Abstract
Objective:
Absent or truncated dystrophin in Duchenne (DMD) and Becker (BMD) muscular dystrophies results in impaired vasodilatory pathways and exercise induced muscle ischemia. Here, we used power Doppler sonography to quantify changes in intramuscular blood flow immediately following exercise in boys with D/BMD
Method:
We quantified changes in intramuscular blood flow following exercise using power Doppler sonography in 14 boys with D/BMD and compared changes in muscle blood flow to disease severity and to historic controls.
Result:
Post exercise blood flow change in the anterior forearm muscles is lower in 1) DMD (median 0.25%; range −0.47 – 2.19%) than BMD (2.46%; 2.02 – 3.38%, p < 0.05) and historical controls (6.59%; 2.16 – 12.40%, p<0.01); 2) in non-ambulatory (0.04%; −0.47 – 0.10%) than ambulatory DMD boys (0.71%; 0.07 – 2.19%, p < 0.05); and 3) in muscle with higher echointensity (rs= −0.7253, p=0.005). The tibialis anterior showed similar findings. We estimate that a single sample clinical trial would require 19 subjects to detect a doubling of blood flow to the anterior forearm after the intervention.
Conclusion:
Post-exercise blood flow is reduced in D/BMD and relates to disease severity.
Significance:
Our protocol for quantifying post-exercise intramuscular blood flow is feasible for clinical trials in D/BMD.
Keywords: Muscle, blood flow, ultrasound, Doppler, dystrophy, exercise
Introduction
Duchenne and Becker Muscular dystrophy (D/BMD) occur in approximately 1:5000 boys (Mendell et al. , 2012) and are caused by mutations in the X-linked dystrophin gene that results in progressive weakness. Becker muscular dystrophy differs from the more severe Duchenne muscular dystrophy due to an in-frame mutation that preserves a truncated, partially functional dystrophin protein (Hoffman et al. , 1988). Absent or dysfunctional dystrophin results in failure of the membrane stabilizing dystroglycan complex and causes disruption of the neuronal nitric oxide synthase (nNOS) vasodilatory signally pathway. nNOS typically mediates pathways that result in vasodilatation following exercise to ensure adequate blood flow to the exercised muscle. In D/BMD, this pathway is impaired (Sander et al. , 2000, Thomas, 2013, Kodippili et al. , 2018). As a result, boys and men with D/BMD suffer from exercise induced, or functional, muscle ischemia. Rescue of the nNOS pathway and repair of the functional muscle ischemia is a potential therapeutic target in treatment of D/BMD. Both animal and human clinical trials in dystrophinopathies have shown that muscle blood flow can be restored following therapeutic correction of the impaired nNOS pathway (Thomas et al. , 2012, Zhang et al. , 2013, Nelson et al. , 2014, Nelson et al. , 2015). A Phase 3 clinical trial of once daily tadalafil, a phosphodiesterase type 5 inhibitor that boosts defective nNOS signaling pathways in skeletal muscle microvessels, showed no effect on the decline in the six minute walk distance in ambulatory boys with DMD compared to placebo over 48 weeks but did show slowed loss of upper extremity function in boys > 10 years of age. (Victor et al. , 2017). In human trials, blood flow to muscles is typically measured indirectly, either through color Doppler evaluation of the brachial artery or through assessment of muscle oxygenation using infrared spectroscopy (Allart et al. , 2012, Martin et al. , 2012). Ultrasound can be used to measure intramuscular blood flow directly but this has required intravenous contrast agents and a rigorously controlled, research protocol that limits its application at the bedside or clinic (Womack et al. , 2009).
We previously reported a novel technique to quantify changes in intramuscular blood flow directly following muscle contraction, i.e., post-contraction hyperemia, using power Doppler that does not require intravenous contrast (Dori et al. , 2016). In healthy controls, intramuscular blood measured using power Doppler increases proportionally to the strength of contraction and reaches a maximal plateau after one minute of exercise with brief, repeated full effort muscle contractions (Dori et al. , 2016). In this study, we measured changes in intramuscular blood flow following repeated muscle contraction at maximal effort in boys with Duchenne and Becker muscular dystrophy. We compared those changes to markers of disease severity including the echointensity (grayscale) level in the muscle, ambulatory status, and by comparing DMD to BMD boys. For reference, we compared intramuscular blood flow in D/BMD to historic healthy controls from our previous study. Our results show that exercise-induced intramuscular blood flow is reduced in D/BMD and relates to disease severity.
Methods
We performed ultrasound of anterior forearm and tibialis anterior (TA) muscles in 11 patients with Duchenne and 3 with Becker muscular dystrophy (dystrophin exons 3–5 and 45–48 deletions) recruited from our neuromuscular clinic at Washington University in St. Louis. This study was approved by the Washington University Institutional Review Board. We obtained informed consent from parents/guardians and assent from all minor participants. AD or CMZ performed the ultrasound evaluation using a Phillips IU22 machine with an L12–5 MHz linear transducer. We performed the ultrasound exam consistent with the previously published protocol and using the same ultrasound system settings and equipment as in the previous study (Dori et al. , 2016). All settings were fixed and remained constant for each exam. We visualized the anterior forearm muscle group (AF) ¼ of the distance from the elbow to the wrist and the TA 1/3 of the distance from the knee to the lateral malleolus of the ankle. The forearm was placed in supination and the fingers and ankle relaxed in neutral position. If possible, patients were placed in a supine position. In non-ambulatory patients, we performed this ultrasound exam in patients in a seated position with the arm supported on a pillow at the level of the heart and the TA with the knee bent at approximately 90 degrees. In boys with severe leg weakness and no dorsiflexion movement at the ankle, we did not perform the examination of the TA.
We performed two types of image quantification on the examined muscles. We measured the average grayscale level from a single still image obtained using B-mode imaging and fixed, standardized settings as per the published protocol (Dori et al. , 2016). We then measured intramuscular blood flow using power Doppler following the previously described protocol (Dori et al. , 2016) before and after a one minute exercise with repeated, maximal voluntary contractions as follows. Patients rested for at least one minute prior to the evaluation. The patient then maximally and rapidly contracted and relaxed the muscle every five seconds for a total of 60 seconds (12 cycles). We provided verbal encouragement to ensure maximal effort. We recorded a 2 minute video of the power Doppler of the muscle including baseline (rest), during 60 seconds of repeated contraction exercise, and following exercise. We took care to minimize transducer pressure on the patient by using copious amounts of ultrasound gel and maintaining a very light touch with the ultrasound transducer on the patient.
We quantified the average grayscale level and power Doppler signal offline using ImageJ version 1.49 (NIH, Bethesda, MD). The target muscle was selected using the region of interest tool including all of the well visualized portions of the muscle/muscle group of interest and avoiding bone or edge artifact, if present. We used the same region of interest for both quantification of the grayscale level and intramuscular blood flow. We measured the mean grayscale of the muscle within the region of interest from the still image of the muscle at baseline. We also measured the amount of intramuscular blood flow detected using power Doppler as previously described (Dori et al. , 2016). Briefly, we selected only the red colored pixels (indicative of blood flow on power Doppler) within the image and measured the percent area of the region of interest occupied by the colored pixels. We applied this procedure to the entire video and exported and graphed results using Microsoft Excel. We then averaged five blood flow measurements at diastole, identified visually as the nadir of the pulsatile blood flow oscillations, before and immediately following 1 minute of exercise. Finally, we calculated the difference between the pre- and post-exercise blood flow. 60 seconds of repetitive maximal muscle contraction/exercise is sufficient to achieve the maximal increase in intramuscular blood flow (Dori et al. , 2016).
We compared patients with DMD to BMD and, for reference, to a group of historical controls published in our previous report (Dori et al. , 2016) using the Mann-Whitney U test (vassarstats.net accessed April and September 2017). We also compared changes in intramuscular blood flow following exercise to the muscle grayscale level and to age using a Spearman’s rank correlation. We performed a sample size calculation for a theoretical clinical trial using G*Power (version 3.0.10).
The historical controls used for this study are previously described in detail (Dori et al. , 2016). In brief, 8 adults (ages 26–57 years; 4 men) and 3 children (ages 6, 8, and 10) without any known diabetes, neuromuscular disease, or vascular disease underwent the exercise protocol described above with ultrasound quantification of intramuscular blood flow.
Results
We enrolled 14 boys with median age of 11 years, 11 boys had DMD (ages 5–17 years) and 3 with BMD (ages 10,12, and 13 years). Eight of 11 (73%) DMD and all (3/3) BMD boys were ambulatory at the time of ultrasound evaluation. All boys with DMD were taking steroids, 7 were taking lisinopril, and 5 were receiving eteplirsen. Dystrophin gene mutations in the boys with DMD were deletions of exon 42, 44, 52, 45–50, 48–50, 46–47, 49–50 (three boys), and nonsense mutations in exon 56 and 70. Two boys with BMD had deletions of exon 3–5 and one had deletion of exon 45–48.
We evaluated 13 anterior forearms and 10 tibialis anterior muscles. We did not image the TA in three non-ambulatory boys with DMD and in the youngest boy with DMD due to inability to complete the exercise portion of the protocol. A technical error resulted in loss of images from one patient’s anterior forearm muscles.
In D/BMD, post-exercise hyperemia is decreased and relates to disease severity. Change in anterior forearm blood flow following exercise was lower in DMD (median 0.25%; range −0.47 – 2.19%) than in BMD (2.46%; 2.02 – 3.38%, p < 0.05) and was lower in both DMD (p<0.01) and BMD (p<0.05) than historical controls (6.59%; 2.16 – 12.40%) (FIGURE 1). Change in blood flow in the TA was also lower in DMD (0.78%; 0.13 – 2.42%) than BMD (5.08%; 2.28 – 9.51%) (p < 0.05) and historical controls (6.35%; 3.91 – 14.17%, p < 0.01) (FIGURE 1 and 2). In DMD boys, change in blood flow in the anterior forearm was lower in non-ambulatory (0.04%; −0.47 – 0.10%) than ambulatory DMD boys (0.71%; 0.07 – 2.19%) (p < 0.05). In boys with D/BMD, the change in blood flow was lower with increased grayscale level in the anterior forearm (rs= −0.73, p=0.005, Figure 3) and showed a non-significant trend in the TA (rs= −0.6, p=0.06). In boys with similar muscle greyscale levels, the change in blood flow was reduced in the boys with DMD compared to those with BMD (Figure 2). Intramuscular blood flow did not vary with age in either the anterior forearm (rs= −0.1, p=0.7) or TA (rs= −0.3, p=0.3) in boys with D/BMD.
Figure 1: Post-Exercise blood flow in Dystrophinopathy and History Healthy Controls.
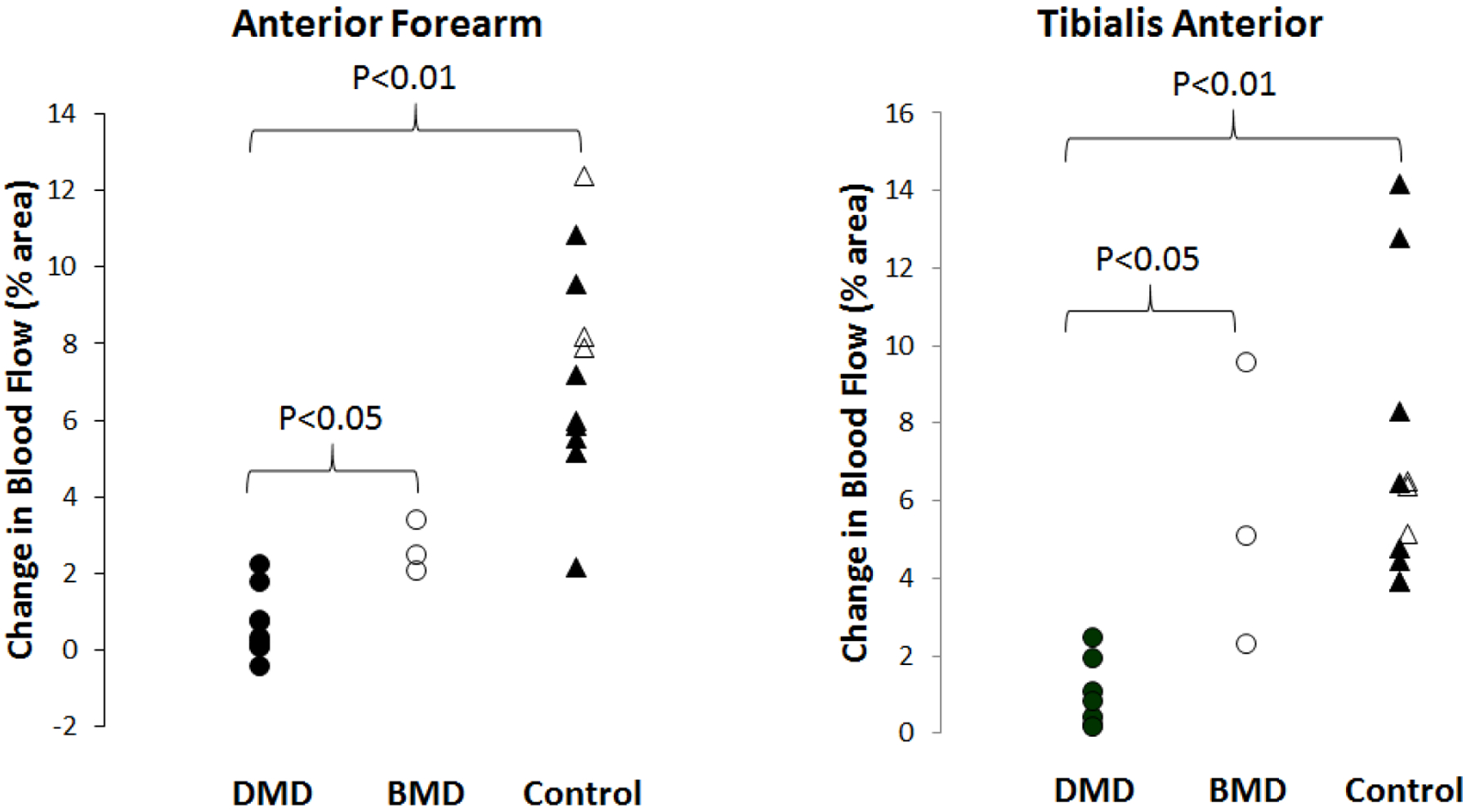
Post-exercise blood flow is severely reduced in DMD compared to the less severely affected BMD and compared to healthy controls, including three children ages 6, 8, and 10 years (open triangles) in both the anterior forearm and tibialis anterior.
Figure 2: Post exercise blood flow in the Tibialis Anterior.
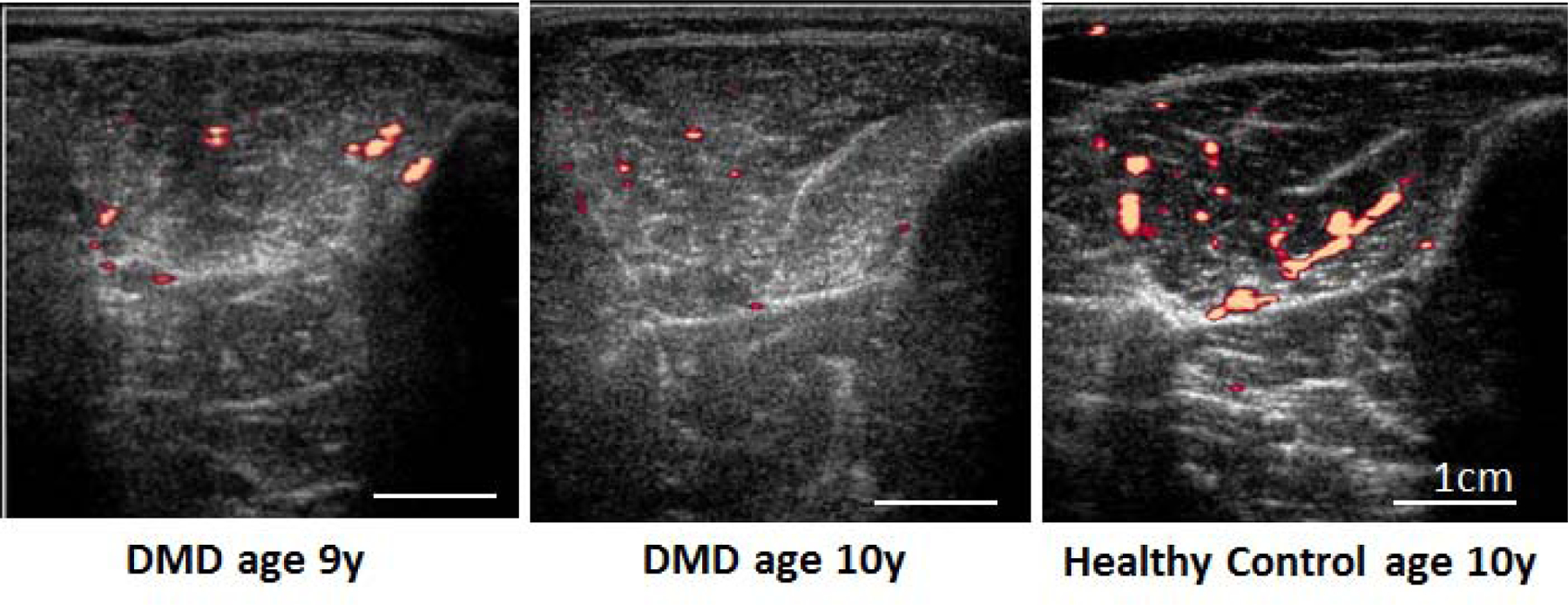
Power Doppler images of the tibialis anterior show reduced blood flow after exercise in a 9 and 10 year old boy with DMD compared to a healthy 10 year old boy.
Figure 3. Intramuscular blood flow compared to gray scale level.
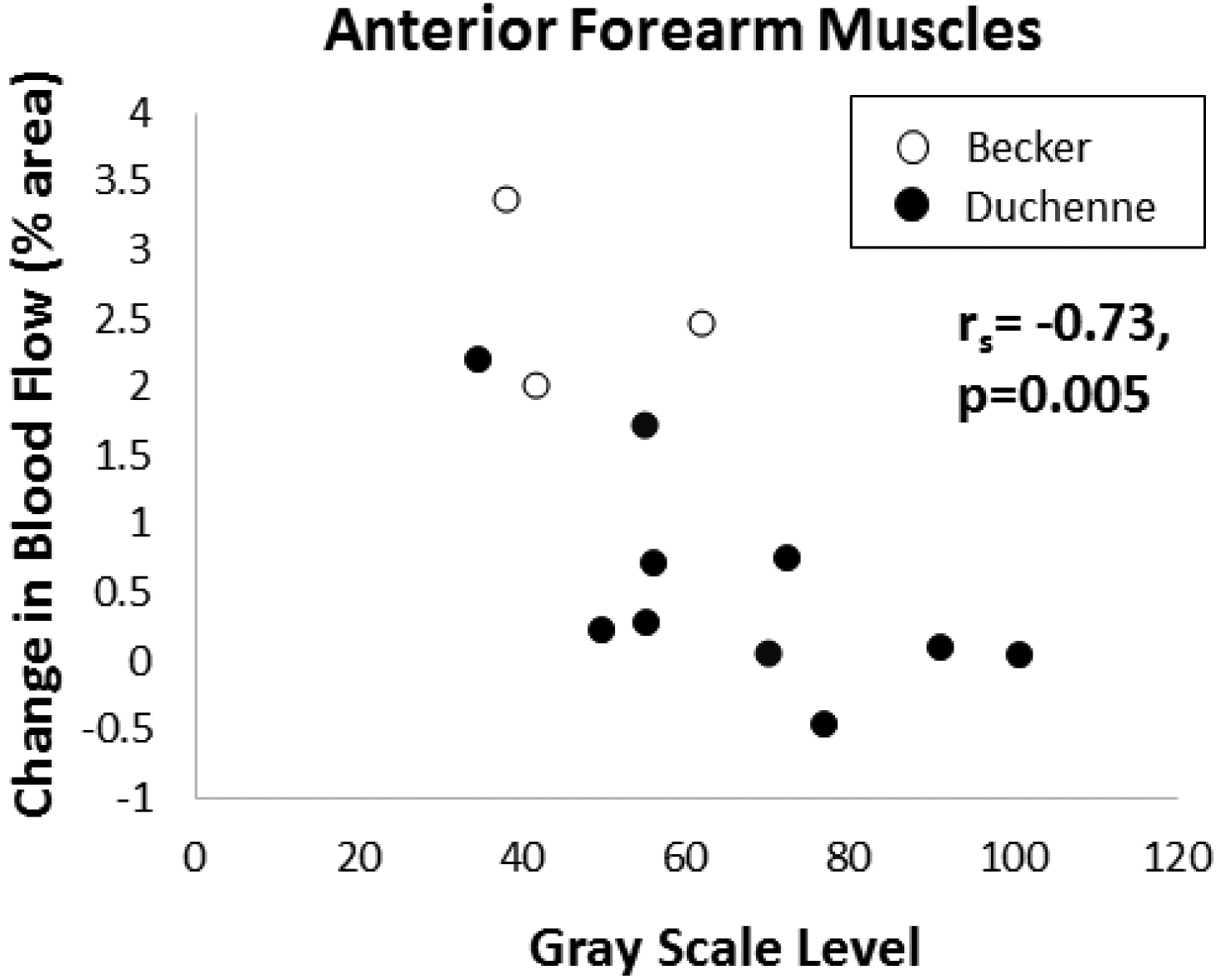
Post-exercise blood flow is more reduced in dystrophic anterior forearm muscles with worse (higher) grayscale echointensity.
For purposes of sample size calculations, we calculated the mean (0.56%) and standard deviation (−0.82) change in anterior forearm blood flow in boys with DMD. Based on this, a single sample clinical trial would require 19 boys with DMD to detect a doubling of blood flow to the anterior forearm after the intervention (80% power, alpha 0.025, one tailed t-test).
Discussion
Ultrasound is an attractive means for assessing intramuscular blood flow as it is non-invasive, painless, uses no radiation, and can be performed using commercially available ultrasound systems and software. In this study, we used power Doppler sonography to quantify changes in intramuscular blood flow immediately following exercise in boys with D/BMD using a protocol that can be performed at the bedside and that is appropriate for use in children. We found that in D/BMD, intramuscular blood flow is reduced compared to controls and is lower in more severe disease as measured by ambulatory status and by worsening muscle grayscale level (Zaidman et al. , 2010, Shklyar et al. , 2015, Zaidman et al. , 2015, Zaidman et al. , 2017). Intramuscular blood flow is also lower in the more severely affected boys with DMD than BMD. Reductions in intramuscular blood flow related more to worse disease severity than increased age. Reduced post-exercise intramuscular blood flow in dystrophic muscle may be due to abnormal nNOS pathway vascular control secondary to dystrophin absence or truncation or may be due to lower muscle contraction strength in D/BMD patients which may reduce activation of the nNOS pathway. Two of our boys with BMD had exon 3–5 deletion, which is predicted to preserve the dystrophin rod domain needed for the sarcolemmal localization of nNOS (Lai et al. , 2009, Anthony et al. , 2011). Additional studies will need to determine the biochemical changes that govern the reduced blood flow in boys with D/BMD, and how intramuscular blood flow measured using power Doppler is affected by treatments such as corticosteroids.
Our protocol for quantifying post-exercise intramuscular blood flow is feasible for clinical trials in children and adults. Our protocol can be performed at the bedside using standard ultrasound equipment, requires only one minute of repeated maximal contraction per muscle, and does not require intravenous contrast agents or rigorous control of the degree of muscle contraction. We have shown that intramuscular blood flow, measured using our protocol, performs as expected: post-contraction intramuscular blood flow increases proportionally with effort and reaches a plateau during exercise in healthy controls (Dori et al. , 2016), and in D/BMD, the exercise induced increase in intramuscular blood flow is reduced compared to healthy subjects and is most reduced in more severely affected patients. We estimate that 19 boys with DMD would be required in a single sample trial to detect a therapeutic response in post-exercise intramuscular blood flow from 0.56% to 1.12%. This effect size is reasonable as it is still lower than the lowest post-exercise increase in intramuscular blood flow seen in normal controls (2.16%) and in BMD (2.02%). Thus, our protocol could be used in future prospective studies to assess the efficacy of medications for improving intramuscular blood flow in Duchenne muscular dystrophy. A limitation of our protocol is that intramuscular blood volume cannot be calculated, as is possible in protocols that employ contrast enhanced ultrasound (Womack et al. , 2009, Blackwood et al. , 2017). Power Doppler signal may also be reduced by technical factors that often co-exist in patients with muscular dystrophy including attenuation of the ultrasound signal/noise cancellation in muscles with very high echointensity. It is unlikely that our findings are due only to increased attenuation/noise cancelation as we found reduced post-exercise blood flow in boys with DMD compared to boys with BMD at similar grey scale values. Additional studies are needed to determine how this protocol for measuring post-exercise intramuscular blood flow performs in other disease conditions where functional muscle ischemia is a suspected mechanism of pathophysiology (Clerk et al. , 2007) and in other neuromuscular conditions without nNOS pathway abnormalities.
This study does have several additional limitations. First, this study does not assess how intramuscular blood flow relates to treatment. Our cross sectional analysis of children with D/BMD, some of whom were taking eteplirsen and /or ACE inhibitor therapy, is unable to assess how these medications affect intramuscular blood flow. A prospective study assessing blood flow before and after administration of medication could determine how therapeutic interventions affect intramuscular blood flow. Prior studies of blood flow in large arterial vessels in boys with DMD have shown that tadalafil is able to increase large vessel arterial flow (Martin et al. , 2012, Nelson et al. , 2014) although this therapy did not ultimately improve function in a phase 3 clinical trial in DMD (Victor et al. , 2017). Second, the sample sizes in our study are small. This may have reduced our power to detect small effects and may explain why some significant differences were seen in the anterior forearm but not in the TA. Finally, we compared children with D/BMD to a control sample that included both children and adults. We feel this is justified as exercise induced increases in intramuscular blood flow is similar between healthy children and adults (Dori et al. , 2016) and did not vary with age in our sample of boys with D/BMD. Larger studies including a wide range of healthy children and adults are required to best understand what factors affect intramuscular blood flow in healthy people.
Highlights.
Post-exercise intramuscular blood flow is reduced in boys with Duchenne muscular. dystrophy (DMD).
Reductions in intramuscular blood flow in Duchenne/Becker MD relate to disease. severity.
Quantifying post-exercise intramuscular blood flow is feasible for clinical trials in DMD.
Acknowledgments
The study was supported by the Washington University in St. Louis Neuromuscular Research Fund, the National Institute of Health Neurological Sciences Academic Development Award (K12 NS00169009) and the Institute of Clinical and Translational Sciences (UL1 TR000448).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
References
- Allart E, Olivier N, Hovart H, Thevenon A, Tiffreau V. Evaluation of muscle oxygenation by near-infrared spectroscopy in patients with Becker muscular dystrophy. Neuromuscul Disord. 2012;22:720–7. [DOI] [PubMed] [Google Scholar]
- Anthony K, Cirak S, Torelli S, Tasca G, Feng L, Arechavala-Gomeza V, et al. Dystrophin quantification and clinical correlations in Becker muscular dystrophy: implications for clinical trials. Brain. 2011;134:3547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood SJ, Dwyer RM, Bradley EA, Keske MA, Richards SM, Rattigan S. Determination of Skeletal Muscle Microvascular Flowmotion with Contrast-Enhanced Ultrasound. Ultrasound Med Biol. 2017;43:2013–23. [DOI] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Barrett EJ, Lankford MF, Lindner JR. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensin-converting enzyme inhibition. Am J Physiol Endocrinol Metab. 2007;293:E1804–9. [DOI] [PubMed] [Google Scholar]
- Dori A, Abbasi H, Zaidman CM. Intramuscular blood flow quantification with power doppler ultrasonography. Muscle Nerve. 2016;54:872–8. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Fischbeck KH, Brown RH, Johnson M, Medori R, Loike JD, et al. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne’s or Becker’s muscular dystrophy. N Engl J Med. 1988;318:1363–8. [DOI] [PubMed] [Google Scholar]
- Kodippili K, Hakim CH, Yang HT, Pan X, Yang NN, Laughlin MH, et al. Nitric oxide-dependent attenuation of noradrenaline-induced vasoconstriction is impaired in the canine model of Duchenne muscular dystrophy. J Physiol. 2018;596:5199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119:624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EA, Barresi R, Byrne BJ, Tsimerinov EI, Scott BL, Walker AE, et al. Tadalafil alleviates muscle ischemia in patients with Becker muscular dystrophy. Sci Transl Med. 2012;4:162ra55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71:304–13. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Rader F, Tang X, Tavyev J, Nelson SF, Miceli MC, et al. PDE5 inhibition alleviates functional muscle ischemia in boys with Duchenne muscular dystrophy. Neurology. 2014;82:2085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Rosenberry R, Barresi R, Tsimerinov EI, Rader F, Tang X, et al. Sodium nitrate alleviates functional muscle ischaemia in patients with Becker muscular dystrophy. J Physiol. 2015;593:5183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, et al. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2000;97:13818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklyar I, Geisbush TR, Mijialovic AS, Pasternak A, Darras BT, Wu JS, et al. Quantitative muscle ultrasound in Duchenne muscular dystrophy: a comparison of techniques. Muscle Nerve. 2015;51:207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD. Functional muscle ischemia in Duchenne and Becker muscular dystrophy. Front Physiol. 2013;4:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Ye J, De Nardi C, Monopoli A, Ongini E, Victor RG. Treatment with a nitric oxide-donating NSAID alleviates functional muscle ischemia in the mouse model of Duchenne muscular dystrophy. PLoS One. 2012;7:e49350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Sweeney HL, Finkel R, McDonald CM, Byrne B, Eagle M, et al. A phase 3 randomized placebo-controlled trial of tadalafil for Duchenne muscular dystrophy. Neurology. 2017;89:1811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol. 2009;53:2175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidman CM, Connolly AM, Malkus EC, Florence JM, Pestronk A. Quantitative ultrasound using backscatter analysis in Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2010;20:805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidman CM, Malkus EC, Connolly AM. Muscle ultrasound quantifies disease progression over time in infants and young boys with duchenne muscular dystrophy. Muscle Nerve. 2015;52:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidman CM, Wu JS, Kapur K, Pasternak A, Madabusi L, Yim S, et al. Quantitative muscle ultrasound detects disease progression in Duchenne muscular dystrophy. Ann Neurol. 2017;81:633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yue Y, Li L, Hakim CH, Zhang K, Thomas GD, et al. Dual AAV therapy ameliorates exercise-induced muscle injury and functional ischemia in murine models of Duchenne muscular dystrophy. Hum Mol Genet. 2013;22:3720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


