Abstract
Background
The long-term physiological consequences of SARS-CoV-2 (severe acute respiratory syndrome coronavirus) infection are not known. The ability of COVID-19 to cause chronic illness, sarcopenia, and physical deconditioning may be underestimated and go beyond the anticipated respiratory sequelae. Myalgia, lethargy, and anorexia are common symptoms even in mild to moderate cases and have the potential to exacerbate frailty. How this impacts on risk-stratification for patients requiring surgery for time-critical conditions, such as malignancy, requires further urgent investigation.
Main body
The deleterious effect of sarcopenia and poor physical capacity are well recognised in cancer surgery. This review commentary highlights current evidence which suggests skeletal muscle as an under recognised cause of COVID-19-related functional deconditioning. The mechanisms behind this are via direct (viral induced myositis, nutritional decline, cytokine-mediated myopathy) and indirect mechanisms (social isolation, inactivity, and psychological consequences).
Conclusion
Further mechanistic research is required to explore the processes behind the deconditioning effects of SARS-CoV-2 infection and how this impacts on treatment of malignant disease.
Keywords: COVID-19, SARS-CoV-2, Functional deconditioning, Sarcopenia, Frailty, Prehabilitation
Main text
High-quality clinical trials are making great strides in the treatment of acute SARS-CoV-2 infection whilst the research world adjusts its focus on long-term consequences of the disease. The term ‘long COVID’ is gaining popularity in the literature and denotes a multisystem deconditioning with symptoms lasting weeks if not months. Surgical teams are encountering increasing numbers of patients who have survived COVID-19. Whilst most patients will fully recover from the illness, some will be left with lasting symptoms and the impact on perioperative risk is currently unknown. Large observational studies have shown myalgia and general weakness is seen in up to 50% of patients which can last for several months [1–6]. Currently, it is unclear why some patients take longer to recover than others but this suggests the ability of SARS-CoV-2 to cause chronic illness and physical deconditioning may go beyond the anticipated respiratory sequelae. The impact of sarcopenia and poor physical function is well described in patients requiring cancer surgery resulting in increased mortality and morbidity [7]. Assessment tools to assess the degree of COVID-related deconditioning and aid risk stratification for cancer surgery are required, especially in vulnerable patients with comorbidity and cachexia. This seems increasingly challenging given that conventional pre-operative assessment tools such as cardiopulmonary exercise testing (CPET) and spirometry are discouraged due to their aerosol risk.
Anorexia, anosmia, and weight loss are also common features of SARS-CoV-2 infection and may exacerbate nutritional deficits and muscle wasting already seen in patients with active malignancy. Some patients may never recover to their baseline functional capacity [8–10]. It is clear that those severely affected by COVID-19 with prolonged convalescence should have cancer surgery delayed where possible. Multimodal prehabilitation and rehabilitation may help to avoid lasting morbidity and reduce perioperative risk [11]. Data is lacking, however, on the deconditioning effects for patients with mild or moderate disease who are treated in the community. Any mild illness with even short periods of inactivity could see skeletal muscle loss of 0.5–6% per day [12]. Patients operated on with asymptomatic COVID-19 had increased risk of perioperative morbidity and mortality. Doglietto et al. showed that 30-day risk of mortality was significantly higher 19.5% vs 2.4%; OR 9.5, CI 1.8–96.5) compared to patient without COVID-19 [13]. As surgeons and oncologists across the world adjust pathways to safely offer treatment for malignant disease, the results from the GlobalSurg-CovidSurg Week study are eagerly anticipated. This international collaborative observational study aims to determine the optimal timing for surgery following SARS-CoV-2 infection. Early signals from around 150 patients show that pre-operative diagnosis of SARS-CoV-2 is associated with poorer peri-operative outcomes even in those who were diagnosed several weeks prior to surgery [14]. The underlying reasons for this remain unclear but could be related to sarcopenia and physical deconditioning following COVID-19. The results of their study will better inform surgical decision-making and identify patient groups that will benefit from multimodal rehabilitation (or prehabilitation).
Skeletal muscle as a potential target
Both muscle weakness and myalgia are well recognised in COVID-19 [15]. Studies of hospitalised patients have described biochemical evidence of muscle damage [16]. Several reports of severe rhabdomyolysis, even in the absence of respiratory symptoms, suggests skeletal muscle tissue is not immune from the disease process [17–19]. Virus-induced myositis is seen with other pathogens including SARS-CoV-1 and influenza but is unclear whether immune-mediated injury due to myotoxic cytokines (such as CXCL-10, IFN-ϒ, IL-1β, IL-6, IL-17, and TNFα) or if direct viral infiltration is the predominant pathological process. SARS-CoV-2 viral particles have been detected by electron microscopy within skeletal muscle and cardiac muscle fibres whilst distressing case reports of fatal cardiac failure in children due to fulminant cardiomyopathy suggests the direct impact on muscle tissue can be profound [20]. SARS-CoV-2 enters host cells via the angiotensin-converting enzyme 2 (ACE2) receptor. Both skeletal and cardiac muscle exhibit robust expression on the ACE2 receptor on their surface as do many other tissues offering a potential viral route for direct tissue damage [21, 22]. Clinical parallels can be drawn from the SARS-CoV-1 pandemic in 2003 owing to its genetic and clinicopathological homology with SARS-CoV-2. Extensive myalgia and muscle dysfunction were reported in SARS patients. Widespread muscle fibre atrophy, fibre necrosis, myofibril disarray, and Z-disc streaming was demonstrated in post-mortem muscle tissue [23]. Furthermore, relatively fit SARS survivors were shown to have objective reductions in hand grip strength (32%) and 6-min walk test (13%) when followed up in clinic [24]. Similar functional outcomes measures in survivors of COVID-19 have also been described with measurable reductions in indices of muscle strength and physical function [25]. How long these impairment last for is unknown.
The indirect ability of the viral pandemic to cause physical deconditioning should also be considered. Some experts predict a pandemic-related increase in surgical morbidity and mortality due to social isolation and reduced activity levels in patients, where operations have been rescheduled or postponed [26, 27]. This deleterious effect will be more striking in the elderly or medically frail who also suffer psychological consequences and demotivation. For patients awaiting major cancer surgery, many community prehabilitation programs and local gymnasiums have closed evoking a rapid response to establish at-home exercise programs and telemedicine to minimise the effects of social isolation and pre-operative inactivity.
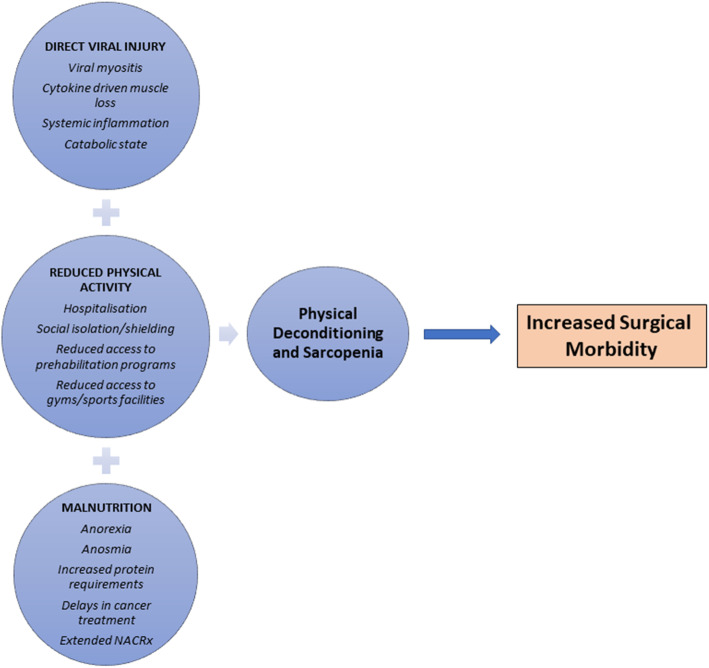
In conclusion, the ability of SARS-CoV-19 to cause physical decline is multifactorial and may be related to periods of convalescence, reduced appetite, chronic cardiorespiratory symptoms, social isolation, and reduced access to physical activity (Fig. 1). The direct and indirect impact on muscle strength, sarcopenia, and frailty may be underestimated and could, in part, explain the poor surgical outcomes seen in patients with previous SARS-CoV-2 infection. Functional outcome scales such as those proposed by Klok et al. may compliment standard assessment tools such as WHO performance status in assessing global functional recovery from the illness but remain unvalidated [28]. Pragmatic approaches to keep physically active prior to cancer surgery, such as home-based exercise programs, will become increasingly important as well as novel rehabilitation techniques for those worse effected by COVID-19 [29]. Going forward, we need a better understanding on how, and to what degree, COVID-19 causes physical deconditioning in mild, moderate, and severe cases alike. Whether this adjusts peri-operative risk for patients requiring cancer surgery requires further investigation to identify best timing for surgery and to tailor focussed prehabilitation for COVID-19 survivors.
Fig. 1.
Schematic of proposed mechanisms causing deterioration in physical function and sarcopenia in patients requiring cancer surgery. Multimodal prehabilitation for COVID survivors may be required to overcome these factors. NACRx, neoadjuvant chemoradiotherapy
Acknowledgements
No specific acknowledgements.
Authors’ contributions
PC—literature review, manuscript production, and revision. YA—literature review, manuscript edit, and revision. JS—review concept, literature review, manuscript edit, and revision. All authors contributed equally and approved the final draft of the manuscript.
Funding
The authors received no funding source in the production of this article.
Availability of data and materials
All data and materials used in the production of this work will be available on request.
Ethics approval and consent to participate
No specific ethical approval required for the production of this review article.
Consent for publication
All contributors give consent for unrestricted publication of this work.
Competing interests
The authors have no competing interests to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Patrick Casey, Email: Patrick.casey@srft.nhs.uk.
Yeng Ang, Email: Yeng.ang@srft.nhs.uk.
Javed Sultan, Email: Javed.sultan@srft.nhs.uk.
References
- 1.Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 2.Nasiri MJ, Haddadi S, Tahvildari A, et al. COVID-19 clinical characteristics, and sex-specific risk of mortality: Systematic Review and Meta-analysis. medRxiv. 2020:2020.03.24.20042903. 10.1101/2020.03.24.20042903. [DOI] [PMC free article] [PubMed]
- 3.Heydari K, Rismantab S, Shamshirian A, et al. Clinical and paraclinical characteristics of covid-19 patients: a systematic review and meta-analysis. medRxiv. 2020:2020.03.26.20044057. 10.1101/2020.03.26.20044057.
- 4.Garner P. Paul Garner: For 7 weeks I have been through a roller coaster of ill health, extreme emotions, and utter exhaustion: BMJ Publishing Group; 2020. https://blogs.bmj.com/bmj/2020/05/05/paul-garner-people-who-have-a-more-protracted-illness-need-help-to-understand-and-cope-with-the-constantly-shifting-bizarre-symptoms/.
- 5.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up; results from a prospective UK cohort. medRxiv. 2020:2020.08.12.20173526. 10.1101/2020.08.12.20173526. [DOI] [PMC free article] [PubMed]
- 6.Carfì A, Bernabei R, Landi F. Group for the GAC-19 P-ACS. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pipek LZ, Baptista CG, Nascimento RFV, et al. The impact of properly diagnosed sarcopenia on postoperative outcomes after gastrointestinal surgery: A systematic review and meta-analysis. PLoS One. 2020;15(8):e0237740. doi: 10.1371/journal.pone.0237740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tu H, Tu S, Gao S, Shao A, Sheng J. Current epidemiological and clinical features of COVID-19; a global perspective from China. J Infect. 2020;81(1):1–9. doi: 10.1016/j.jinf.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippi G, Wong J, Henry BM. Myalgia may not be associated with severity of coronavirus disease 2019 (COVID-19) World J Emerg Med. 2020;11(3):193–194. doi: 10.5847/wjem.j.1920-8642.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J. Rehabilitation management of patients with COVID-19: lessons learned from the first experience in China. Eur J Phys Rehabil Med. 2020;56(3):335–338. doi: 10.23736/s1973-9087.20.06292-9. [DOI] [PubMed] [Google Scholar]
- 12.Kortebein P, Frontera W, DeLisa J, JR B. Delisa’s physical medicine and rehabilitation: principals and practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2019. Physical inactivity: physiologic impairments and related clinical conditions. [Google Scholar]
- 13.Doglietto F, Vezzoli M, Gheza F, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155(8):691–702. doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVIDSurgCollaborative. GlobalSurg-CovidSurg week: determining the optimum timing for surgery following SARS-CoV-2 infection. https://globalsurg.org/globalsurg-covidsurg-week/. Published 2020.
- 15.Carod-Artal FJ. Neurological complications of coronavirus and COVID-19. Rev Neurol. 2020;70(9):311–322. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- 16.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26(7):1618–1620. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borku Uysal B, Ikitimur H, Yavuzer S, Islamoglu MS, Cengiz M. Case report: a COVID-19 patient presenting with mild rhabdomyolysis. Am J Trop Med Hyg. 2020;103(2):847–850. doi: 10.4269/ajtmh.20-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Shan KS, Minalyan A. A rare presentation of coronavirus disease 2019 (COVID-19) induced viral myositis with subsequent rhabdomyolysis. Cureus. 2020;12(5):e8074. https://www.cureus.com/articles/31422-a-rare-presentation-of-coronavirus-disease-2019-covid-19-induced-viral-myositis-with-subsequent-rhabdomyolysis. [DOI] [PMC free article] [PubMed]
- 20.Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Heal. 2020;0(0). 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed]
- 21.Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol. 2020. 10.1152/japplphysiol.00321.2020. [DOI] [PMC free article] [PubMed]
- 22.Disser NP, De Micheli AJ, Schonk MM, et al. Musculoskeletal consequences of COVID-19. JBJS. 2020;102(14):1197-1204. https://journals.lww.com/jbjsjournal/Fulltext/2020/07150/Musculoskeletal_Consequences_of_COVID_19.1.aspx. [DOI] [PMC free article] [PubMed]
- 23.Leung TW, Wong KS, Hui AC, et al. Myopathic changes associated with severe acute respiratory syndrome: a postmortem case series. Arch Neurol. 2005;62(7):1113–1117. doi: 10.1001/archneur.62.7.1113. [DOI] [PubMed] [Google Scholar]
- 24.Lau HM-C, Lee EW-C, Wong CN-C, Ng GY-F, Jones AY-M, Hui DS-C. The impact of severe acute respiratory syndrome on the physical profile and quality of life. Arch Phys Med Rehabil. 2005;86(6):1134–1140. doi: 10.1016/j.apmr.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paneroni M, Simonelli C, Saleri M, et al. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med & Rehabil. 2020. 10.1097/phm.0000000000001641. [DOI] [PubMed]
- 26.Silver JK. Prehabilitation may help mitigate an increase in COVID-19 peripandemic surgical morbidity and mortality. Am J Phys Med Rehabil. 2020;99(6):459–463. doi: 10.1097/PHM.0000000000001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silver JK. Prehabilitation could save lives in a pandemic. BMJ. 2020;369:m1386. doi: 10.1136/bmj.m1386. [DOI] [PubMed] [Google Scholar]
- 28.Klok FA, Boon GJAM, Barco S, et al. The post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56(1). 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed]
- 29.Nakamura K, Nakano H, Naraba H, Mochizuki M, Hashimoto H. Early rehabilitation with dedicated use of belt-type electrical muscle stimulation for severe COVID-19 patients. Crit Care. 2020;24(1):342. doi: 10.1186/s13054-020-03080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials used in the production of this work will be available on request.