Abstract
OBJECTIVE
To determine the impact of skin-to-tumor (STT) distance on the risk for treatment failure following percutaneous cryoablation (PCA).
METHODS
We retrospectively reviewed patients who underwent PCA with documented T1a recurrent renal cell carcinoma (RCC) at 2 academic centers between 2005 and 2015. Patient demographics, tumor characteristics, and perioperative and postoperative course variables were collected. Additionally, we measured the STT distance by averaging the distance from the skin to the center of the tumor at 0°, 45°, and 90° on preoperative computed tomography imaging.
RESULTS
We identified 86 patients with documented T1a RCC. The mean age at the time of surgery was 69 years (range: 37–91 years), and the mean tumor size was 2.7 cm (range: 1.0–4.0 cm). With a mean follow-up of 24 months (range: 3–63 months), 11 (12.8%) treatment failures occurred. Patients with treatment failure had significantly higher mean STT distance than those without: 11.0 cm (range: 6.3–20.1 cm) compared to 8.4 cm (range: 4.4–15.2 cm), respectively (P = .002). STT distance was an independent predictor of treatment failure (odds ratio: 1.32, 95% confidence interval: 1.04–1.69, P = .029). STT distance greater than 10 cm had a fourfold increased risk of tumor treatment failure (odds ratio: 4.43, 95% confidence interval: 1.19–16.39, P = .018). Tumor size, R.E.N.A.L. Nephrometry score, and number of cryoprobes placed were not associated with treatment failure.
CONCLUSION
STT, an easily measured preoperative variable, may inform the risk of RCC treatment failure following PCA.
Renal cell carcinoma (RCC) is the 14th most common cancer in the world, with an estimated 61,000 people diagnosed in the United States in 2015.1 The widespread use of diagnostic imaging has increased the detection of T1 a RCC variants, allowing for more timely intervention and thereby better cancer-specific survival.1,2 While extirpation remains the gold standard for treatment of small renal masses (SRM), percutaneous cryoablation (PCA) is an alternative, minimally invasive approach that is effective in select patients.3–5 The American Urological Association and European Association of Urology guidelines support the use of ablation modalities in patients with T1a (<4 cm) disease, those at increased risk of multiple tumors (ie, Von HippelLindau syndrome) solitary kidney, or in patients with significant comorbidities who are poor surgical candidates.6,7 However, although durable disease response is favorable in patients who have undergone PCA, reported local treatment failure rates remain relatively high, from 5% to 30% in comparison to less than 2% following partial nephrectomy.8–10
Tumor and patient characteristics such as tumor size, location, depth, and patient body mass index (BMI) account for the complexity of a procedure and potentially lead to higher treatment failure and complication rates.11–13 Current reports support the assertion that PCA should only be applied to T1a (<4 cm) renal cell carcinoma variants, with early evidence corroborating that tumor size directly affects oncologic outcomes.14 Further, the location of the tumor such as anterior or upper pole lesions may have significant impact on the difficulty of needle deployment and proper lesion targeting.12,15 In addition, proximity of the tumor to hilar vessels has also been hypothesized to contribute to treatment failure due to the possibility of "heat sink" or the inability for the juxtavascular probe to reach temperatures low enough to induce complete tumor necrosis.11,12,14 Treatment algorithms that take into account the size, location, and proximity to surrounding retroperitoneal and abdominal structures have been developed that are predictive of treatment difficulty and complications; however, few studies combining patient and tumor-specific variables to predict long-term procedural outcomes have been conducted.16 Pareek and colleagues first introduced the concept of skin-to-target (stone) distance as a predictor of outcomes for stone disease in the setting of shockwave lithotripsy.17 Subsequently, Blute and colleagues described skin-to-tumor (STT) distance for renal cortical neoplasms in a heterogeneous population of patients with benign, malignant, and indeterminate tumors of all sizes.13 Herein we evaluated STT distance as a predictor of treatment failure following PCA in patients with biopsy-proven T1a RCC. It is our hypothesis that tumors deeper in the body may be harder to eradicate with contemporary cryoablation.
METHODS
Study Design
After institutional review board approval, we conducted a retrospective chart review of all patients with biopsy-proven T1a RCC who underwent primary treatment with PCA at 2 academic institutions between December 2005 and June 2015. Only patients with available preoperative imaging were included in this analysis. We collected and analyzed patient demographics and peri- and postoperative characteristics to determine preoperative factors predictive of treatment failure following primary PCA.
Measurements
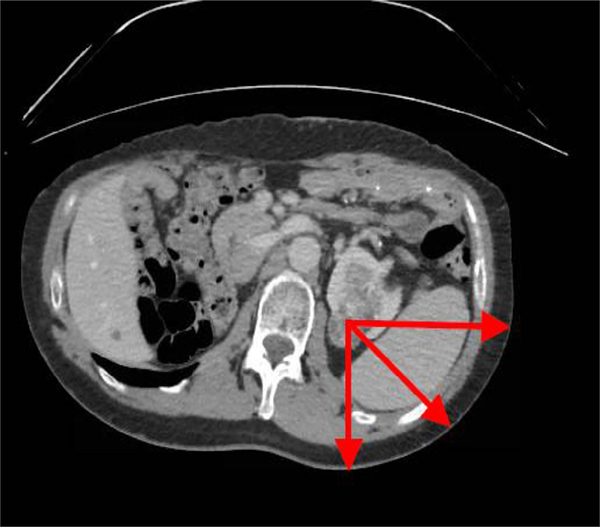
Preoperative computed tomography (CT) and magnetic resonance imaging scans were used to determine STT distances for all patients according to the methods of Pareek and colleagues (2005). The average of the 3 measurements at 0° posteriorly and 45° and 90° laterally from the skin to the center of the tumor was recorded as the STT distance17 (Fig. 1). Probe distance was taken as the average distance from the surface of the skin to the tip of the probe for each probe based on interprocedural CT images.
Figure 1.
Axial imaging demonstrating presence of renal tumor and method of measuring skin-to-tumor at 0°, 45°, and 90°.
Tumor size (ie, largest axial diameter), tumor polarity, and tumor depth were recorded. With regard to polarity, tumors that crossed the midline between the upper and lower poles were classified as interpolar. Tumors protruding more than 50% from the renal parenchyma were categorized as exophytic, whereas those that were protruding less than or equal to 50% were deemed mesophytic. Tumors entirely confined within the renal parenchyma were categorized as endophytic. A R.E.N.A.L. Nephrometry score was determined for each tumor based on the method of Kutikov and colleagues.18
Surgical Technique
At both institutions, all PCAs were performed in a hospitalbased interventional radiology suite as a combined effort between interventional radiologist and urologist. The PCA technique used at both institutions involved a double freezethaw cycle as previously described.17,19 The total number of probes placed was based on the tumor size. Probe placement was confirmed prior to each procedure with CT imaging. Treatment success was determined by an interprocedural CT scan documenting extension of the ice ball at least 1 cm beyond the tumor in every dimension. A diagnostic CT with intravenous contrast was performed immediately following the procedure.
Imaging Follow-up
Following the procedure, CT or magnetic resonance imaging was obtained at 3–6 months, 1 year, and then annually. Treatment failure was defined as enhancement in the region of the ablated tumor or tumor growth on follow-up imaging. Patients with persisting tumors were offered a variety of options: surveillance, repeat cryoablation, partial nephrectomy, or radical nephrectomy. All patients with persistent tumors elected to undergo either repeat PCA or laparoscopic partial nephrectomy.
Statistical Analysis
The primary outcome measure was absence of enhancement or tumor growth on follow-up imaging. Chi-square analysis was used to compare frequency and distribution of treatment failure and R.E.N.A.L. Nephrometry scores. Pearson correlations and Fisher exact tests were used to assess correlation between STT, probe distance, and BMI. Finally, predictive preoperative and patient and diseasespecific variables were used to determine treatment failure using logistic regression analysis. Statistical significance was defined as a P value of ≤.05.
RESULTS
A total of 169 patients underwent PCA for an SRM. Of these, we identified 86 patients with biopsy-proven T1a RCC. The mean age at the time of surgery was 69 years (range: 37–91 years), and the mean tumor size was 2.7 cm (range: 1.0–4.0 cm). Patient demographics and clinical characteristics are presented in Table 1.
Table 1.
Demographics and disease characteristics
| Overall |
Treatment Failure |
Successful Treatment |
P | ||||
|---|---|---|---|---|---|---|---|
| No. of patients | 86 | 11 | 75 | ||||
| Mean age (range), years | 69 | (37–91) | 62 | (47–79) | 71 | (37–91) | .014 |
| Gender, m(f) | 64(22) | 10(1) | 54(21) | .173* | |||
| ASA score | |||||||
| I, II | 40 | 51% | 4 | 40% | 36 | 53% | <.001† |
| III | 38 | 49% | 6 | 60% | 32 | 47% | |
| Solitary kidney | 7 | 8% | 3 | 27% | 4 | 5% | .042† |
| Mean (range) BMI, kg/m2 | 28.4 | (18.0–42.4) | 31.3 | (24.7–40.6) | 27.9 | (18.0–42.4) | .131 |
| Mean (range) tumor size, cm | 2.70 | (1.0–4.0) | 2.80 | (1.0–4.0) | 2.70 | (1.0–4.0) | .600 |
| Polarity | .805† | ||||||
| Upper | 20 | 24% | 3 | 27% | 17 | 23% | |
| Interpolar | 33 | 40% | 5 | 46% | 28 | 39% | |
| Lower | 30 | 36% | 3 | 27% | 27 | 38% | |
| Tumor depth | .191† | ||||||
| Exophytic | 44 | 51% | 3 | 27% | 42 | 56% | |
| Mesophytic | 28 | 33% | 6 | 55% | 21 | 28% | |
| Endophytic | 14 | 16% | 2 | 18% | 12 | 16% | |
| Mean (range) R.E.N.A.L. Nephrometry score | 6.69 | (4–11) | 7 | (4–10) | 6.64 | (4–11) | .536† |
| Low (4–6) | 40 | 47% | 5 | 46% | 35 | 45% | |
| Moderate (7–9) | 38 | 44% | 4 | 36% | 36 | 47% | |
| High (10–12) | 8 | 9% | 2 | 18% | 6 | 8% | |
| RCC subtype | |||||||
| Clear cell | 57 | 66% | 7 | 64% | 50 | 67% | |
| Papillary | 15 | 17% | 2 | 18% | 13 | 17% | |
| Chromophobe | 5 | 6% | 0 | 0 | 5 | 7% | |
| Not specified | 9 | 11% | 2 | 18% | 7 | 9% | |
| Grade | |||||||
| 1 | 16 | 19% | 3 | 27% | 13 | 17 % | |
| 2 | 33 | 38% | 3 | 27% | 30 | 40% | |
| 3 | 19 | 22% | 2 | 18% | 17 | 23% | |
| 4 | 1 | 1% | 0 | 0 | 1 | 1% | |
| Not specified | 17 | 20% | 3 | 27% | 14 | 19% | |
| Mean (range) skin-to-tumor, cm | 8.7 | (4.4–20.1) | 11.0 | (6.3–20.1) | 8.4 | (4.4–15.2) | .002 |
| Mean probe distance (range), cm | 9.9 | (5.2–18.9) | 11.3 | (8.2–18.9) | 9.7 | (5.2–14.2) | .040 |
| Mean no. of probes | 2.47 | (1–8) | 2.46 | (1–4) | 2.47 | (1–7) | .864 |
| Mean (range) no. of probes/cm tumor | 1.3 | (0.5–3.7) | 1.3 | (0.7–2.2) | 1.3 | (0.5–3.7) | .885 |
ASA, American Society of Anesthesiologists; BMI, body mass Index; RCC, renal celi carcinoma.
Fisher exact test.
Pearson chi-square.
With a mean follow-up of 24 months (range: 3–64 months), there were 11 (12.8%) treatment failures. The mean time identification of treatment failure was 15 months (range: 6–24 months). Patients with treatment failure had a mean age of 62 years (range: 47–79 years), whereas patients without treatment failure were older (ie, 71 years; range: 37–91 years, P = .014). A greater proportion of patients with treatment failure were American Society of Anesthesiologists score III, 60% vs 49% (P < .001). A greater proportion of patients with treatment failure had a solitary kidney (3 of 11 or 27%) vs those who underwent successfu treatment (4 of 75 or 5%) (P = .042). Lesion size and R.E.N.A.L. Nephrometry score did not correlate with treatment failure (P = .600 and P = .536, respectively). Similarly tumor depth (endophytic, mesophytic, or exophytic character), polarity (upper vs lower), and nearness to the renal hilum were not significant (P = .191, P = .805, P = .518, respectively). The number of probes used was not significantly different between patients with and without treatment failure (P = .864), nor was the number of probes per centimeter of tumor (P = .885) (Supplementary Table S1). There was no significant difference in probe per centimeter of tumor in patients with paired and solitary kidney (P = .331), and similarly probe per centimeter of tumor in patients with solitary kidney was not different between those with treatment failure and those without (P = .102). The mean STT distance in patients with treatmen failure was significantly greater than in patients whose disease was successfully eradicated (11.0 and 8.4 cm, respectively, P = .002). Patients with solitary kidney had a significantly longer STT distance compared with patients with a paired kidney (10.8 cm vs 8.6 cm, P = .037). However, there was no significant difference in STT distance in patients with a solitary kidney who failed compared with those whose treatment was successful (11.6 and 10.1 cm, respectively, P = .561). In all patients, probe distance was highly correlated with STT distance (Pearson correlation coefficient: 0.746, P < .001). Similar to STT distance, probe distance was also greater in patients with treatment failure (11.3 cm [8.2–18.9 cm] vs 9.7 cm [5.214.2 cm], P = .040). Finally, patients with treatment failure trended toward a higher BMI (mean: 31.3 [24.7–40.6] vs 27.9 [18.0–42.4], P = .131). Pearson correlation revealed that STT distance was highly correlated with BMI (Pearson correlation coefficient: 0.55, P < .001) (Table 1).
When treated as a continuous variable, STT distance was significantly associated with treatment failure on univariate logistic regression analysis (odds ratio [OR]: 1.37, 95% confidence interval [CI]: 1.08–1.72, P = .008) and multivariate analysis (OR: 1.32, 95% CI: 1.03–1.69, P = .029), indicating a 32% increased risk of treatment failure for every increased centimeter of STT. Overall, a total of 6 treatment failures occurred in 21 patients (28.6%) with an STT distance greater than 10 cm. Treatment failure rate among patients with an STT distance less than or equal to 10 cm was 7.7% (5 of 65). Supplementary Table S1 indicates the distribution of STT distance among patients with and without treatment failure, and Supplementary Table S2 indicates the percentage of treatment failures corresponding to every centimeter of STT distance. When treated as a dichotomous variable, STT distance greater than 10 cm was associated with a fourfold increased risk of treatment failure (OR: 4.43, 95% CI: 1.20–16.39, P = .018). BMI was not significantly associated with tumor treatment failure on univariate analysis (OR: 1.11, 95% CI: 0.97–1.22, P = .126). Whereas a tumor in a solitary kidney was associated with higher treatment failures on univariate analysis (OR: 6.656, 95% CI: 1.26–35.20, P = .026), it was not significant on multivariate analysis (P = .173). Finally, on multivariate analysis, younger age at the time of surgery was associated with an increased risk of treatment failure (OR: 0.94, 95% CI: 0.88–0.999, P = .047) (Table 2 and Supplementary Table S2).
Table 2.
Univariate logistic regression
| Patient Characteristics Variable | Odds Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Skin-to-tumor (continuous) | 1.37 | (1.08–1.72) | .008 |
| Skin-to-tumor (dichotomous variable, >10 cm) | 4.43 | (1.20–16.39) | .018 |
| Body mass index | 1.11 | (0.97–1.22) | 0.126 |
| Age at surgery | 0.95 | (0.88–0.99) | .019 |
| Solitary kidney | 6.66 | (1.26–35.20) | .026 |
Ten complications (12%) were noted (Supplementary Table S3). Neither R.E.N.A.L. score nor STT distance was associated with complication rate (P = .099 and P = .85, respectively).
Two of the 11 patients who experienced treatment failure underwent subsequent partial nephrectomy successfully. One elected active surveillance and ultimately underwent radical nephrectomy due to tumor progression. The remainder underwent successful repeat cryoablation, with no patient progressing to metastasis or tumor-related death (mean follow-up of 21 months, range: 3–36 months).
DISCUSSION
Current guidelines recommend surgical extirpation for all renal cortical masses despite the finding that upwards of 30% of these lesions are benign or of low malignant potential.20 Over the past decade, PCA has emerged as an effective, less morbid, alternative treatment modality. PCA preserves renal parenchyma and minimizes morbidity, convalescence time, and costs vs surgical excision.19,21 Moreover, PCA offers similar cancer-specific and metastasisfree survival to the gold standard, partial nephrectomy.9 Still, reported local treatment failure after PCA is higher than with surgical extirpation, indicating that better patient selection or improved ablation techniques are needed. To this end, we compared patient and tumor characteristics relevant to PCA procedural planning and complexity in patients with documented T1a disease.
Previous partial nephrectomy studies have demonstrated that tumor-specific variables such as size, depth within the kidney, and tumor polarity and location relative to the renal hilum significantly impact outcomes including perioperative complications and oncologic outcomes.11,12,22 However, this does not seem to be the case for PCA. Indeed, in our study of pathologic T1a renal cancers, treatment failure rates following PCA were not dependent on the aforedescribed tumor-specific variables. This is consistent with previous findings that PCA may be used to successfully treat even anteriorly located tumors independent of tumor size and proximity to the renal hilum.11,23–25 It is important to note that the selection criteria of the current manuscript include only T1a RCC variants, which are, by definition, all ≤4 cm. As such, we continue to prefer to limit cryoablation to tumors that are 3 cm or smaller. Although the relevance of tumor size and location for SRM remains concerning, the use of multiple probes, facile use of intraoperative monitoring of ablative margins, and surgeon's experience appear to contribute to successful ablation.
The R.E.N.A.L. Nephrometry score is a proven metric of complexity that has utility for surgical planning and has been demonstrated to be predictive of outcomes following either PN or PCA.26,27 In a study of 751 mixed renal tumors, Schmit and colleagues found that a R.E.N.A.L. Nephrometry score greater than 8 correlated with risk of early and overall treatment failure following both PCA and radiofrequency ablation (RFA).24 Camacho and colleagues similarly found that the R.E.N.A.L. Nephrometry score was significantly associated with treatment failure following PCA and RFA.22 In contrast, in the present study, tumor complexity, as indicated by the R.E.N.A.L. Nephrometry score, was not a significant predictor of treatment failure after PCA. One reason for this discrepancy could be that in both studies evaluated PCA and RFA together; as such, the Nephrometry score may indeed be less predictive for PCA when considered alone. Also, it is also possible that our negative Nephrometery results are secondary to a pretreatment selection bias. In general, tumors chosen for PCA in the current series were small (ie, all T1a) and of low to moderate complexity; hence, we may have had too few patients with a higher Nephrometry score to allow for a more balanced analysis.
It is clear that an important variable that contributes to complexity of the PCA procedure is intracorporeal distance. In 2005, Pareek and colleagues introduced the concept of skin-to-stone distance, changing the management of renal stones by demonstrating that skin-to-stone distance greater than 10 cm predicts extracorporeal shockwave lithotripsy failure.17 In 2012, Blute and colleagues found STT distance, similarly measured by taking the average of the distances from the center of the tumor to the surface of the skin at 0°, 45°, and 90° on axial imaging, predicted treatment failure after PCA in a heterogeneous patient population with malignant, benign, and indeterminate tumors of all sizes.13 The present study is the first to test the impact of STT distance specifically on the treatment of pathologic T1a renal cancer with PCA.
In agreement with the earlier study by Blute and colleagues, we noted that increased STT distance predicts subsequent treatment failure. Indeed, with every centimeter of increased STT distance, the risk of treatment failure rose by 32%. Moreover, in patients with STT distance greater than 10 cm, risk of treatment failure was increased fourfold vs those with an STT distance less than 10 cm. These findings are consistent with recent observations by Prince and colleagues, which demonstrate that greater STT distance is associated with higher failure rates of percutaneous renal biopsy.28 Taken together, it is likely that greater STT distance complicates effective targeting of renal lesions be it for biopsy or for PCA.
It is also possible that increased tumor depth within the body may result in a heat sink phenomenon along the cryoablation probe itself. Previous observations have revealed that the use of multipoint temperature sensing needles allows for precise measurement of the lethal freeze temperature within the target lesion. With the insurance that lethal temperatures have been reached, treatment failure rates may be all but eliminated.29 Unfortunately, it is not standard of practice to employ multipoint temperature sensing needles, and relative freeze temperatures cannot be confirmed within the current cohort.
Notably, increased BMI was not significantly associated with treatment failure. This finding is consistent with previous findings by Vricella and colleagues.30 Prince and colleagues also failed to find an association between BMI and success of renal biopsy.28 An explanation is that BMI fails to represent the distribution of adipose tissue and thus does not reliably reflect the distance over which a biopsy or cryoablation needle must travel. Further exploration of the relationship between the amount of perirenal fat, flank adipose tissue, and BMI is needed to more completely explain this discordance.
Based on our results, we believe that STT distance may be used as a measure of technical difficulty that can inform treatment choice and procedural planning, counseling, and follow-up. In patients with STT distance greater than 10 cm, physicians may consider partial nephrectomy or, if PCA is to be done, alterations in ablation technique. These alterations could include use of additional cryoablation needles, placement of multipoint temperature sensing needles, or altering patient positioning in order to minimize STT distance.
Another variable that may have significant impact on treatment outcomes is presence of a solitary kidney. We observed a disproportionate number of failures among patients with a solitary kidney. Previously, our team showed that solitary kidney patients who underwent cryoablation had higher tumor recurrence rates compared with those who underwent PN.31 It is possible that the presence of a solitary kidney impacts how aggressively the tumor is treated with PCA. In the current study, there was no difference in the number of probes per centimeter of tumor used in patients with a solitary kidney compared with those with a paired kidney. Importantly, patients with a solitary kidney had a greater STT distance than patients with a paired kidney; therefore, the above finding may reflect the impact of STT rather than the presence of a solitary kidney.
Finally, in the current study, younger age at the time of surgery was a predictor of treatment failure. Older age has previously been implicated as a worse prognostic indicator in patients with T1a disease.32 Paradoxically, consistent with our findings, a recent epidemiologic study showed a survival benefit in patients between the ages of 50 and 59 undergoing partial nephrectomy compared with tumor ablation.33 Further exploration of the relationships between age, tumor type, and ablative outcomes is very much needed.
Limitations of our study are most certainly related to its retrospective design. Also, the relatively small number of treatment failures limits the statistical power of the analysis and may introduce bias into our findings. Certainly the creation of a national tumor registry that would separate percutaneous ablation between cryoablation and RFA is needed, as currently in Surveillance, Epidemiology, and End, Results databases as well as other databases the 2 are lumped together. Finally, a larger, prospective analysis is recommended to further test the findings in the present study.
CONCLUSION
STT is an easily measured preoperative variable that is linearly associated with increased risk of RCC treatment failure following PCA of T1a tumors. An STT distance of >10 cm is predictive of a fourfold higher rate of treatment failure.
Supplementary Material
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
APPENDIX
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.urology.2017.06.026.
References
- 1.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–530. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen MM, Gill IS, Ellison LM. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J Urol. 2006;176:2397–2400. [DOI] [PubMed] [Google Scholar]
- 3.Thompson RH, Atwell T, Schmit G, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67:252–259. [DOI] [PubMed] [Google Scholar]
- 4.Long CJ, Kutikov A, Canter DJ, et al. Percutaneous vs surgical cryoablation of the small renal mass: is efficacy compromised? BJU Int 2011;107:1376–1380. [DOI] [PubMed] [Google Scholar]
- 5.Sewell P, Shingleton WB. Five-year treatment success and survival of patients treated with percutaneous MRI guided and monitored renal cell carcinoma cryoablation. BJU Int. 2004;94:106. (Abstract). [Google Scholar]
- 6.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. [DOI] [PubMed] [Google Scholar]
- 7.Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 2010;58:398–406. [DOI] [PubMed] [Google Scholar]
- 8.Mues AC, Landman J. Image-guided percutaneous ablation of renal tumors: outcomes, technique, and application in urologic practice. Curr Urol Rep. 2010;11:8–14. [DOI] [PubMed] [Google Scholar]
- 9.Pierorazio PM, Johnson MH, Patel HD, et al. Management of renal masses and localized renal cancer: systemic review and meta-analysis. J Urol. 2016;196:989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer. 2008;113:2671–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malcolm JB, Berry TT, Williams MB, et al. Single center experience with percutaneous and laparoscopic cryoablation of small renal masses. J Endourol. 2009;23:907–911. [DOI] [PubMed] [Google Scholar]
- 12.Strom KH, Derweesh I, Stroup SP, et al. Second prize: recurrence rates after percutaneous and laparoscopic renal cryoablation of small renal masses: does the approach make a difference? J Endourol. 2011;25:371–375. [DOI] [PubMed] [Google Scholar]
- 13.Blute ML, Okhunov Z, Moreira DM, et al. Image-guided percutaneous renal cryoablation: preoperative risk factors for recurrence and complications. BJU Int. 2012;111:e181–e185. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Allaf ME, Kavoussi LR, et al. Computerized tomography guided percutaneous renal cryoablation with the patient under conscious sedation: initial clinical experience. J Urol. 2006;175:447–452.. [DOI] [PubMed] [Google Scholar]
- 15.Permpongkosoi S, Link RE, Kavoussi LR, Solomon SB. Percutaneous computerized tomography guided cryoablation for localized renal cell carcinoma: factors influencing success. J Urol. 2006;176:1963–1968.. [DOI] [PubMed] [Google Scholar]
- 16.Schmit GD, Kurup A, Weisbrod AJ, et al. ABLATE: a renal ablation planning algorithm. AJR AM J Roentgenol. 2014;202:894–903. [DOI] [PubMed] [Google Scholar]
- 17.Pareek G, Hedican SP, Lee FT Jr, Nakada SY. Shock wave lithotripsy success determined by skin-to-stone distance on computed tomography. Urology. 2005;66:941–944. [DOI] [PubMed] [Google Scholar]
- 18.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. [DOI] [PubMed] [Google Scholar]
- 19.Bandi G, Hedican S, Moon T, et al. Comparison of postoperative pain, convalescence, and patient satisfaction after laparoscopic and percutaneous ablation of small renal masses. J Endourol. 2008;22:963–967.. [DOI] [PubMed] [Google Scholar]
- 20.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170(6 Pt 1):2217–2220. [DOI] [PubMed] [Google Scholar]
- 21.Badwan K, Maxwell K, Venkatesh R, et al. Comparison of laparoscopic and percutaneous cryoablation of renal tumors: a cost analysis. J Endourol 2008;22:1275–1277. [DOI] [PubMed] [Google Scholar]
- 22.Camacho JC, Kokabi N, Xing M, et al. R.E.N.A.L. (Radius, Endophytic/Exophytic, nearness to Collecting System or Sinus, Anterior/Posterior, and Location Relative to Polar Lines) nephrometry score predicts early tumor recurrence and complications after percutaneous ablative therapies for renal cell carcinoma: a 5- year experience. J Vasc Interv Radiol. 2015;26:686–693. [DOI] [PubMed] [Google Scholar]
- 23.Atwell TD, Farrell MA, Leibovich BC, et al. Percutaneous renal cryoablation: experience treating 115 tumors. J Urol. 2008;179:2136–2140.. [DOI] [PubMed] [Google Scholar]
- 24.Schmit GD, Thompson RH, Kurup AN, et al. Usefulness of R.E.N.A.L. Nephrometry scoring system for predicting outcomes and complications of percutaneous ablation of 751 renal tumors. J Urol. 2013;189:30–35. [DOI] [PubMed] [Google Scholar]
- 25.Hruby GRK, Venkatesh R, Yan Y, Landman J. Comparison of laparoscopic partial nephrectomy and laparoscopic cryoablation for renal hilar tumors. J Urol. 2006;67:50–54. [DOI] [PubMed] [Google Scholar]
- 26.Kutikov A, Smaldone MC, Egleston BL, et al. Anatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL nephrometry score. Eur Urol. 2011;60:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simhan J, Smaldone MC, Tsia KJ, et al. Objective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomy. Eur Urol. 2011;60:724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prince J, Bultman E, Hinshaw L, et al. Patient and tumor characteristics can predict nondiagnostic renal mass biopsy findings. J Urol. 2015;193:1899–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin J, Patel RM, Okhunov Z, Vyas A, Vajgrt D, Clayman RV. Multipoint thermal sensors associated with improved oncologic outcomes following cryoablation. J Endourol. 2017;31:355–360.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vricella GJ, Haaga JR, Adler BL, et al. Percutaneous cryoablation of renal masses: impact of patient selection and treatment parameters on outcomes. Urology. 2011;77:649–654. [DOI] [PubMed] [Google Scholar]
- 31.Mues AC, Korets R, Graversen JA, et al. Clinical, pathologic, and functional outcomes after nephron-sparing surgery in patients with a solitary kidney: a multicenter experience. J Endourol. 2012;26:1361–1366.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crestani A, Rossanese M, Calandriello M, Sioletic S, Giannarini G, Ficarra V. Introduction to small renal tumours and prognostic indicators. Int J Surg. 2016;36:495–503. [DOI] [PubMed] [Google Scholar]
- 33.Moskowitz D, Chang J, Ziogas A, Anton-Culver H, Clayman RV. Treatment for T1a renal cancer substratified by size: "less is more". J Urol. 2016;196:1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.