Abstract
The authors present a case of a 55-year-old gentleman with a medical history of atrial fibrillation on amiodarone who presented with weight loss, palpitations and exertional dyspnoea. Thyroid function tests revealed thyrotoxicosis with a free thyroxine (T4) of 117 pmol/L and a thyroid-stimulating hormone (TSH) of <0.008 mIU/L. Interleukin-6 level was low. The negative TSH-receptor antibody status, the presence of a small thyroid gland with heterogeneous echotexture and decreased internal vascularity on ultrasound together with the relatively quick drop in free T4 and free tri-iodothyronine (T3) levels once prednisolone therapy was added to carbimazole suggested that this was typical of amiodarone-induced thyrotoxicosis (AIT) type 2. Subsequently, carbimazole was discontinued and treatment with prednisolone was continued. This case highlights that AIT management may be challenging and it is of paramount importance to establish the type of AIT present as this will guide management and is key to improving prognosis.
Keywords: endocrinology, thyroid disease, cardiovascular system
Background
Amiodarone therapy may lead to both subclinical as well as overt thyroid dysfunction. While amiodarone-induced hypothyroidism (AIH) is usually relatively simple to manage with levothyroxine replacement therapy, management of amiodarone-induced thyrotoxicosis (AIT) may be more challenging. In AIT, the physician needs to classify the type, namely type 1, type 2 or mixed/indefinite type AIT, as this will guide management with thionamides, glucocorticoids or both, respectively. In addition, unlike AIH, AIT may develop abruptly without any previous subclinical thyroid function changes and can progress quickly. Hence, physicians should be aware of the effects of amiodarone on thyroid physiology and monitor thyroid function regularly in patients on current or previous amiodarone treatment. Furthermore, patients on amiodarone should be educated to seek medical advice promptly when symptoms of thyrotoxicosis occur, since AIT can present in an unpredictable manner at any stage during or after amiodarone therapy.
Case presentation
A 55-year-old gentleman with a medical history of left ventricular impairment, atrial fibrillation and alcohol misuse presented to the accident and emergency department with a 3-week history of increasing dyspnoea on exertion, as well as bilateral lower limb oedema and increasing lethargy. The patient also admitted to an 8 kg weight loss over the past month. The patient denied any symptoms of upper respiratory tract infections, gastrointestinal infections, and neck pain or swelling. Before coming to the accident and emergency department, the patient had already visited his general practitioner who requested routine blood tests including a thyroid function test which showed a free T4 of 52 pmol/L and a thyroid-stimulating hormone (TSH) of <0.008 mIU/L. The patient was advised to start carbimazole 15 mg twice a day by his general practitioner. However, the patient’s symptoms did not improve and this prompted his visit to the accident and emergency department. The patient had been on amiodarone since 2 years after an unsuccessful attempt at DC cardioversion. On physical examination, the patient had an irregularly irregular pulse of 110 bpm, signs of fluid overload and minimal fine tremor of the outstretched hands. He denied neck tenderness. A repeat thyroid function test was requested and it was noted that the free T4 had now risen to 117 pmol/L. Antibodies for TSH receptor and thyroid peroxidase and an interleukin-6 level were taken and sent for analysis. An ultrasound of his thyroid revealed a small thyroid gland with heterogeneous echotexture and decreased internal vascularity.
Given his presentation with worsening thyrotoxicosis and the ultrasound findings, the patient was prescribed prednisolone 40 mg daily in addition to his carbimazole, pending the IL-6 levels and antibody results. Furthermore, amiodarone was stopped following discussion with the patient’s caring cardiologist and he was administered carvedilol instead to control his fast AF. His diuretic dose was also increased.
Outcome and follow-up
The patient was discharged after improvement of his symptoms. The patient was followed closely as an outpatient and after 2 weeks, he was feeling much better and his thyroid function tests revealed marked improvement with a free T4 that decreased from 117 pmol/L to 49 pmol/L and a free tri-iodothyronine (T3) drop from 24 pmol/L to 8.7 pmol/L. In addition, laboratory data revealed negative TSH receptor antibody and thyroid peroxidase antibody status and a normal interleukin-6 level at 2.2 pg/mL (normal value: <7 pg/mL).
The significant and relatively quick drop in free T4 and free T3 once prednisolone therapy was added to carbimazole, together with the laboratory findings and ultrasound findings, suggested that this was typical of amiodarone induced thyrotoxicosis type 2. Thus, prednisolone was continued at 40 mg daily and carbimazole was stopped. Following another 4 weeks of treatment with prednisolone, there was further biochemical improvement in his thyrotoxicosis and the prednisolone was gradually tailed down over 2 months. On follow-up, he remained clinically and biochemically euthyroid. Table 1 and figure 1 represent the trend of free T4, free T3 and TSH from the patient’s initial presentation with thyrotoxicosis, with recovery to a euthyroid state following treatment modification.
Table 1.
Free T4, free T3 and TSH trend from the patient’s initial presentation with thyrotoxicosis, with recovery to a euthyroid state following treatment modification
| Date | TSH (mIU/L) | Free T4 (pmol/L) | Free T3 (pmol/L) | Treatment |
| 09/08/2019 | <0.008 | 52 | 12.4 | Initiation of carbimazole therapy |
| 26/08/2019 | <0.008 | 117 | 24.4 | Addition of prednisolone to carbimazole therapy |
| 30/08/2019 | <0.008 | 96 | 18.6 | |
| 09/09/2019 | <0.008 | 49 | 8.7 | Cessation of carbimazole therapy |
| 10/10/2019 | 0.012 | 21 | 4.5 | |
| 12/11/2019 | 3.008 | 13 | 3.5 | |
| 27/12/2019 | 1.506 | 19 | 5.4 | Cessation of prednisolone |
| 17/02/2020 | 1.040 | 17 | 5.2 |
T3, tri-iodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
Figure 1.
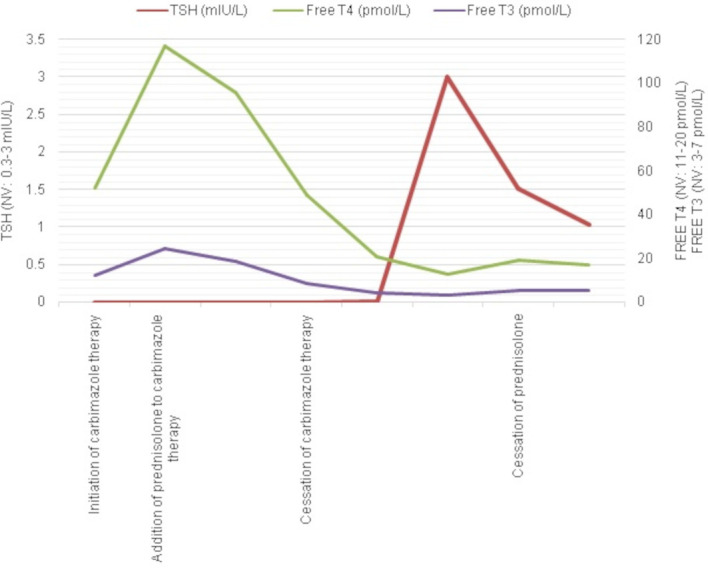
Graph illustrating the trend of free T4, free T3 and thyroid-stimulating hormone (TSH) from the patient’s initial presentation with thyrotoxicosis, with recovery to a euthyroid state following treatment modification.
Discussion
Amiodarone is an iodine-rich benzofuranic agent which is widely used in the management of several cardiac arrhythmias.1 Amiodarone may result in thyroid disease secondary to two main mechanisms: amiodarone’s intrinsic drug effects as well as effects secondary to amiodarone’s iodine-rich content.2
Amiodarone’s intrinsic drug effects include the following: (1) Amiodarone inhibits the type 1′5-deiodinase enzyme hence leading to reduced T4 to T3 conversion.3 (2) Amiodarone inhibits pituitary type 2 deiodinase with a resultant reduced intracellular T3 concentration.4 (3) Amiodarone inhibits the nuclear binding of T3 in a dose-dependent fashion leading to reduced expression of some thyroid hormone genes.5 (4) Amiodarone has a direct toxic effect on thyroid follicular cells.6
Furthermore, amiodarone contains two iodine atoms. This means that it contains circa 37.5% of organic iodine by weight, 10% of which is de-iodinated as part of amiodarone’s metabolism hence releasing a large amount of iodine into the systemic circulation.1 The recommended daily intake of iodine is approximately 0.2 mg/day. Hence, a maintenance dose of 200 mg of amiodarone per day will provide 7 mg of iodine per day. Treatment with amiodarone results in a 50–100-fold excess daily iodine intake.7 This expansion in the iodine pool leads to several changes in thyroid hormone dynamics.
Under normal circumstances, there are autoregulatory mechanisms that prevent hyperthyroidism after exposure to such an iodine load. This is known as the Wolff-Chaikoff effect. When the intra-thyroidal iodine concentration reaches a critical level, iodine transport and synthesis of thyroid hormones decreases.1 2 Thyroid dysfunction is related to this Wolff-Chaikoff effect. If there is failure to escape from the Wolff-Chaikoff effect, this leads to hypothyroidism.1 On the other hand, if the Wolff-Chaikoff effect is defective, the thyroid is not protected from the increased iodine pool, and hyperthyroidism may occur.6 8 This mostly occurs in patients with underlying thyroid cell abnormalities that predisposes them to autonomy of function.9 When the number of such abnormal cells becomes significant and the iodine pool increases, these subjects may develop thyrotoxicosis.9 These include patients with nodule formation or those with latent Graves’ disease. This is termed the Jod-Basedow effect.10
In addition, another characteristic that leads to difficulty with amiodarone-induced thyroid dysfunction is related to amiodarone’s long half-life. The elimination of amiodarone averages 52±23 days whereas its metabolite desethylamiodarone has a half-life of 61±31 days after cessation of amiodarone therapy.8 Hence, despite amiodarone withdrawal, the drug and its metabolites remain active for a considerable period of time. This explains why amiodarone-induced dysfunction may occur many months after cessation of treatment.6
Type 1 AIT and type 2 AIT are characterised by distinct pathophysiological mechanisms. Type 1 AIT is characterised by the increased synthesis of thyroid hormones whereas type 2 AIT is the result of excessive pre-formed thyroid hormone release due to a destructive thyroiditis mediated by amiodarone’s direct toxic effect on the thyroid follicular cells.11
The diagnostic criteria of type 1 and 2 AIT are summarised in table 2. Differentiating between the two types of AIT may be challenging and sometimes AIT may consist of an overlap of both types, that is, type 1 and type 2 AIT.12 13 This is termed as a mixed/indefinite type.11 13
Table 2.
Comparison between AIT type 1 versus AIT type 2
| Criteria | AIT 1 | AIT 2 |
| Mechanism | Excessive hormone production (true hyperthyroidism) | Destructive thyroiditis |
| Underlying thyroid abnormality | Yes | Usually no |
| Colour-flow Doppler ultrasound | Increased vascularity | No hypervascularity |
| Thyroid radio-iodine uptake | Decreased/normal/increased | Decreased |
| Thyroid autoantibodies | Present if AIT is due to Graves’ disease | Usually absent |
| Onset time after starting amiodarone | Short (median 3 months) | Long (median 30 months) |
| Spontaneous remission | No | Possible |
| Subsequent hypothyroidism | No | Possible |
| First-line medical treatment | Thionamides | Oral glucocorticoids |
| Subsequent definitive thyroid treatment | Generally yes | No |
Adapted with permission from Bartalena L, Bogazzi F, Chiovato L, et al. 2018 European Thyroid Association (ETA) guidelines for the management of amiodarone-associated thyroid dysfunction. Eur Thyroid J 2018;7:55–66. Copyright 2018 S. Karger AG, Basel.11
AIT, amiodarone-induced thyrotoxicosis.
Interleukin-6 (IL-6) levels are classically significantly elevated in type 2 AIT. However, it is not a reliable marker of type 2 AIT as exceptions to the aforementioned rule are common, with type 2 AIT patients having unexpectedly low IL-6 levels. Hence, IL-6 levels should be used to follow-up patients with type 2 AIT with significantly elevated IL-6 levels. At present, colour flow Doppler sonography offers a more rapid and efficient method of differentiating between type 1 and type 2 AIT.3 12 14 15
Restoration of euthyroidism in patients with AIT should be achieved promptly especially in those patients with serious cardiac abnormalities.16 However, this depends on defining the pathogenic mechanism of AIT in order to select the appropriate optimal treatment. In practice, most patients initially present with an indefinite form of AIT; at least until the necessary laboratory and imaging investigations are performed. Han et al suggest an initial regimen of carbimazole and prednisolone therapy for 2 weeks, followed by measurement of serum free T3. If the serum free T3 levels decrease by 50%, then this is more typical of type 2 AIT and carbimazole may be discontinued. On the other hand, if the serum free T3 levels do not change, then prednisolone may be stopped and carbimazole continued based on the assumption that this is more in keeping with type 1 AIT.17 Hence, Han et al uses the relatively quick drop in serum free T3 concentration after prednisolone therapy as an additional marker of type 2 AIT. Indeed, a study by Osman et al highlights that patients with type 2 AIT also had significantly lower serum free T4 concentrations at 12 weeks when compared with type 1 AIT patients (14.9 pmol/L (12–18 pmol/L) vs 20.4 pmol/L (16–37 pmol/L), respectively).18 This may reflect the self limiting nature of type 2 AIT.18
Due to the underlying pathological mechanism of a destructive thyroiditis, treatment of type 2 AIT is with oral glucocorticoids, usually prednisolone at an initial dose of 0.5–0.7 mg/kg bodyweight which is then tapered down and eventually discontinued over the course of 3 months. Thionamides are not used in the treatment of AIT type 2 as the underlying mechanism does not involve increased hormone synthesis. However, these may be useful in mixed/indefinite type AIT.16 Response to glucocorticoids is usually dramatic as one can observe in this case. It is possible for patients to develop subsequent hypothyroidism after resolution of the thyrotoxicosis episode due to the underlying destructive thyroiditis. Hence, these patients need to be followed up and if hypothyroidism develops, appropriate levothyroxine replacement therapy should be prescribed.
Patients who are refractory to glucocorticoid therapy should be referred for thyroidectomy.19 Surgical candidates with inadequately controlled thyrotoxicosis are at serious risk.20 Thus, the decision to proceed to thyroidectomy needs to be made carefully by a multidisciplinary team where the risk versus benefit ratio of each patient is carefully assessed.11
Learning points.
Amiodarone-induced thyrotoxicosis (AIT) can be classified into type 1 AIT, type 2 AIT and a mixed/indefinite type of AIT.
Patients receiving amiodarone therapy should be educated to seek medical advice promptly when symptoms of thyrotoxicosis occur, since AIT can present in an unpredictable manner at any stage during or after amiodarone therapy.
Interleukin-6 may not always be elevated in patients with type 2 AIT and thus may be an unhelpful test in differentiating between type 1 and type 2 AIT.
Colour flow doppler sonography offers a more rapid and efficient method of differentiating between type 1 and type 2 AIT.
Medical management with oral glucocorticoids is the first-line treatment for patients with type 2 AIT, whereas surgical treatment (thyroidectomy) is reserved for those who do not respond to glucocorticoids.
Footnotes
Contributors: DP and SM were responsible for literature review and manuscript preparation. AA and SF contributed towards editing and review of the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Loh KC. Amiodarone-induced thyroid disorders: a clinical review. Postgrad Med J 2000;76:133–40. 10.1136/pmj.76.893.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narayana SK, Woods DR, Boos CJ. Management of amiodarone-related thyroid problems. Ther Adv Endocrinol Metab 2011;2:115–26. 10.1177/2042018811398516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsang W, Houlden RL. Amiodarone-induced thyrotoxicosis: a review. Can J Cardiol 2009;25:421–4. 10.1016/S0828-282X(09)70512-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosene ML, Wittmann G, Arrojo E, et al. Inhibition of the type 2 iodothyronine deiodinase underlies the elevated plasma TSH associated with amiodarone treatment. Endocrinology 2010;151:5961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklyn JA, Davis JR, Gammage MD, et al. Amiodarone and thyroid hormone action. Clin Endocrinol 1985;22:257–64. 10.1111/j.1365-2265.1985.tb03238.x [DOI] [PubMed] [Google Scholar]
- 6.Martino E, Bartalena L, Bogazzi F, et al. The effects of amiodarone on the thyroid. Endocr Rev 2001;22:240–54. 10.1210/edrv.22.2.0427 [DOI] [PubMed] [Google Scholar]
- 7.Sudheer Ahamed P, Mathew A. A case of amiodarone-induced thyrotoxicosis. Sultan Qaboos Univ Med J 2009;9:319–23. [PMC free article] [PubMed] [Google Scholar]
- 8.Ursella S, Testa A, Mazzone M, et al. Amiodarone-induced thyroid dysfunction in clinical practice. 10. [PubMed] [Google Scholar]
- 9.Stanbury JB, Ermans AE, Bourdoux P, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid 1998;8:83–100. 10.1089/thy.1998.8.83 [DOI] [PubMed] [Google Scholar]
- 10.Newman CM, Price A, Davies DW, et al. Amiodarone and the thyroid: a practical guide to the management of thyroid dysfunction induced by amiodarone therapy. Heart 1998;79:121–7. 10.1136/hrt.79.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartalena L, Bogazzi F, Chiovato L, et al. 2018 European Thyroid Association (ETA) guidelines for the management of amiodarone-associated thyroid dysfunction. Eur Thyroid J 2018;7)::55–66. Mar;. 10.1159/000486957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan A, Puttanna A, Raskauskiene D. Amiodarone-induced thyrotoxicosis: type 1 or type 2? Case Reports 2014;2014:bcr2014204485 10.1136/bcr-2014-204485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macchia PE, Feingold KR, Thyrotoxicosis AI. Amiodarone induced thyrotoxicosis : Feingold KR, Anawalt B, Boyce A, et al., Endotext [Internet]. South Dartmouth (MA: MDText.com, Inc., 2000. http://www.ncbi.nlm.nih.gov/books/NBK279030/ [Google Scholar]
- 14.Eaton SEM, Euinton HA, Newman CM, et al. Clinical experience of amiodarone-induced thyrotoxicosis over a 3-year period: role of colour-flow Doppler sonography. Clin Endocrinol 2002;56:33–8. 10.1046/j.0300-0664.2001.01457.x [DOI] [PubMed] [Google Scholar]
- 15.Bogazzi F, Martino E, Dell'Unto E, et al. Thyroid color flow Doppler sonography and radioiodine uptake in 55 consecutive patients with amiodarone-induced thyrotoxicosis. J Endocrinol Invest 2003;26:635–40. 10.1007/BF03347021 [DOI] [PubMed] [Google Scholar]
- 16.Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab 2010;95:2529–35. 10.1210/jc.2010-0180 [DOI] [PubMed] [Google Scholar]
- 17.Han TS, Williams GR, Vanderpump MPJ. Benzofuran derivatives and the thyroid. Clin Endocrinol 2009;70:2–13. 10.1111/j.1365-2265.2008.03350.x [DOI] [PubMed] [Google Scholar]
- 18.Osman F, Franklyn JA, Sheppard MC, et al. Successful treatment of amiodarone-induced thyrotoxicosis. Circulation 2002;105:1275–7. 10.1161/circ.105.11.1275 [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto K, Ota M, Irie T, et al. A case of type 2 amiodarone-induced thyrotoxicosis that underwent total thyroidectomy under high-dose steroid administration. Case Rep Endocrinol 2015;2015:1–6. 10.1155/2015/416145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buget MI, Sencan B, Varansu G, et al. Anaesthetic management of a patient with thyrotoxicosis for nonthyroid surgery with peripheral nerve blockade. Case Rep Anesthesiol 2016;2016:1–3. 10.1155/2016/9824762 [DOI] [PMC free article] [PubMed] [Google Scholar]


