence. Subsequently, the growth medium was replaced by differentiation medium composed of DMEM and 2% horse serum (Gibco; Thermo Fisher Scientific, Inc.) to induce myoblast differentiation. The myoblasts were cultured for another 4 days at 37°C to allow their differentiation into myotubes, and the differentiation medium was replaced by growth medium. At the same time, CT26 cells were seeded (20,000 cells/cm2) into Transwell inserts (Corning Life Sciences) on a different 6-well plate containing growth medium. After a further 24 h of culture at 37°C, the inserts were placed into the wells containing myotubes, and the medium was changed to growth medium with or without the calpain inhibitors CAST (cat. no. 208902; 1 µM; EMD Millipore) and calpeptin (CAPT; cat. no. C8999; 50 µM; Sigma-Aldrich; Merck KGaA). After 24 h of co-culture at 37°C, the myotubes were fixed for immunocytochemistry or harvested for further analysis.
The co-culture combinations consisted of 5 groups: i) Sham myotubes (without CT26 cells in the insert) without calpain inhibitors (NC group); ii) myotubes co-cultured with CT26 cells but without calpain inhibitors (CO group); iii) myotubes with CT26 cells and CAST (CAST group); iv) myotubes with CT26 cells and CAPT (CAPT group); and v) myotubes with CT26 cells and CAST plus CAPT (CC group).
Immunocytochemical analysis
Myotubes were fixed, permeabilized, blocked and incubated with anti-myosin heavy chain (MHC) primary antibody (1:200; cat. no. ab91506; Abcam) followed by Alexa Fluor 488-conjugated goat anti-rabbit IgG secondary antibody (1:500; cat. no. ab150077; Abcam) as previously described (16). The myotubes were then mounted with Fluoroshield Mounting Medium containing DAPI (cat. no. ab104139; Abcam). Images were acquired with a fluorescence microscope (Nikon Corporation; magnification, ×100) and analyzed with ImageJ software (version 1.46r; National Institutes of Health).
Myotube diameters were measured as previously described (17). Briefly, 20 images/well were captured, and the diameters of the five largest myotubes (those containing ≥3 nuclei when viewed at ×100 magnification) in each image were measured. Next, the mean diameter of a single myotube was calculated based on three independent measurements. The measurement points were separated by 200 µm. This method was also used to calculate the mean diameter ± SD of the 100 largest myotubes in each well.
Isolation of myotube mitochondria
The Mitochondria Isolation kit for Cultured Cells (cat. no. ab110170; Abcam) was used to isolate myotube cytoplasm and mitochondria according to the manufacturer's protocol as previously described (18). Both cytoplasm and mitochondria were collected for further analysis.
Western blot analysis
Myotube cytosolic and mitochondrial proteins were extracted and separated via SDS-PAGE, blocked and stained with primary antibodies and HRP-conjugated secondary antibodies (1:2,000; cat. nos. ab97051 and ab6789; Abcam), and visualized as previously described (19). Images of the membranes were recorded with ChemiDoc XRS+ system (Bio-Rad Laboratories, Inc.) and analyzed using Quantity One software (version 4.6.6; Bio-Rad Laboratories, Inc.). The following primary antibodies were used: MHC (1:1,000; cat. no. ab124937), calpain-1 (1:1,000; cat. no. ab108400), calpain-2 (1:2,000; cat. no. ab126600), CAST (1:1,000; cat. no. ab28252), NADH dehydrogenase (ubiquinone) iron-sulfur protein 3, mitochondrial (NDUFS3; 1:2,000; cat. no. ab177471), cyclophilin D (CYCD; 1:2,000; cat. no. ab181983), phosphorylated- (p-)AKT S473 (1:1,000; cat. no. ab81283), AKT (1:5,000; cat. no. ab179463), p-mTOR S2448 (1:1,000; cat. no. ab109268), mTOR (1:1,000; cat. no. ab32028), p-eukaryotic translation initiation factor 4E-binding protein 1 (p-4EBP1) T37 (1:1,000; cat. no. ab75767), 4EBP1 (1:2,000; cat. no. ab32024), p-FoxO3a S253 (1:1,000; cat. no. ab31109), FoxO3a (1:1,000; cat. no. ab70315), atrogin1 (1:2,000; cat. no. ab168372), muscle-specific RING finger protein 1 (MuRF1; 1:2,000; cat. no. ab172479), GAPDH (1:2,000; cat. no. ab181602) and voltage-dependent anion-selective channel protein 1 (VDAC; 1:1,000; cat. no. ab154856), all purchased from Abcam; calpain-3 (1:1,000; cat. no. sc-365277) and spectrin [cat. no. sc-46696; detects both full-length (F)-spectrin and cleaved (C)-spectrin] from Santa Cruz Biotechnology, Inc.; and apoptosis-inducing factor (AIF; 1:1,000; cat. no. 4642S) from Cell Signaling Technology, Inc.
Calpain activity assay
A calpain activity assay kit (cat. no. QIA120; EMD Millipore) was used to measure calpain activity in samples of myotube cytoplasm and mitochondria, according to the manufacturer's protocol as previously described (20). The assay plates were read using a SpectraMax M5 microplate reader with a wavelength of 380 nm (Molecular Devices, LLC).
Complex I enzyme activity assay
The Complex I Enzyme Activity Microplate Assay kit (cat. no. ab109721; Abcam) was used to measure mitochondrial complex I enzyme activity in samples of myotube mitochondria according to the manufacturer's protocol as previously described (18). The assay plates were read using a SpectraMax M5 microplate reader with a wavelength of 450 nm.
JC-1 staining assay
Myotubes from 2 wells were collected using trypsin (cat. no. 25200072; Gibco; Thermo Fisher Scientific, Inc.) and plated in 96-well dark plates. JC-1 dye (cat. no. C2006; Beyotime Institute of Biotechnology) was used to detect the mitochondrial membrane potential (Δψm) according to the manufacturer's protocol. Δψm was measured by quantifying the fluorescence emission shift from green (530 nm) monomers to red (590 nm) aggregates (21). The assay plates were read using a SpectraMax M5 microplate reader. Data were expressed as fold increase in the red/green ratio.
Mitochondrial permeability transition pore (MPTP) opening
Myotubes were collected and plated in 96-well dark plates. MitoProbe Transition Pore Assay kit (cat. no. M34153; Thermo Fisher Scientific, Inc.) was used to measure MPTP opening according to the manufacturer's protocol as previously described (21). Myotubes were incubated with calcein AM dye (included in the kit) for 15 min and then incubated with CoCl2 for 15 min at 37°C. Ionomycin was used as a positive control. The assay plates were read using a SpectraMax M5 microplate reader with a wavelength of 480 nm.
Statistical analysis
Each experiment was repeated at least three times, and all data were analyzed using SPSS version 19.0 (IBM Corp.). Results are shown as the mean ± SD. Statistical comparisons between groups were analyzed using one-way ANOVA followed by Tukey's post-hoc test when equal variances were assumed or Dunnett's T3 test when equal variances were not assumed. P<0.05 was considered to indicate a statistically significant difference.
Results
Calpain inhibitors decrease myotube atrophy
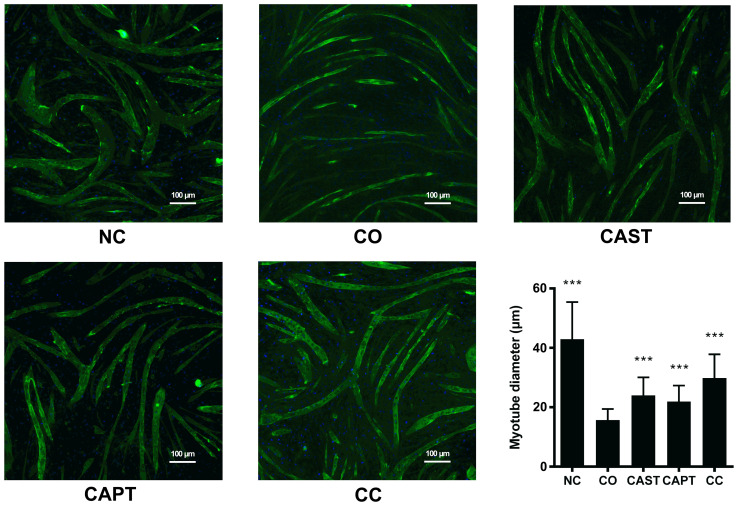
Myotube diameter was significantly decreased following 24 h of co-culture (CO group) compared with the NC group (Fig. 1), suggesting that the cell co-culture model can simulate muscle atrophy. Compared with untreated myotubes (CO group), both CAST and CAPT treatment, as well as the combination of both treatments (CC group), significantly increased myotube diameter, indicating that inhibition of calpain attenuated myotube atrophy during co-culture of myoblasts and colon carcinoma cells.
Figure 1.
Effect of calpain inhibitors in myotube atrophy. Immunofluorescence staining for anti-MHC antibody in mouse C2C12 myotubes. MHC staining outlines the myotubes (green). DAPI was used to stain nuclei (blue). Magnification, ×100. Scale bar, 100 µm. The myotube diameter was measured for each group and is represented in the bar graph. Data are represented as mean ± SD. ***P<0.001 vs. CO. MHC, myosin heavy chain; NC group, sham myotubes without CT26 cells and without calpain inhibitors; CO group, myotubes with CT26 cells without calpain inhibitors; CAST group, myotubes with CT26 cells and CAST; CAPT group, myotubes with CT26 cells and CAPT; CC group, myotubes with CT26 cells and CAST plus CAPT; CAST, calpastatin; CAPT, calpeptin.
Calpain inhibitors prevent calpain activation during co-culture
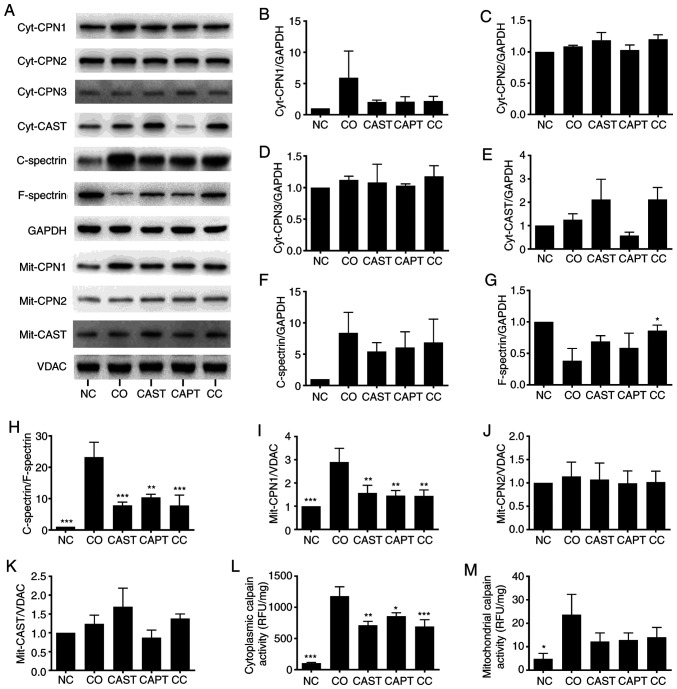
Co-culture activated both cytosolic and mitochondrial calpains in myotubes. Both cytosolic calpain-1 (cyt-CPN1) and mitochondrial calpain-1 (mit-CPN1) contents were increased by co-culture compared with the NC group (Fig. 2A, B and I). Direct enzyme activity assays revealed that cytosolic and mitochondrial calpain activities were also increased by co-culture (Fig. 2L and M). Cytosolic calpain activation can also be measured by calculating the C-spectrin/F-spectrin ratio in myotubes (22). The results indicated that this ratio was significantly increased in co-cultured myotubes, indicating the increased formation of C-spectrin (Fig. 2A and F-H). Compared with the CO group, CAST, CAPT and CC treatment markedly decreased both CPN-1 expression in the western blots (Fig. 2A and B) and cytosolic calpain activity (Fig. 2L), the formation of C-spectrin (Fig. 2F-H) and the mit-CPN1 content (Fig. 2A and I). Cyt-CPN1 content (Fig. 2A and B) and mitochondrial calpain activity (Fig. 2M) were also decreased by CAST, CAPT and CC treatment, but did not reach a statistically significant difference. These data indicated that CAST, CAPT and CC treatment attenuated cytosolic and mitochondrial calpain activation. Cyt-CPN-2, cyt-CPN3, cyt-CAST, mit-CPN-2 and mit-CAST were also detected in myotubes, but their protein expression levels were not altered by co-culture, CAST, CAPT or CC treatment (Fig. 2A, C-E, J and K).
Figure 2.
Effect of calpain inhibitors in the activation of calpain during co-culture. (A) Western blot results. Quantification of (B) cyt-CPN1, (C) cyt-CPN2, (D) cyt-CPN3 and (E) cyt-CAST normalized to GAPDH. (F) Quantification of cytosolic C-spectrin normalized to GAPDH. (G) Quantification of cytosolic F-spectrin normalized to GAPDH. (H) Quantification of C-spectrin/F-spectrin ratio. (I) Quantification of mit-CPN1 normalized to VDAC. Quantification of (J) mit-CPN2 and (K) mit-CAST normalized to VDAC. (L) Quantification of cytosolic calpain activity. (M) Quantification of mitochondrial calpain activity. Data are represented as the mean ± SD. *P<0.05, **P<0.01 and ***P<0.001 vs. CO. C-spectrin, cleaved spectrin; F-spectrin, full-length spectrin; NC group, sham myotubes without CT26 cells and without calpain inhibitors; CO group, myotubes with CT26 cells without calpain inhibitors; CAST group, myotubes with CT26 cells and CAST; CAPT group, myotubes with CT26 cells and CAPT; CC group, myotubes with CT26 cells and CAST plus CAPT; CAST, calpastatin; CAPT, calpeptin; cyt-, cytosolic; CPN, calpain; VDAC, voltage-dependent anion-selective channel protein 1; mit-, mitochondrial; RFU, relative fluorescence unit.
Calpain inhibitors improve complex I activity in myotube mitochondria following co-culture
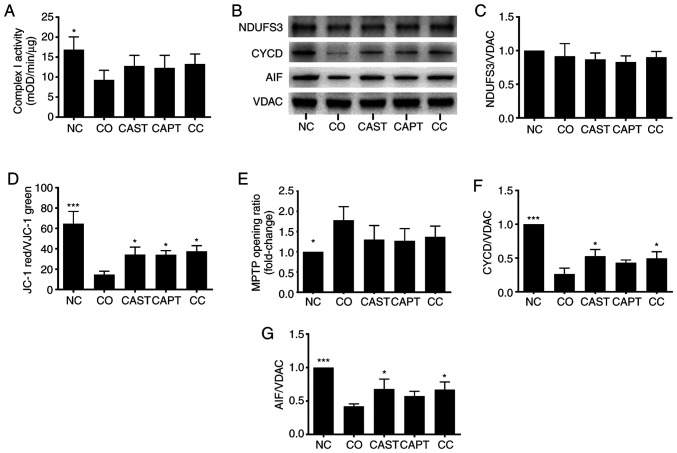
Complex I is the first respiratory complex of the mitochondrial electron transport chain (13). Activation of mitochondrial calpain damages complex I activity, which impairs energy generation, leads to MPTP opening and cardiac injury during reperfusion (13). Complex I activity was significantly decreased in mitochondria following co-culture compared with the NC control (Fig. 3A). CAST, CAPT and CC treatment slightly improved complex I activity, but did not reach statistical significance (Fig. 3A). Additionally, NDUFS3 is a core subunit of complex I that is essential for NADH oxidation and subsequent electron transfer through the complex, maintaining the activity of complex I (13). NDUFS3 content was not altered by co-culture, CAST, CAPT or CC treatment (Fig. 3B and C).
Figure 3.
Effect of calpain inhibitors on complex I activity and MPTP opening in myotubes during co-culture with colon carcinoma cells. (A) Quantification of mitochondrial complex I activity. (B) Western blot results. (C) Quantification of mitochondrial NDUFS3 normalized to VDAC. (D) Quantification of JC-1 red/green ratio. (E) Quantification of MPTP opening ratio. (F) Quantification of mitochondrial CYCD normalized to VDAC. (G) Quantification of mitochondrial AIF normalized to VDAC. Data are represented as the mean ± SD. *P<0.05 and ***P<0.001 vs. CO. NC group, sham myotubes without CT26 cells and without calpain inhibitors; CO group, myotubes with CT26 cells without calpain inhibitors; CAST group, myotubes with CT26 cells and CAST; CAPT group, myotubes with CT26 cells and CAPT; CC group, myotubes with CT26 cells and CAST plus CAPT; CAST, calpastatin; CAPT, calpeptin; VDAC, voltage-dependent anion-selective channel protein 1; NDUFS3, NADH dehydrogenase (ubiquinone) iron-sulfur protein 3, mitochondrial; AIF, apoptosis-inducing factor; CYCD, cyclophilin D; MPTP, mitochondrial permeability transition pore; mOD, mean optical density.
Calpain inhibitors increase the Δψm in myotubes following co-culture
JC-1 was used to assess the Δψm in myotubes (21). The Δψm was reflected by the JC-1 red/green ratio. Compared with the NC group, the JC-1 red/green ratio in the CO group was significantly decreased by 70% (Fig. 3D), indicating damage of the Δψm following co-culture. However, treatment with CAST, CAPT or CC significantly improved this ratio compared with the CO group (Fig. 3D), indicating that the activation of calpain during co-culture is able to damage the Δψm in myotubes.
Calpain inhibitors decrease the MPTP opening in myotube following co-culture
The opening of the MPTP results in a permeation of the mitochondrial membrane that leads to the loss of Δψm (21). Thus, the MitoProbe assay was used to assess the MPTP opening in myotubes (21). The MPTP opening was significantly increased in the CO group compared with in the NC group (Fig. 3E), indicating the increased MPTP opening following co-culture. However, CAST, CAPT and CC treatment seemed to decrease the MPTP opening compared with the CO group, although there were no significant differences (Fig. 3E).
CYCD is a key factor that regulates MPTP opening (23). Compared with the NC group, co-culture significantly decreased the CYCD content in myotube mitochondria (Fig. 3B and F), whereas CAST and CC treatment significantly increased the CYCD content compared with co-culture alone. The present results suggested that the activation of calpain during co-culture decreased the CYCD content, which led to MPTP opening in mitochondria.
Calpain inhibitors preserve AIF content in myotube mitochondria
In addition to promoting the MPTP opening, calpain can cleave AIF and induce its release from the mitochondria to the cytoplasm (24,25). Similarly, the current results demonstrated that co-culture significantly decreased the AIF content in myotube mitochondria compared with the NC group, and that this decrease was significantly attenuated by CAST and CC treatment (Fig. 3B and G). However, assays failed to detect the cleaved AIF in both cytoplasm and mitochondria (data not shown).
Calpain inhibitors increase the AKT/mTOR activity and decrease FoxO3a activity in myotubes following co-culture
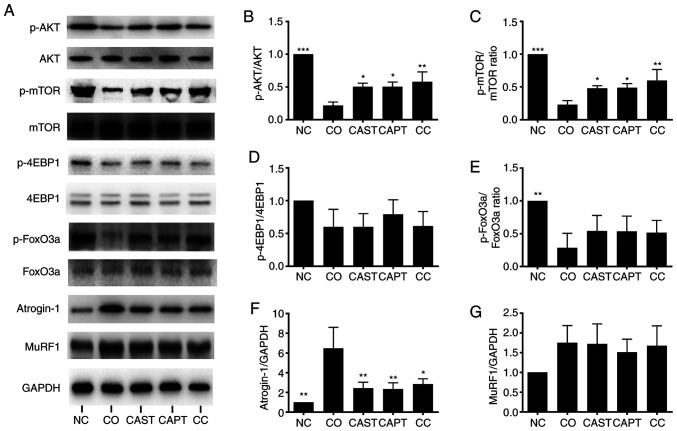
AKT/mTOR and FoxO3a serve a critical role in regulating muscular protein metabolism (26). Foxo3a is a transcription factor that activates the expression of ubiquitin ligase Atrogin-1 and MuRF1; Akt can phosphorylate FoxO3a and decrease its transcriptional activity (26). Co-culture significantly decreased the ratios of p-AKT/AKT, p-mTOR/mTOR and p-FoxO3a/FoxO3a in myotubes compared with the NC control group (Fig. 4A-C and E). Treatment with CAST, CAPT and CC significantly increased p-AKT/AKT and p-mTOR/mTOR ratios compared with the CO group (Fig. 4A-C), but not the p-FoxO3a/FoxO3a ratio (Fig. 4E). The p-4EBP1/4EBP1 ratio was not significantly affected by co-culture or treatment with the calpain inhibitors CAST or CAPT (Fig. 4A and D). The present results suggested that activation of calpain during co-culture decreased AKT/mTOR activity and increased FoxO3a activity in myotubes.
Figure 4.
Effect of calpain inhibitors in AKT/FoxO3a signaling pathway activity and atrogin-1 content in myotubes during co-culture with colon carcinoma cells. (A) Western blot results. (B) Quantification of p-AKT/AKT ratio. (C) Quantification of p-mTOR/mTOR ratio. (D) Quantification of p-4EBP1/4EBP1 ratio. (E) Quantification of p-FoxO3a/FoxO3a ratio. (F) Quantification of atrogin-1 normalized to GAPDH. (G) Quantification of MuRF1 normalized to GAPDH. Data are represented as the mean ± SD. *P<0.05, **P<0.01 and ***P<0.001 vs. CO. p-, phosphorylated; NC group, sham myotubes without CT26 cells and without calpain inhibitors; CO group, myotubes with CT26 cells without calpain inhibitors; CAST group, myotubes with CT26 cells and CAST; CAPT group, myotubes with CT26 cells and CAPT; CC group, myotubes with CT26 cells and CAST plus CAPT; CAST, calpastatin; CAPT, calpeptin; 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1; MuRF1, muscle-specific RING finger protein 1.
Calpain inhibitors decrease atrogin-1 content in myotubes following co-culture
Atrogin-1 and MuRF1 are two muscle-specific ubiquitin ligases that drive muscle protein degradation (8). As shown in Fig. 4A and F, co-culture significantly increased atrogin-1 content in myotubes compared with the NC control group, whereas CAST, CAPT and CC treatment significantly ameliorated this change. However, MuRF1 content was not significantly affected by co-culture or treatment with the calpain inhibitors CAST or CAPT (Fig. 4A and G).
Discussion
Cancer cachexia is a multifactorial syndrome that affects ~50–80% of patients with cancer, depending on the tumor type (27,28). In patients with gastric or pancreatic cancer, the incidence is >80%, whereas ~50% of patients with colon, lung or prostate cancer are affected, and ~40% of patients with breast cancer or some leukemias develop the syndrome (27,28). In the present study, calpain-1, calpain-2 and CAST were detected in mouse myotube mitochondria. In addition, the results revealed that co-culture of myoblasts with colon carcinoma cells activated calpain in myotube mitochondria, caused MPTP opening and Δψm damage, which led to mitochondrial injury. Moreover, co-culture activated calpain in myotube cytoplasm, caused the deactivation of AKT/mTOR and the activation of FoxO3a/atrogin-1. Calpain inhibitors (CAST, CAPT or CC) prevented calpain activation in both cytoplasm and mitochondria during co-culture, accompanied by inhibition of Δψm damage and atrogin-1 expression. Therefore, the present results indicated that calpain inhibitors protected the myotube by inhibiting both cytosolic and mitochondrial calpain activity.
CT26 colorectal adenocarcinoma cell conditioned medium is often used to induce myotube atrophy to simulate CCMA in vitro (29,30). In addition, CT26 colorectal adenocarcinoma and BALB/c mice can be used to develop cancer cachectic tumor-bearing mice, as previously described (4). Thus, in the present study, CT26 cells were used to develop a cell co-culture model with myoblasts to simulate CCMA.
Complex I is the first respiratory complex of the mitochondrial electron transport chain (31). Activation of mitochondrial calpain damages complex I activity, which impairs energy generation, leads to MPTP opening and cardiac reperfusion injury (13,31). In the present study, co-culture impaired complex I activity in myotube mitochondria, whereas calpain inhibitors slightly improved complex I activity, indicating that the activation of mitochondrial calpain impairs complex I activity, which may lead to MPTP opening and energy generation impairment.
The mechanisms of complex I damage involves complex I subunit damage and post-translational modifications (32). NDUFS3 is a core subunit of complex I that is essential for NADH oxidation and subsequent electron transfer through the complex, maintaining the activity of complex I (13). In the present study, NDUFS3 content in myotube mitochondria was not altered by co-culture or by CAST, CAPT and CC treatment, indicating that the decreased complex I activity was not due to altered NDUFS3 content. It has been reported that complex I activity can be damaged by a conformational change (induced by a sulfhydryl oxidation of the second cysteine residue in complex I) (33). Therefore, the decreased complex I activity upon co-culture in the current study may be due to this post-translational modification, although this requires further investigation.
The MPTP is a non-selective pore located on the membrane of the mitochondria (34). MPTP opening increases the permeability of the mitochondrial membrane and leads to the loss of Δψm and to mitochondrial injury, which in turn causes CCMA (27). In mouse hearts, calpain inhibitors attenuate the ischemia-reperfusion and induce MPTP opening and Δψm depolarization (20). Additionally, calpain inhibitors ameliorate the microcystin-LR-induced MPTP opening and Δψm depolarization in cultured hepatocytes (35), suggesting that calpain activation contributes to MPTP opening and Δψm depolarization. Consistent with the aforementioned findings, the results of the present study demonstrated that co-culture activated calpain in myotube mitochondria, causing MPTP opening and Δψm depolarization. By contrast, administration of CAST, CAPT or CC ameliorated these changes, suggesting that the activation of mitochondrial calpain induces MPTP opening and Δψm depolarization. Furthermore, co-culture decreased the CYCD content in myotube mitochondria, whereas treatment with calpain inhibitors improved CYCD content, suggesting that the activation of mitochondrial calpain may increase MPTP opening through the cleavage of CYCD. However, no cleaved band of CYCD was detected in myotubes.
AIF is a mitochondrial flavoprotein that functions as an antioxidant in the mitochondrial intermembrane space (11). Activation of mitochondrial calpain cleaves AIF to truncated AIF (t-AIF) and releases t-AIF from the mitochondria to the cytoplasm, allowing it to translocate to the nucleus, inducing DNA degradation and apoptosis (13,36). In the present study, co-culture decreased AIF content in myotube mitochondria, which was then reversed by calpain inhibitors, suggesting that activation of mitochondrial calpain induced the loss of AIF in the mitochondria. However, t-AIF was not detected in myotubes in the present study, suggesting that co-culture may induce myotube apoptosis via other mechanisms, which should be further investigated in future studies.
AKT/mTOR and FoxO3a serve a critical role in regulating muscular protein synthesis and proteolysis (26). AKT phosphorylates mTOR to increase its activity and promote protein synthesis (37). Activated mTOR in turn phosphorylates 4EBP1 (a negative regulator of the eukaryotic translation initiation factor 4E) to inhibit its activity (38). Additionally, AKT phosphorylates FoxO3a, inhibiting its activation and nuclear entrance, which decreases the expression levels of atrogin-1 and MuRF1 (6,26). In the present study, co-culture decreased AKT and mTOR activity and increased FoxO3a activity and atrogin-1 content in myotubes, whereas calpain inhibitors ameliorated these changes, suggesting that the activation of cytosolic calpain acted through AKT/mTOR and FoxO3a/atrogin-1 to disrupt muscular protein metabolism.
Activation of autophagy contributes to muscle wasting (7,39). Therefore, future studies should investigate whether the autophagy-lysosome system may be involved in the atrophy of myotubes.
To the best of our knowledge, calpain-1, calpain-2 and CAST were for the first time demonstrated to be present in mouse myotube mitochondria in the present study. Calpain inhibitors protected the myotubes during co-culture by inhibiting both cytosolic and mitochondrial calpain activity. CAST is an endogenous calpain inhibitor, whereas CAPT is a commonly used non-selective and reversible calpain inhibitor; however, they both have off-target effects (9,40,41). Therefore, a genetic approach is required to further clarify the potential role of mitochondrial calpain in CCMA. Investigation of mitochondrial calpain will provide new insights to understand the mechanism of CCMA. The present results will further help to develop focused approaches to attenuate CCMA by manipulating the mitochondrial and cytosolic calpain activity.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- 4EBP1
eukaryotic translation initiation factor 4E-binding protein 1
- AIF
apoptosis-inducing factor
- CAPT
calpeptin
- CAST
calpastatin
- CCMA
cancer cachectic muscle atrophy
- CYCD
cyclophilin D
- MHC
myosin heavy chain
- MPTP
mitochondrial permeability transition pore
- Δψm
mitochondrial membrane potential
Funding
The present was supported by Joint Funds for The Innovation of Science and Technology, Fujian province (grant no. 2018Y9083).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XZ drafted the main manuscript and performed the main experiments. XL and SC conceived and designed the experiments. LZ helped with cell culture, western blotting and immunofluorescence staining experiments, and was responsible for analyzing the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 2.Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: Mechanisms and clinical implications. Gastroenterol Res Pract. 2011;2011:601434. doi: 10.1155/2011/601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida T, Semprun-Prieto L, Sukhanov S, Delafontaine P. IGF-1 prevents ANG II-induced skeletal muscle atrophy via Akt- and Foxo-dependent inhibition of the ubiquitin ligase atrogin-1 expression. Am J Physiol Heart Circ Physiol. 2010;298:H1565–H1570. doi: 10.1152/ajpheart.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin XY, Chen SZ. Calpain inhibitors ameliorate muscle wasting in a cachectic mouse model bearing CT26 colorectal adenocarcinoma. Oncol Rep. 2017;37:1601–1610. doi: 10.3892/or.2017.5396. [DOI] [PubMed] [Google Scholar]
- 5.Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int J Biochem Cell Biol. 2013;45:2215–2229. doi: 10.1016/j.biocel.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Tsubouchi H, Yanagi S, Miura A, Matsumoto N, Kangawa K, Nakazato M. Ghrelin relieves cancer cachexia associated with the development of lung adenocarcinoma in mice. Eur J Pharmacol. 2014;743:1–10. doi: 10.1016/j.ejphar.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Penna F, Costamagna D, Pin F, Camperi A, Fanzani A, Chiarpotto EM, Cavallini G, Bonelli G, Baccino FM, Costelli P. Autophagic degradation contributes to muscle wasting in cancer cachexia. Am J Pathol. 2013;182:1367–1378. doi: 10.1016/j.ajpath.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Goll DE, Neti G, Mares SW, Thompson VF. Myofibrillar protein turnover: The proteasome and the calpains. J Anim Sci. 2008;86(Suppl):E19–E35. doi: 10.2527/jas.2007-0395. [DOI] [PubMed] [Google Scholar]
- 9.Dókus LE, Yousef M, Bánóczi Z. Modulators of calpain activity: Inhibitors and activators as potential drugs. Expert Opin Drug Discov. 2020;15:471–486. doi: 10.1080/17460441.2020.1722638. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Zhu X. The molecular mechanisms of calpains action on skeletal muscle atrophy. Physiol Res. 2016;65:547–560. doi: 10.33549/physiolres.933087. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki T, Tomita H, Tamai M, Ishiguro S. Characteristics of mitochondrial calpains. J Biochem. 2007;142:365–376. doi: 10.1093/jb/mvm143. [DOI] [PubMed] [Google Scholar]
- 12.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Thompson J, Hu Y, Dean J, Lesnefsky EJ. Inhibition of the ubiquitous calpains protects complex I activity and enables improved mitophagy in the heart following ischemia-reperfusion. Am J Physiol Cell Physiol. 2019;317:C910–C921. doi: 10.1152/ajpcell.00190.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kar P, Chakraborti T, Roy S, Choudhury R, Chakraborti S. Identification of calpastatin and mu-calpain and studies of their association in pulmonary smooth muscle mitochondria. Arch Biochem Biophys. 2007;466:290–299. doi: 10.1016/j.abb.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 16.Zeng X, Chen S, Yang Y, Ke Z. Acylated and unacylated ghrelin inhibit atrophy in myotubes co-cultured with colon carcinoma cells. Oncotarget. 2017;8:72872–72885. doi: 10.18632/oncotarget.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–C1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XL, Wang ZZ, Shao QH, Zhang Z, Li L, Guo ZY, Sun HM, Zhang Y, Chen NH. RNAi-mediated knockdown of DJ-1 leads to mitochondrial dysfunction via Akt/GSK-3β and JNK signaling pathways in dopaminergic neuron-like cells. Brain Res Bull. 2019;146:228–236. doi: 10.1016/j.brainresbull.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Zeng X, Chen S, Lin Y, Ke Z. Acylated and unacylated ghrelin inhibit apoptosis in myoblasts cocultured with colon carcinoma cells. Oncol Rep. 2018;39:1387–1395. doi: 10.3892/or.2018.6213. [DOI] [PubMed] [Google Scholar]
- 20.Thompson J, Hu Y, Lesnefsky EJ, Chen Q. Activation of mitochondrial calpain and increased cardiac injury: Beyond AIF release. Am J Physiol Heart Circ Physiol. 2016;310:H376–H384. doi: 10.1152/ajpheart.00748.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendriks KDW, Joschko CP, Hoogstra-Berends F, Heegsma J, Faber KN, Henning RH. Hibernator-derived cells show superior protection and survival in hypothermia compared to non-hibernator cells. Int J Mol Sci. 2020;9:1864. doi: 10.3390/ijms21051864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudo-Sakamoto Y, Akazawa H, Ito K, Takano J, Yano M, Yabumoto C, Naito AT, Oka T, Lee JK, Sakata Y, et al. Calpain-dependent cleavage of N-cadherin is involved in the progression of post-myocardial infarction remodeling. J Biol Chem. 2014;289:19408–19419. doi: 10.1074/jbc.M114.567206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fakharnia F, Khodagholi F, Dargahi L, Ahmadiani A. Prevention of cyclophilin D-mediated mPTP opening using cyclosporine-A alleviates the elevation of necroptosis, autophagy and apoptosis-related markers following global cerebral ischemia-reperfusion. J Mol Neurosci. 2017;61:52–60. doi: 10.1007/s12031-016-0843-3. [DOI] [PubMed] [Google Scholar]
- 24.Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, Clark RS, Graham SH, Chen J. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polster BM, Basañez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280:6447–6454. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- 26.Clavel S, Siffroi-Fernandez S, Coldefy AS, Boulukos K, Pisani DF, Dérijard B. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol Cell Biol. 2010;30:470–480. doi: 10.1128/MCB.00666-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: Understanding the molecular basis. Nat Rev Cancer. 2014;14:754–762. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 28.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Lokireddy S, Wijesoma I, Bonala S, Wei M, Sze S, McFarlane C, Kambadur R, Sharma M. Retraction: Myostatin is a novel tumoral factor that induces cancer cachexia. Biochem J. 2016;473:1111. doi: 10.1042/BJ4731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun R, Zhang S, Hu W, Lu X, Lou N, Yang Z, Chen S, Zhang X, Yang H. Valproic acid attenuates skeletal muscle wasting by inhibiting C/EBPβ-regulated atrogin1 expression in cancer cachexia. Am J Physiol Cell Physiol. 2016;311:C101–C115. doi: 10.1152/ajpcell.00344.2015. [DOI] [PubMed] [Google Scholar]
- 31.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC, Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18:239–250. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollander JM, Thapa D, Shepherd DL. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: Influence of cardiac pathologies. Am J Physiol Heart Circ Physiol. 2014;307:H1–H14. doi: 10.1152/ajpheart.00747.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galkin A, Abramov AY, Frakich N, Duchen MR, Moncada S. Lack of oxygen deactivates mitochondrial complex I: Implications for ischemic injury? J Biol Chem. 2009;284:36055–36061. doi: 10.1074/jbc.M109.054346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Ding WX, Shen HM, Ong CN. Calpain activation after mitochondrial permeability transition in microcystin-induced cell death in rat hepatocytes. Biochem Biophys Res Commun. 2002;291:321–331. doi: 10.1006/bbrc.2002.6453. [DOI] [PubMed] [Google Scholar]
- 36.Ye H, Cande C, Stephanou NC, Jiang S, Gurbuxani S, Larochette N, Daugas E, Garrido C, Kroemer G, Wu H. DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat Struct Biol. 2002;9:680–684. doi: 10.1038/nsb836. [DOI] [PubMed] [Google Scholar]
- 37.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 39.Tardif N, Klaude M, Lundell L, Thorell A, Rooyackers O. Autophagic-lysosomal pathway is the main proteolytic system modified in the skeletal muscle of esophageal cancer patients. Am J Clin Nutr. 2013;98:1485–1492. doi: 10.3945/ajcn.113.063859. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida M, Miyasaka Y, Ohuchida K, Okumura T, Zheng B, Torata N, Fujita H, Nabae T, Manabe T, Shimamoto M, et al. Calpain inhibitor calpeptin suppresses pancreatic cancer by disrupting cancer-stromal interactions in a mouse xenograft model. Cancer Sci. 2016;107:1443–1452. doi: 10.1111/cas.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsujinaka T, Kajiwara Y, Kambayashi J, Sakon M, Higuchi N, Tanaka T, Mori T. Synthesis of a new cell penetrating calpain inhibitor (calpeptin) Biochem Biophys Res Commun. 1988;153:1201–1208. doi: 10.1016/S0006-291X(88)81355-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.