Abstract
Background
The COVID-19 pandemic has focused attention on the challenges and risks faced by frontline healthcare workers (HCW). This study aimed to describe the clinical outcomes and risk factors for SARS-CoV-2 infection in HCW.
Methods
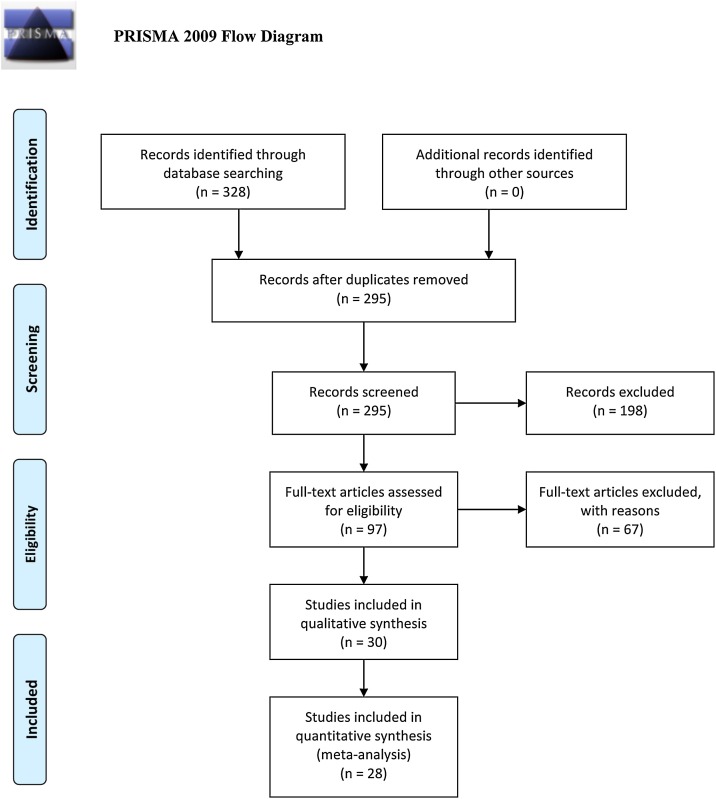
Three databases were surveyed and 328 articles were identified. Of these, 225 articles did not meet inclusion criteria; therefore, 97 full-text article were reviewed. Finally, after further revision, 30 articles were included in the systematic review and 28 were used for meta-analysis.
Results
Twenty-eight studies were identified involving 119,883 patients. The mean age of the patients was 38.37 years (95% CI 36.72–40.03) and males comprised 21.4% (95% CI 12.4–34.2) of the population of HCW. The percentage of HCW who tested positive for COVID-19 was 51.7% (95% CI 34.7–68.2). The total prevalence of comorbidities in seven studies was 18.4% (95% CI 15.5–21.7). The most prevalent symptoms were fever 27.5% (95% CI 17.6–40.3) and cough 26.1% (95% CI 18.1–36). The prevalence of hospitalisation was 15.1% (95% CI 5.6–35) in 13 studies and of death was 1.5% (95% CI 0.5–3.9) in 12 studies. Comparisons of HCW with and without infection showed an increased relative risk for COVID-19 related to personal protective equipment, workplace setting, profession, exposure, contacts, and testing.
Conclusion
A significant number of HCW were reported to be infected with COVID-19 during the first 6 months of the COVID-19 pandemic, with a prevalence of hospitalisation of 15.1% and mortality of 1.5%. Further data are needed to track the continued risks in HCW as the pandemic evolves and health systems adapt.
Keywords: COVID-19, SARS-CoV2, Healthcare workers, Meta-analysis, Occupational health, Infectious disease transmission
Introduction
On 21 December 2019, a pneumonia-like outbreak of an unknown cause or origin was found to be emerging in Wuhan, Hubei Province, China. Due to the rapidly increasing cases and unclear protocol regarding medical care, bronchoalveolar lavage samples of patients were isolated and analysed by 03 January 2020. The reports showcased a new strain of coronavirus, initially termed 2019-nCoVs by the Chinese Center for Disease Control and Prevention (CDC) (Zhang, 2020) and then later named SARS-CoV-2 by the International Committee on Taxonomy of Viruses. On 11 March 2020, the World Health Organization declared the COVID-19 outbreak a pandemic, sending millions into a state of panic and emergency, with many federal governments developing strategies to protect their citizens (World Health Organization).
With limited understanding of this novel coronavirus strain and being at the frontline, healthcare workers (HCW) were soon deemed as one of the groups with the highest risk of exposure to COVID-19 infection. By late January 2020, CDC China reported transmission of COVID-19 to 16 healthcare workers, as a result of being in contact with patients from the outbreak (Li, 2020c). It was speculated that HCW infection could potentially contribute to exacerbating the chain of transmission in hospitals and outside health facilities, and therefore proper protection of HCW against COVID-19 through mandating protective protocols had to be prioritised (Black et al., 2020).
Along with focusing on the impact of COVID-19 on the general population, numerous studies have since been published in different parts of the world outlining the implication of this virus on healthcare systems, pertaining to the challenges and risks faced by the frontline and high-risk HCW. The focus of these research studies has ranged from describing clinical characteristics of HCW with COVID-19, investigating the risk factors involved in acquiring the infection, transmission dynamics among HCW, to stating the observed complications and outcomes of the infection.
This study aimed to combine a systematic review of the published data, with a meta-analysis to determine the risk and clinical outcomes of infection in HCWs at the frontline of diagnosing and caring for COVID-19 infected patients. Furthermore, as part of the qualitative discussion, it aimed to explore the risk factors that may have been involved in the transmission of COVID-19 to HCW.
Methods
Study protocol
The protocol for this study was generated according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) recommendations. The PRISMA checklist was used to guide the reporting (Shamseer et al., 2015).
Information sources and search strategy
PubMed, Scopus and Google Scholar were the three databases that were searched, from 01 May to 09 July 2020, by five independent researchers. All five researchers independently evaluated the search results after finishing the database search process. The search keywords were broadly grouped into four categories: “healthcare”, “risk”, “COVID-19” and “miscellaneous” (Supplementary Table 1).
Eligibility criteria
Full-text, peer reviewed articles from 01 January to 09 July 2020 discussing SARS-CoV-2 only amongst HCW populations were included. Articles that were not in English or an English translation was not available, and articles without comprehensive data, comments or viewpoints related to HCW were excluded from the analysis.
Study selection
Full texts of the selected articles were compared with the pre-determined inclusion and exclusion criteria after the initial search results were screened by title and abstract.
Data collection
The following variables were obtained for all the selected papers: name of authors, year and date of publication, study design, publishing country, and total number of HCW in the study. Regarding the quantitative part of the study, information from the selected articles was extracted by the five independent researchers and then pooled together. Data pertaining to demographics (age and gender), comorbidities (diabetes mellitus, cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), and hypertension), clinical manifestations (fever, cough, fatigue, sputum, headache, haemoptysis, sore throat, diarrhoea, nausea and vomiting), blood investigations (anaemia, white blood cells, high lactate dehydrogenase (LDH), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and high creatinine), complications (unilateral pneumonia, bilateral pneumonia, reactive airway disease, RNA anaemia, shock, hospitalisation, discharge and death) were extracted to Microsoft Excel. Data were screened by a single researcher for duplicates. For the qualitative analysis of the study, 30 articles were thoroughly reviewed by six independent researchers to identify risk factors contributing to COVID-19 infection of HCW.
Statistical approach
The distribution of the categorical dichotomous variables was described by calculating percentages. The mean and 95% confidence intervals (CI) were calculated for continuous data. For studies reporting the mean with 95% CI or the range of the data, the formula (upper limit-lower limit)/4 was used to extract the standard deviation. Meta-analysis using the random-effect model was performed to estimate the pooled prevalence and 95% CI. The pooled percentage, prevalence and corresponding 95% CI were calculated in order to indicate the weighted effect size for all binary variables. The measure of heterogeneity was reported by including Cochran’s Q statistics and I2 index, with the level of heterogeneity defined as poor <25, moderate >50, and high >75, and the Tau square (T2) test. Publication bias was assessed with a funnel plot and Egger’s test.
Results
Search results
Three databases–PubMed, Scopus and Google Scholar–were searched from 01 May 2020 to 09 July 2020 using predefined keywords and a search strategy (Supplementary Table 1). The literature retrieval flowchart is represented in Figure 1 . During the initial phase of the search, 328 articles were identified; 33 duplicates were removed. After screening the abstracts, 198 articles were further excluded due to failure to meet the inclusion criteria. Ninety-seven full-text articles were downloaded and reviewed. Of these, 67 were excluded due to lack of sufficient data, comment or viewpoint, as well as three articles that were in languages other than English and where an English translation of the article was not available. The final count of articles for systematic review was 30, and 28 of those articles, published from February 2020 to June 2020, were used for meta-analysis (Figure 1 and Table 1 ).
Figure 1.
PRISMA flowchart of study selection process.
Table 1.
Summary of characteristics of articles included in the study.
| No. | Author | Journal | Date (MM/YY) | Country | Study type | N (total population) | N HCW with COVID-19 | Quality score | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Zhan et al. | N Engl J Med | 02/20 | China | Cross-sectional | 23 | 23 | 8 | Zhan (2020) |
| 2 | Chu et al. | J Med Virol | 03/20 | China | Retrospective cohort | 54 | 38 | 10 | Chu (2020) |
| 3 | Xing et al. | Euro Surveill | 03/20 | China | Case series | 2 | 2 | 8 | Xing et al. (2020) |
| 4 | Marjolein et al. | JAMA Netw Open | 03/20 | Netherlands | Cross-sectional | 1353 | 86 | 8 | Marjolein (2020) |
| 5 | Zheng et al. | Clin Infect Dis | 03/20 | China | Cross-sectional | 2457 | 2457 | 8 | Zheng et al. (2020) |
| 6 | Li YK et al. | Curr Med Sci | 03/20 | China | Retrospective cohort | 148 | 12 | 10 | Li et al. (2020) |
| 7 | Reusken et al. | Euro Surveill | 03/20 | Netherlands | Cross-sectional | 1097 | 45 | 10 | Reusken et al. (2020) |
| 8 | Ran et al. | Clin Infect Dis | 03/20 | China | Retrospective cohort | 72 | 28 | 11 | Ran et al. (2020) |
| 9 | McMichael et al. | N Engl J Med | 03/20 | United States of America | Retrospective cohort | 50 | 50 | 9 | McMichael (2020) |
| 10 | Sun et al. | J Infect | 03/20 | China | Cross-sectional | 32 | 32 | 7 | Sun et al. (2020) |
| 11 | Burrer et al. | MMWR Morb Mortal Wkly Rep | 04/20 | United States of America | Cross-sectional | 8945 | 8495 | 10 | Burrer (2020) |
| 12 | Wei et al. | J Microbiol Immunol Infect | 04/20 | China | Prospective cohort | 14 | 12 | 10 | Wei et al. (2020) |
| 13 | Kimball et al. | MMWR Morb Mortal Wkly Rep | 04/20 | United States of America | Cross-sectional | – | 1 | 9 | Kimball et al. (2020) |
| 14 | Wang et al. | J Hosp Infect | 04/20 | China | Cross-sectional | 80 | 80 | 8 | Wang et al. (2020) |
| 15 | Schwierzeck et al. | Dtsch Arztebl Int | 04/20 | Germany | Cross-sectional | 957 | 52 | 9 | Schwierzeck et al. (2020) |
| 16 | Canova et al. | Swiss Med Wkly | 04/20 | Switzerland | Cross-sectional | 21 | 0 | 8 | Canova et al. (2020) |
| 17 | Tostmann et al. | Euro Surveill | 04/20 | Netherlands | Cross-sectional | 803 | 90 | 9 | Tostmann et al. (2020) |
| 18 | Heinzerling et al. | MMWR Morb Mortal Wkly Rep | 04/20 | United States of America | Cross-sectional | 43 | 43 | 8 | Heinzerling et al. (2020) |
| 19 | Breazzano et al. | J Clin Invest | 04/20 | United States of America | Cross-sectional | 264 | 101 | 9 | Breazzano et al. (2020) |
| 20 | Nguyen et al. | Lancet Public Health | 05/20 | United States of America | Prospective cohort | 99,795 | 1922 | 11 | Nguyen et al. (2020b) |
| 21 | Lai et al. | JAMA Netw Open | 05/20 | China | Case-series | 110 | 110 | 9 | Lai et al. (2020) |
| 22 | Chow et al. | JAMA Netw Open | 05/20 | United States of America | Cross-sectional | 48 | 48 | 8 | Chow et al. (2020) |
| 23 | Korth et al. | J Clin Virol | 05/20 | Germany | Cross-sectional | 316 | 5 | 9 | Korth et al. (2020) |
| 24 | Felice et al. | J Community Health Res | 05/20 | Italy | Cross-sectional | 388 | 18 | 9 | Felice et al. (2020) |
| 25 | Jin et al. | Mil Med Res | 05/20 | China | Cross-sectional | 103 | 84 | 8 | Jin et al. (2020) |
| 26 | Cabas et al. | Res Social Adm Pharm | 05/20 | Italy | Cross-sectional | 1632 | 15 | 9 | Cabas et al. (2021) |
| 27 | Chen et al. | J Infect | 05/20 | China | Prospective cohort | 105 | 18 | 11 | Chen et al. (2020) |
| 28 | Garzaro et al. | Med Lav | 05/20 | Italy | Cross-sectional | 830 | 80 | 9 | Garzaro et al. (2020) |
| 29 | Guo et al. | J Bone Joint Surg Am | 05/20 | China | Cross-sectional | 24 | 24 | 10 | Guo et al. (2020) |
| 30 | Rivera-Izquierdo et al. | Int J Environ Res Public Health | 06/20 | Spain | Prospective cohort | 76 | 76 | 11 | Rivera-Izquierdo et al. (2020) |
Table 1 provides a summary of characteristics of the included articles. A great variety of articles from different countries were noted: 13 were from China, seven from USA, three each from Netherlands and Italy, two from Germany, and one from Spain. The most common study type amongst the articles was cross-sectional (n = 19) and the remaining were a mix of retrospective and prospective cohort studies, with the exception of one case-series article (Table 1).
Twenty-nine variables were included in the meta-analysis (Table 2, Table 3, Table 4, Table 5, Table 6 ). Most of the studies showed considerable heterogeneity (I2 > 75%) (Table 2). Fewer studies had evidence of bias, as demonstrated by Egger’s test (p > 0.05) (Table 2).
Table 2.
Meta-analysis of healthcare workers.
| Item | No. of studies | Prevalence% | 95% CI | n | Q | I2 | T2 | p-value | Egger’s test |
|---|---|---|---|---|---|---|---|---|---|
| Demographical characteristics | |||||||||
| Age (years, mean) | 24 | 38.73 | 37.83–39.63 | 23 | 2,326.49 | 99.01 | 3.104 | <0.001 | 0.0572 |
| Male | 27 | 21.4 | 12.4–34.2 | 26 | 7,356.1 | 99.6 | 2.796 | <0.001 | 0.8925 |
| Comorbidity | 7 | 18.4 | 15.5–21.7 | 6 | 25.30 | 76.29 | 0.0.037 | <0.001 | 0.5678 |
| DM | 9 | 1.5 | 0.3–8.2 | 8 | 763.46 | 98.95 | 6.311 | <0.001 | 0.2436 |
| Hypertension | 7 | 2.5 | 0.2–27.9 | 6 | 584.15 | 98.90 | 12.69 | <0.001 | 0.2374 |
| CVD | 5 | 2.4 | 0.7–7.5 | 4 | 8.01 | 50.06 | 0.878 | 0.091 | 0.0743 |
| COPD | 5 | 2.4 | 0.9–6.4 | 4 | 6.83 | 41.43 | 0.519 | 0.145 | 0.1083 |
| Clinical manifestations | |||||||||
| Tested positive | 28 | 51.7 | 34.7–68.2 | 27 | 2,611.19 | 98.97 | 2.908 | <0.001 | 0.0001 |
| Fever | 20 | 24.6 | 12.2–43.4 | 19 | 11,287.11 | 99.83 | 3.61 | <0.001 | 0.2096 |
| Cough | 21 | 23.3 | 13.6–37 | 20 | 9,652.89 | 99.79 | 2.15 | <0.001 | 0.1306 |
| Fatigue | 16 | 22.1 | 14.9–31.6 | 15 | 3,139.76 | 99.52 | 0.805 | <0.001 | 0.2816 |
| Sputum | 5 | 17.6 | 10.1–28.8 | 4 | 10.33 | 61.27 | 0.28 | 0.035 | 0.8148 |
| Headache | 16 | 15.1 | 9.0–22.6 | 15 | 3,241.08 | 99.54 | 0.82 | <0.001 | 0.2982 |
| Sore throat | 15 | 13.7 | 9.4–19.5 | 14 | 1,507.93 | 99.07 | 0.49 | <0.001 | 0.1912 |
| Diarrhoea | 16 | 9.8 | 5.3–17.2 | 15 | 3,142.05 | 99.52 | 1.53 | <0.001 | 0.1981 |
| Nausea and vomiting | 10 | 11.8 | 5.8–22.6 | 9 | 143.86 | 93.74 | 1.19 | <0.001 | 0.9016 |
| Laboratory findings | |||||||||
| Leucocytosis | 7 | 49.4 | 10.3–89.2 | 6 | 122.59 | 95.11 | 7.53 | <0.001 | 0.9856 |
| Leukopenia | 4 | 13 | 5.5–27.8 | 3 | 20.92 | 85.66 | 0.75 | <0.001 | 0.1634 |
| Lymphopenia | 4 | 29.1 | 12–55.1 | 3 | 40.52 | 92.60 | 1.112 | <0.001 | 0.4001 |
| High creatinine | 2 | 22.6 | 7.2–52.5 | 1 | 18.44 | 94.58 | 0.869 | <0.001 | NA |
| High LDH | 2 | 12.2 | 0.4–84.3 | 1 | 35.59 | 97.19 | 6.753 | <0.001 | NA |
| High CRP | 3 | 17.3 | 5.1–45 | 2 | 25.33 | 92.11 | 1.321 | <0.001 | 0.6070 |
| Complications | |||||||||
| Unilateral Pneumonia | 2 | 26.8 | 19.4–35.8 | 1 | 0.505 | 0 | 0 | 0.4736 | NA |
| Bilateral Pneumonia | 5 | 78.7 | 43.9–94.6 | 4 | 43.26 | 90.75 | 90.75 | <0.001 | 0.2858 |
| Ground glass Opacity | 5 | 67.5 | 41.4–86 | 4 | 28.46 | 85.95 | 1.092 | <0.001 | 0.3812 |
| ARDS | 2 | 12.2 | 0–97.8 | 1 | 15.74 | 93.65 | 16.344 | <0.001 | NA |
| Outcomes | |||||||||
| Hospitalisation | 13 | 15.1 | 5.6–35 | 14 | 176.195 | 93.19 | 3.167 | <0.001 | 0.4417 |
| Discharge | 7 | 47.5 | 10.9–87 | 6 | 96.035 | 93.74 | 6.289 | <0.001 | 0.7948 |
| Death | 12 | 1.5 | 0.5–3.9 | 11 | 80.56 | 86.35 | 2.066 | <0.001 | 0.1700 |
Abbreviations: No., number; CI, confidence interval; LDH, lactate dehydrogenase; ARDS, acute respiratory distress syndrome; n, degree of freedom; CRP, C-reactive protein; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ARDS, acute respiratory distress syndrome.
Q, Cochran's Q statistic for heterogeneity.
T2, Tau-squared measure of heterogeneity.
I2, Index for the degree of heterogeneity.
Table 3.
Demographical characteristics and comorbidities.
| Author | Date | N | Mean age | Age range | Male | N (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comorbidities | Diabetes | Hypertension | Cardiovascular disease | COPD/lung disease | ||||||
| Burrer et al. | 04/2020 | 8945 | 42 | 16–>65 | 2464 (27.5%) | 1779 (19.9%) | N/A | N/A | N/A | N/A |
| Chu et al. | 03/2020 | 54 | 39 | 26–73 | 36 (66.7%) | N/A | N/A | N/A | N/A | N/A |
| Wei et al. | 04/2020 | 14 | 36 | 27–51 | 4 (28.6%) | 1 (7.7%) | 0 | 0 | 0 | 0 |
| Xing et al. | 03/2020 | 2 | 30 | 20–40 | 1 (50%) | N/A | N/A | N/A | N/A | N/A |
| Zhan et al. | 02/2020 | 23 | 55 | 29–72 | 17 (73.9%) | 5 (21.7%) | N/A | N/A | N/A | N/A |
| Marjolein et al. | 03/2020 | 1353 | 49 | 22–66 | 15 (1.1%) | N/A | N/A | N/A | N/A | N/A |
| Wang et al. | 04/2020 | 80 | 39 | N/A | 31 (38.7%) | N/A | 1 (1.2%) | 10 (12.5%) | 2 (2.5%) | 1 (1.2%) |
| Nguyen et al. | 05/2020 | 99,795 | 42 | N/A | 16,965 (17%) | N/A | 2495 (2.5%) | N/A | 1597 (1.6%) | 13,073 (13.1%) |
| Zheng et al. | 03/2020 | 2457 | N/A | N/A | 681 (27.7%) | N/A | N/A | N/A | N/A | N/A |
| Schwierzeck et al. | 04/2020 | 957 | 35 | N/A | 370 (38.7%) | N/A | N/A | N/A | N/A | N/A |
| Canova et al. | 04/2020 | 21 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Ran et al. | 03/2020 | 72 | 31 | 21–66 | 22 (30.6%) | N/A | N/A | N/A | N/A | N/A |
| Lai et al. | 05/2020 | 110 | 36.5 | N/A | 31 (28.2%) | 14 (12.7%) | 1 (0.9%) | 12 (10.9%) | 0 (0%) | 2 (1.8%) |
| Chow et al. | 05/2020 | 48 | 43 | 22–79 | 11 (22.9%) | N/A | N/A | N/A | N/A | N/A |
| Tostmann et al. | 04/2020 | 803 | N/A | N/A | 19 (2.4%) | N/A | N/A | N/A | N/A | N/A |
| Reusken et al. | 03/2020 | 1097 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Heinzerling et al. | 04/2020 | 43 | 39 | 27–60 | 7 (16.3%) | N/A | N/A | N/A | N/A | N/A |
| Breazzano et al. | 04/2020 | 264 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Korth et al. | 05/2020 | 316 | N/A | N/A | 112 (35.4%) | N/A | N/A | N/A | N/A | N/A |
| Felice et al. | 05/2020 | 388 | N/A | N/A | N/A | 63 (16.2%) | N/A | N/A | N/A | N/A |
| McMichael et al. | 03/2020 | 50 | 43.5 | 21–79 | 12 (24%) | 18 (36%) | 5 (10%) | 4 (8%) | 4 (8%) | N/A |
| Jin et al. | 05/2020 | 103 | 35 | N/A | 39 (37.9%) | N/A | N/A | N/A | N/A | N/A |
| Cabasa et al. | 05/2020 | 1632 | 40.7 | 30–60 | 336 (20.6%) | 269 (16.5%) | N/A | N/A | N/A | N/A |
| Sun et al. | 03/2020 | 32 | 33.8 | 22−56 | 4 (12.5%) | N/A | N/A | N/A | N/A | N/A |
| Chen et al. | 05/2020 | 105 | 30 | 26−39.5 | 2 (1.9%) | N/A | N/A | N/A | N/A | N/A |
| Rivera-Izquierdo et al. | 06/2020 | 76 | 45.8 | N/A | 23 (30.3%) | N/A | 5 (6.6%) | 8 (10.5%) | N/A | 5 (6.6%) |
| Garzaro et al. | 05/2020 | 830 | 46 | N/A | 276 (33.2%) | N/A | N/A | N/A | N/A | N/A |
| Guo et al. | 05/2020 | 24 | 36.1 | 25−48 | 23 (95.8%) | N/A | 1 (4.2%) | N/A | N/A | N/A |
Abbreviations: COPD, chronic obstructive pulmonary disease; N/A, not available; N, number.
Table 4.
Clinical manifestations.
| Author | Date | N | N (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever N (%) | Cough N (%) | Sore throat N (%) | Fatigue N (%) | Sputum N (%) | Headache N (%) | Haemoptysis N (%) | Diarrhoea N (%) | Nausea and vomiting N (%) | |||
| Burrer et al. | 04/2020 | 8945 | 3196 (35.7%) | 3694 (41.3%) | 1790 (20%) | 3122 (34.9%) | N/A | 3048 (34%) | N/A | 1507 (16.8%) | 923 (10.3%) |
| Chu et al. | 03/2020 | 54 | 36 (66.7%) | 17 (31.5%) | 1 (1.8%) | 9 (16.7%) | 3 (5.6%) | N/A | N/A | 3 (5.6%) | 1 (1.8%) |
| Wei et al. | 04/2020 | 14 | 12 (85.7%) | 10 (71.4%) | 7 (50%) | 14 (100%) | 5 (35.7%) | 8 (57.1%) | N/A | 9 (64.3%) | 2 (14.3%) |
| Xing et al. | 03/2020 | 2 | 1 (50%) | 0 (0%) | 1 (50%) | 1 (50%) | 0 (0%) | 1 (50%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Zhan et al. | 02/2020 | 23 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Marjolein et al. | 03/2020 | 1353 | 46 (3.4%) | 66 (4.9%) | 34 (2.5%) | 65 (4.8%) | N/A | 49 (3.6%) | N/A | 16 (1.2%) | 15 (1.1%) |
| Wang et al. | 04/2020 | 80 | 65 (81.2%) | 47 (58.7%) | N/A | 28 (35%) | 19 (23.7%) | 8 (10%) | N/A | 15 (18.7%) | N/A |
| Nguyen et al. | 05/2020 | 99,795 | 2795 (2.8%) | 6986 (7.0%) | 10,079 (10.1%) | 13,772 (13.8%) | N/A | 12,275 (12.3%) | N/A | 3493 (3.5%) | N/A |
| Zheng et al. | 03/2020 | 2457 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Schwierzeck et al. | 04/2020 | 957 | 78 (8.1%) | 345 (36%) | 309 (32.3%) | 11 (1.1%) | N/A | 191 (20%) | N/A | 35 (3.7%) | N/A |
| Canova et al. | 04/2020 | 21 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 21 |
| Ran et al. | 03/2 020 | 72 | 24 (33.3%) | 17 (23.6%) | 2 (2.8) | N/A | N/A | 2 (2.8%) | N/A | 2 (2.8%) | N/A |
| Lai et al. | 05/2020 | 110 | 67 (60.9%) | 62 (56.4%) | 55 (50%) | 66 (60%) | 16 (14.5%) | 33 (30%) | 1 (0.9%) | 39 (35.4%) | 15 (13.6%) |
| Chow et al. | 05/2020 | 48 | 36 (75%) | 42 (87.5%) | 12 (25%) | 14 (29.2%) | N/A | 20 (41.7%) | N/A | 16 (33.3%) | 8 (16.7%) |
| Tostmann et al. | 04/2020 | 803 | 51 (6.3%) | 53 (6.6%) | 36 (4.5%) | 57 (7.1%) | N/A | 64 (8%) | N/A | N/A | N/A |
| Reusken et al. | 03/2020 | 1097 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Heinzerling et al. | 04/2020 | 43 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Breazzano et al. | 04/2020 | 264 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Korth et al. | 05/2020 | 316 | 1 (0.3%) | 0 (0%) | 0 (0%) | 1 (0.3%) | N/A | 2 (0.6%) | N/A | 1 (0.3%) | N/A |
| Felice et al. | 05/2020 | 388 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| McMichael et al. | 03/2020 | 50 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Jin et al. | 05/2020 | 103 | 50 (48.5%) | 35 (34%) | N/A | 43 (41.7%) | N/A | N/A | N/A | N/A | 3 nausea (2.9%) 2 vomiting (1.9%) |
| Cabasa et al. | 05/2020 | 1632 | 127 (7.8%) | 137 (8.4%) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sun et al. | 03/2020 | 32 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Chen et al. | 05/2020 | 105 | 2 (1.9%) | 6 (5.7%) | 3 (2.9%) | N/A | N/A | 2 (1.9%) | N/A | 3 (2.9%) | N/A |
| Rivera-Izquierdo et al. | 06/2020 | 76 | 34 (44.7%) | 47 (61.8%) | 34 (44.7%) | 64 (84.2%) | N/A | 48 (63.2%) | N/A | 31 (40.8%) | 17 nausea (22.4%) 7 vomiting (9.2%) |
| Garzaro et al. | 05/2020 | 830 | 152 (18.3%) | 305 (36.7%) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Guo et al. | 05/2020 | 24 | 20 (83.3%) | 15 (62.5%) | 2 (8.3%) | 17 (70.8%) | N/A | 8 (33.3%) | N/A | 9 (37.5%) | N/A |
Abbreviations: N, number; N/A, not available.
Table 5.
Laboratory investigations.
| Author | Date | N | N (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Leucocytosis N (%) | Leukopenia N (%) | Lymphopenia N (%) | High creatinine N (%) | High LDH N (%) | High CRP N (%) | |||
| Burrer et al. | 04/2020 | 8945 | N/A | N/A | N/A | N/A | N/A | N/A |
| Chu et al. | 03/2020 | 54 | N/A | N/A | N/A | N/A | N/A | N/A |
| Wei et al. | 04/2020 | 14 | N/A | N/A | N/A | N/A | N/A | N/A |
| Xing et al. | 03/2020 | 2 | N/A | N/A | N/A | N/A | N/A | N/A |
| Zhan et al. | 02/2020 | 23 | N/A | N/A | N/A | N/A | N/A | N/A |
| Marjolein et al. | 03/2020 | 1353 | N/A | N/A | N/A | N/A | N/A | N/A |
| Wang et al. | 04/2020 | 80 | 5 (6.2%) | 19 (23.7%) | 38 (47.5%) | 19 (23.7%) | 37 (46.2%) | N/A |
| Nguyen et al. | 05/2020 | 99,795 | N/A | N/A | N/A | N/A | N/A | N/A |
| Zheng et al. | 03/2020 | 2457 | N/A | N/A | N/A | N/A | N/A | N/A |
| Schwierzeck et al. | 04/2020 | 957 | N/A | N/A | N/A | N/A | N/A | N/A |
| Canova et al. | 04/2020 | 21 | N/A | N/A | N/A | N/A | N/A | N/A |
| Ran et al. | 03/2020 | 72 | N/A | N/A | N/A | N/A | N/A | N/A |
| Lai et al. | 05/2020 | 110 | N/A | N/A | N/A | N/A | N/A | N/A |
| Chow et al. | 05/2020 | 48 | N/A | N/A | N/A | N/A | N/A | N/A |
| Tostmann et al. | 04/2020 | 803 | N/A | N/A | N/A | N/A | N/A | N/A |
| Reusken et al. | 03/2020 | 1097 | N/A | N/A | N/A | N/A | N/A | N/A |
| Heinzerling et al. | 04/2020 | 43 | N/A | N/A | N/A | N/A | N/A | N/A |
| Breazzano et al. | 04/2020 | 264 | N/A | N/A | N/A | N/A | N/A | N/A |
| Korth et al. | 05/2020 | 316 | N/A | N/A | N/A | N/A | N/A | N/A |
| Felice et al. | 05/2020 | 388 | N/A | N/A | N/A | N/A | N/A | N/A |
| McMichael et al. | 03/2020 | 50 | N/A | N/A | N/A | N/A | N/A | N/A |
| Jin et al. | 05/2020 | 103 | N/A | 24.1% | 39.5% | N/A | N/A | 31.8% |
| Cabasa et al. | 05/2020 | 1632 | N/A | N/A | N/A | N/A | N/A | N/A |
| Sun et al. | 03/2020 | 32 | N/A | N/A | N/A | N/A | N/A | N/A |
| Chen et al. | 05/2020 | 105 | N/A | N/A | N/A | N/A | N/A | N/A |
| Rivera-izquierdo et al. | 06/2020 | 76 | N/A | N/A | N/A | N/A | N/A | N/A |
| Garzaro et al. | 05/2020 | 830 | N/A | N/A | N/A | N/A | N/A | N/A |
| Guo et al. | 05/2020 | 24 | N/A | 3 (12.5%) | 14 (58.3%) | N/A | N/A | 6 (25%) |
Abbreviations: LDH,lactate dehydrogenase; CRP, C-reactive protein; N/A, not available.
Table 6.
Course of disease and complications.
| Author | Date | N | N (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unilateral pneumonia N (%) | Bilateral pneumonia N (%) | Ground-glass opacity N (%) | ARDS N (%) | Hospitalisation N (%) | Discharge N (%) | Death N (%) | |||
| Burrer et al. | 04/2020 | 8945 | N/A | N/A | N/A | N/A | 723 (8.1%) | N/A | 27 (0.3%) |
| Chu et al. | 03/2020 | 54 | N/A | 46 (85.2%) | 39 (72.2%) | N/A | 54 (100%) | 15 (27.8%) | 1 (1.8%) |
| Wei et al. | 04/2020 | 14 | N/A | 12 (85.7%) | 12 (85.7%) | N/A | 14 (100%) | 14 (100%) | 0 (0%) |
| Xing et al. | 03/2020 | 2 | 1 (50%) | 0 (0%) | 0 (0%) | N/A | 2 (100%) | 2 (100%) | 0 (0%) |
| Zhan et al. | 02/2020 | 23 | N/A | N/A | N/A | 16 (69.6%) | 23 (100%) | 0 (0%) | 23 (100%) |
| Marjolein et al. | 03/2020 | 1353 | N/A | N/A | N/A | N/A | 27 (2%) | N/A | N/A |
| Wang et al. | 04/2020 | 80 | N/A | 79 (98.7%) | N/A | 3 (4%) | 80 (100%) | 78 (97.5%) | 1 (1.25%) |
| Nguyen et al. | 05/2020 | 99,795 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Zheng et al. | 03/2020 | 2457 | N/A | N/A | N/A | N/A | N/A | N/A | 17 (0.7%) |
| Schwierzeck et al. | 04/2020 | 957 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Canova et al. | 04/2020 | 21 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Ran et al. | 03/2020 | 72 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lai et al. | 05/2020 | 110 | 29 (26.4%) | 49 (44.5%) | 45 (40.9%) | N/A | N/A | N/A | N/A |
| Chow et al. | 05/2020 | 48 | N/A | N/A | N/A | N/A | 3 (6.2%) | N/A | 0 (0%) |
| Tostmann et al. | 04/2020 | 803 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Reusken et al. | 03/2020 | 1097 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Heinzerling et al. | 04/2020 | 43 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Breazzano et al. | 04/2020 | 264 | N/A | N/A | N/A | N/A | 2 (0.8%) | 2 (0.8%) | 0 (0%) |
| Korth et al. | 05/2020 | 316 | N/A | N/A | N/A | N/A | 0 (0%) | N/A | N/A |
| Felice et al. | 05/2020 | 388 | N/A | N/A | N/A | N/A | 1 (0.3%) | N/A | N/A |
| McMichael et al. | 03/2020 | 50 | N/A | N/A | N/A | N/A | 3 (6%) | N/A | 0 (0%) |
| Jin et al. | 05/2020 | 103 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Cabasa et al. | 05/2020 | 1632 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sun et al. | 03/2020 | 32 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Chen et al. | 05/2020 | 105 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Rivera-Izquierdo et al. | 06/2020 | 76 | N/A | N/A | N/A | N/A | 11 (14.5%) | N/A | N/A |
| Garzaro et al. | 05/2020 | 830 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Guo et al. | 05/2020 | 24 | N/A | N/A | 21 (87.5%) | N/A | 15 (62.5%) | 16 (66.7%) | 0 (0%) |
Meta-analysis results
Demographic characteristics
The total number of patients analysed across the 28 studies was 119,883. The mean age of the patients was 38.37 years (95% CI 36.72–40.03) and males comprised 21.4% (95% CI 12.4–34.2) of the population of HCW (Table 2).
Comorbidities
The total prevalence of comorbidities in the seven included studies was 18.4% (95% CI 15.5–21.7), the most prevalent being hypertension 2.5% (95% CI 0.2–27.2), CVD 2.4% (95% CI 0.7–7.5), COPD 2.4 (95% CI 0.9–6.4), and diabetes 1.4% (95% CI 0.1–12.9) (Table 2).
Clinical manifestations
Across 28 studies, 51.7% (95% CI 34.7–68.2) of HCW tested positive for COVID-19. Regarding the symptoms of COVID-19 amongst HCW, the most prevalent finding was fever 27.5% (95% CI 17.6–40.3), followed by cough 26.1% (95% CI 18.1–36), fatigue 23.4% (95% CI 12.7–39), sputum 17.6% (95% CI 10.1–28.8), headache 15.1% (95% CI 9.0–24.1), sore throat 13.3% (95% CI 8.2–20.9), nausea and vomiting 11.8% (95% CI 5.8–22.6), and diarrhoea 10.6% (95% CI 5.9–18.4) (Table 2).
Blood investigations and imaging
The most prevalent laboratory finding was leucocytosis 49.4% (95% CI 10.3–89.2), followed by lymphopenia 29.1% (95% CI 12–55.1), high creatinine 22.6% (95% CI 7.2–52.5), high CRP 17.3% (95% CI 5.1–45), leukopenia 13% (95% CI 5.5–27.8), and high LDH 12.2% (95% CI 0.4–84.3). Regarding radiological imaging, the most common pneumonia finding was bilateral pneumonia, with a prevalence of 78.7% (95% CI 43.9–94.6). Other findings included ground-glass opacity with a prevalence of 67.5% (95% CI 41.4–86) and unilateral pneumonia with a prevalence of 26.8% (95% CI 19.4–35.8) (Table 2).
Course of illness, complications and outcomes
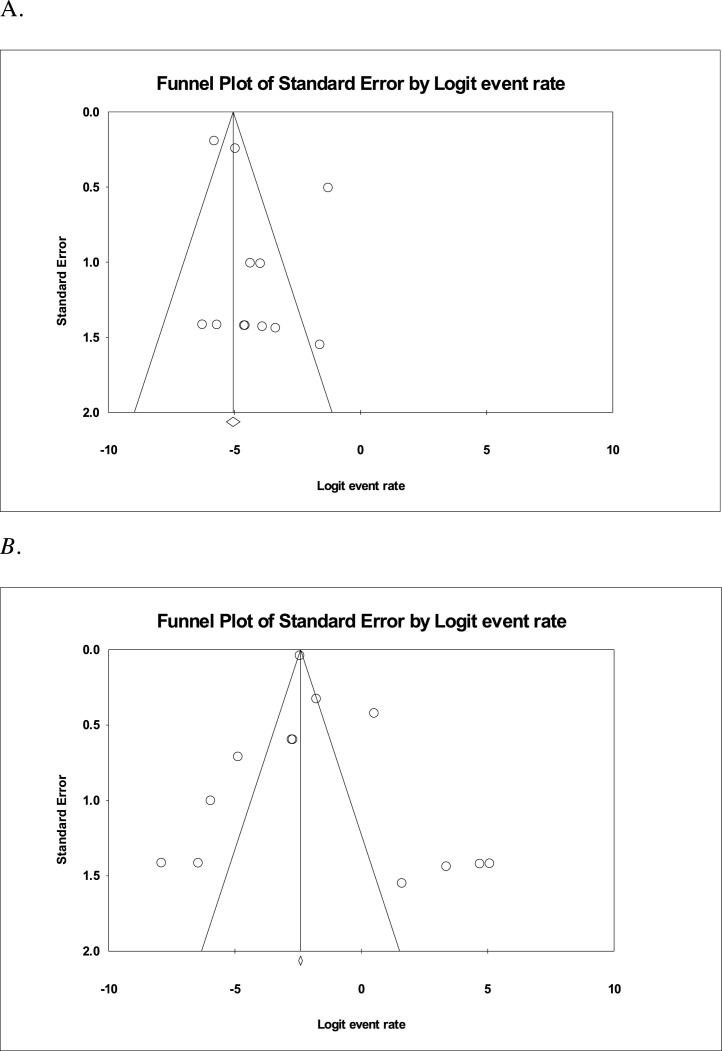
Two studies reported ARDS as a complication of COVID-19 infection, with a prevalence of 12.2% (95% CI 0–97.8). Across 13 studies, using the random-effect model to find the pooled prevalence and 95% CI, the prevalence of hospitalisation of HCW was 15.1% (95% CI 5.6–35) and across seven studies, prevalence of discharge from the hospital was 47.5% (95% CI 10.9–87). In 12 studies, prevalence of death was 1.5% (95% CI 0.5–3.9) (Table 2). Funnel plots of hospitalisations and deaths are shown in Figure 2 , and these indicate a minimal risk of bias related to death rates, but more potential bias in terms of reporting hospitalisation rates.
Figure 2.
(A) Funnel plot showing death among healthcare workers. The standard errors are well distributed showing minimal bias. (B) Funnel plot showing hospitalisation among HCW with COVID-19. The standard errors are widely distributed and demonstrates more potential bias.
Risk factors
Thirty articles were thoroughly revised by six independent researchers looking for risk factors contributing to HCW COVID-19 infection. Of 30 articles, seven yielded information regarding the pertinent risk factors. A summary of the main points regarding risk factors in the respective articles can be found in Table 7 . The identified risk factors were categorised into the following six entities: personal protective equipment (PPE), workplace setting, profession, exposure, contacts, and testing (Table 7).
Table 7.
Risk factors of COVID-19 infection in healthcare worker populations.
| Reference | Risk factor category | Data |
|---|---|---|
| Felice et al. (2020) | Personal protective equipment (PPE) | PPEs were more readily available in high-risk specialty sectors OR 1.96 (95% CI 0.98–3.94) vs. less likely for HCW with recent onset of symptoms OR 0.48 (95% CI 0.28–0.83) |
| Nguyen et al. (2020a, Nguyen et al., 2020 | PPE | Compared with HCW with adequate PPE supply: |
|
||
|
||
|
||
|
||
| Ran et al. (2020) | PPE | Compared to HCW without infection: |
|
||
|
||
|
||
|
||
| Chen et al. (2020) | PPE | Face mask use reduced risk of infection OR 0.127 (95% CI 0.017–0.968) |
| Guo et al. (2020) | PPE |
|
|
||
|
||
|
||
|
||
| Felice et al. (2020) | Exposure | Those reporting typical symptoms during the last two weeks were more likely, but not statistically significant, to come from high-prevalence regions OR 1.48 (95% CI 0.93–2.37) |
| Korth et al. (2020) | Exposure | Seroprevalence: Higher in the intermediate-risk (with daily non-COVID-19 patient contact) vs. high-risk group (daily contact to COVID-19 patients on the designated wards and on the intensive care units) OR 0.22 (confidence interval (95% CI 0.04–1.35) |
| Nguyen et al. (2020a) | Workplace setting | Compared with risk for the general community, risk for front-line healthcare workers was increased in all healthcare settings, but was highest for those working in inpatient settings (adjusted HR 24.30, 95% CI 21.83–27.06) and nursing homes (16.24, 13.39–19.70) |
| Ran et al. (2020) | Workplace setting | High-risk department (with interventional medical or surgical procedures that generate respiratory aerosols, including the respiratory department, infection department, ICU and the surgical department) vs. general department group (crude RR 2.13, 95% CI 1.45–3.95, p < 0.05). |
| Garzaro et al. (2020) | Workplace setting | Sharing work environment was associated with increased risk OR 2.63 (95% CI 1.34–5.32) |
| Chen et al. (2020) | Profession | Risk of infection highest in physicians exposed to positive COVID-19 patients OR 346.83 (95% CI 8.924–13479.434) compared with nurses OR 19.523 (95% CI 0.667–571.463) or general service employees OR 13.294 (95% CI 0.265–666.605) |
| Garzaro et al. (2020) | Profession |
|
|
||
| Ran et al. (2020) | Contacts |
|
|
||
| Suspected patient: RR 0.49 (95% CI 0.27–0.89, p < 0.05) | ||
| Felice et al. (2020) | Testing |
|
Discussion
This systematic review and meta-analysis summarised the available clinical information and characteristics of HCW with COVID-19, as well as the risk factors involved in making them more susceptible to the infection. The PRISMA guidelines were followed and 30 articles were filtered in three online databases (Shamseer et al., 2015). This article analysed 119,883 HCW, with a 51.7% prevalence of testing positive for COVID-19 from the analysable reports. Note that many of these reports included only HCW with COVID-19 infections. The articles were primarily from China, and additional countries included USA, Netherlands, Italy, Germany, and Spain (Table 1).
Of the HCW who were analysed, a wide spectrum of symptoms, comorbidities and complications were observed. As a group, HCW were generally found to be a young working age population (mean age 38.73 years), and the clinical characteristics of this group were likely similar to others in this age distribution. It was found that the predominant symptoms in HCW with COVID-19 included fever, closely followed by cough and fatigue (Guan et al., 2020, Li et al., 2020b, Sun et al., 2020). Patients with comorbidities have been shown to have a greater risk of symptomatic infection with COVID-19, with a worse prognosis than those without (Sanyaolu et al., 2020). In this study, 18.4% of the infected healthcare workers had pre-existing conditions. While hypertension was deemed to be the most prevalent (2.5%), CVD and COPD closely followed with a prevalence of 2.4%, and diabetes was present in 1.4%. These findings contrast with preliminary data related to comorbidities in the general population of COVID-19 patients found in a metanalysis of reports from China, where the prevalence of these comorbidities was higher: hypertension in 15.8%, CVD in 11.7%, diabetes in 9.4%, and COPD in 1.4%. The generally lower prevalence rates of comorbidities in HCW compared with the general population is likely explained by ‘The Healthy Worker Effect’ phenomenon, which has been described by some as “the reduction of mortality or morbidity of occupational cohorts when compared with the general population” (Shah, 2009).
Along with comorbidities, this study additionally explored the main laboratory findings in COVID-19 infection: leucocytosis, lymphopenia and an elevated CRP. In line with the laboratory results in this study, other studies have reported a decrease in CD4+ and CD8+ cells, attributed to lymphocyte consumption during the infection process, and an increased cytokine release, which is co-related with disease severity and mortality (Huang et al., 2020, Li et al., 2020a, Qin et al., 2020, Ruan et al., 2020). Bilateral pneumonia was the most observed imaging finding within the current analysis for HCW, followed by ground-glass opacity. Ground-glass opacity was found to be the most common finding amongst patients in the general population, whereas consolidations were more frequently seen amongst those who were deemed to be severely ill (Li et al., 2020b). No results for the presence of shock, anaemia or elevated ESR were described in the analysed papers.
The outcomes of COVID-19 in HCW remained markedly better compared with those reported from most studies from the general population. Overall, 15% required hospitalisation, approximately 50% were discharged and death was reported in 1% of HCW with COVID-19. Factors in favour of undesirable outcomes amongst COVID-19 patients included presence of previous comorbidities, especially CVD, secondary infection, and elevated inflammatory markers on laboratory analysis (Ruan et al., 2020). This is in contrast with prior reports of SARS-CoV-1 and MERS amongst HCW. Between 2012 and 2018, 415 MERS-CoV-positive HCW were reported to the WHO, amongst which 24 (5.8%) died as a direct result of the infection (Elkholy et al., 2020). At that time, HCW with renal impairment were noted to be at highest risk of death (Shalhoub et al., 2018). Due to the limited available data on SARS until 2003, with a relatively low total number of cases reported by the WHO (8096 cases and 774 deaths), comparison with the current pandemic trend is difficult (WHO| Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003, 2015). Evidence of a definitive mortality rate for HCW infected with Sars-CoV-1 was not found. Xiao et al. estimated that deaths in HCW due to SARS-CoV-1 could be up to 164 of the total 774 deaths (21%), although they stated that this number might have been exaggerated due to factors of younger age and good immunity of the frontline HCW (Xiao et al., 2020).
The largest reported series came from a study reaching HCW using a novel smartphone “Covid Symptom Study” application that was used by 2,035,395 individuals in the United Kingdom and the United States (Nguyen et al., 2020a). Among these were 99,795 individuals who identified themselves as HCW and reported information related to symptoms and use of PPE. Of the identified HCW there were 1922 (1.9%) that reported testing positive for Covid-19 compared with 3623 (0.18%) general population subjects testing positive for Covid-19. Reported rates of comorbidities in HCW were higher in this series, especially for the presence of lung disease. The methods for obtaining these novel self-reported data will need further verification, and data related to hospitalisation and death were not reported.
This review of risk factors amongst HCW who tested positive for SARS-CoV-2 infection found measurements of risk for the following factors: PPE, workplace setting, profession, exposure, contacts, and testing (Table 7). Face masks were shown to be protective, and having worn one at all times decreased the risk of infection (Chen et al., 2020, Guo et al., 2020). PPE training has been reported to be a protective factor, while lack of N95 masks, reused PPE and suboptimal hand hygiene practices were risk factors for infection with COVID-19 (Guo et al., 2020, Nguyen et al., 2020b, Ran et al., 2020). The highest risk was reported to be among physicians exposed to COVID-19-positive patients when compared with nurses and general service employees. Physicians at highest risk were those involved in interventional or surgical procedures that generated respiratory aerosols, including within respiratory departments, infection control departments, ICU and surgical departments (Ran et al., 2020). There was no association between risk of infection and length of exposure or distance with positive patients (Garzaro et al., 2020). An overall increased risk of infection was noted in frontline HCW in all healthcare settings as compared with the general community, with a higher risk in HCW working in inpatient settings and nursing homes (Nguyen et al., 2020b). Most of the extracted risk factor data have been reported from China, followed by Italy, US, UK, and Germany. Data reported around December–February by Guo et al. reflected PPE training as the concerned risk factor as opposed to papers published later on from March to May, which reported inadequate PPE availability, work environments and contact exposure as the primary risk factors for HCW (Guo et al., 2020).
With the rapid global spread of this novel coronavirus strain, it soon became evident that much research is needed to understand and contain this infection, particularly for frontline HCW. The data encompassed by this review reflect the first 6 months after the official declaration of COVID-19 as a pandemic and the early experience of the disease in HCW with a previously unknown virus. The overall global magnitude of COVID-19 in HCWs was recently documented by a survey of members of the ID-IRI (Infectious Diseases International Research Initiative) from 37 countries through 15 August 2020 (Erdem and Lucey, 2021). They documented 2736 HCW deaths with a mortality rate of 0–0.90/100,000 in the reporting countries. Further data will be needed to continue to understand the evolving implication of this pandemic on the health and well-being of healthcare workers internationally.
Conflict of interest
None declare.
Funding source
None declare.
Ethical approval
Approval was not required for this study.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2021.01.013.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Black J.R.M., Bailey C., Przewrocka J., Dijkstra K.K., Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418–1420. doi: 10.1016/S0140-6736(20)30917-X. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breazzano M.P., Shen J., Abdelhakim A.H., Dagi Glass L., Horowitz J., Xie S.X. New York City COVID-19 resident physician exposure during exponential phase of pandemic. J Clin Invest. 2020;(May) doi: 10.1172/JCI139587. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC COVID-19 Response Team. Burrer S.L. Characteristics of health care personnel with COVID-19–United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477–481. doi: 10.15585/mmwr.mm6915e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabas P., di Bella S., Giuffrè M., Rizzo M., Trombetta C., Luzzati R. Community pharmacists’ exposure to COVID-19. Res Social Adm Pharm. 2021;17(1):1882–1887. doi: 10.1016/j.sapharm.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canova V., Lederer Schläpfer H., Piso R.J., Droll A., Fenner L., Hoffmann T. Transmission risk of SARS-CoV-2 to healthcare workers-observational results of a primary care hospital contact tracing. Swiss Med Wkly. 2020;150:w20257. doi: 10.4414/smw.2020.20257. PMID: 32333603. [DOI] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020:368. doi: 10.1136/bmj.m1091. https://www.bmj.com/content/368/bmj.m1091 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow E.J., Schwartz N.G., Tobolowsky F.A., Zacks R.L.T., Huntington-Frazier M., Reddy S.C. Symptom screening at illness onset of health care personnel with SARS-CoV-2 infection in King County, Washington. JAMA. 2020;323(20):2087–2089. doi: 10.1001/jama.2020.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Jiaojiao. Clinical characteristics of 54 medical staff with COVID‐19: a retrospective study in a single center in Wuhan, China. J Med Virol. 2020;92(7):807–813. doi: 10.1002/jmv.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkholy A.A., Grant R., Assiri A., Elhakim M., Malik M.R., Van Kerkhove M.D. MERS-CoV infection among healthcare worker and risk factors for death: retrospective analysis of all laboratory-confirmed cases reported to WHO from 2012 to 2 June 2018. J Infect Public Health. 2020;13(3) doi: 10.1016/j.jiph.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem H., Lucey D.R. Healthcare worker infections and deaths due to COVID-19: a survey from 37 nations and a call for WHO to post national data on their website. Int J Infect Dis. 2021;102:239–241. doi: 10.1016/j.ijid.2020.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice C., di Tanna G.L., Zanus G., Grossi U. Impact of COVID-19 outbreak on healthcare workers in Italy: results from a national e-survey. J Community Health. 2020;45:675–683. doi: 10.1007/s10900-020-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzaro G., Clari M., Ciocan C., Grillo E., Mansour I., Godono A. COVID-19 infection and diffusion among the healthcare workforce in a large university-hospital in Northwest Italy. Med Lav. 2020;111(3):184–194. doi: 10.23749/mdl.v111i3.9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Wang J., Hu D., Wu L., Gu L., Wang Y. Survey of COVID-19 disease among orthopaedic surgeons in Wuhan, People’s Republic of China. J Bone Joint Surg Am. 2020;102(10):847–854. doi: 10.2106/JBJS.20.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling A., Stuckey M.J., Scheuer T., Xu K., Perkins K.M., Resseger H. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient–Solano County, California, February 2020. Morb Mortal Wkly Rep. 2020;69(15):472–476. doi: 10.15585/mmwr.mm6915e5. http://www.ncbi.nlm.nih.gov/pubmed/32298249 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.-H., Huang Q., Wang Y.-Y., Zeng X.-T., Luo L.-S., Pan Z.-Y. Perceived infection transmission routes, infection control practices, psychosocial changes, and management of COVID-19 infected healthcare workers in a tertiary acute care hospital in Wuhan: a cross-sectional survey. Mil Med Res. 2020;7(1):24. doi: 10.1186/s40779-020-00254-8. https://mmrjournal.biomedcentral.com/articles/10.1186/s40779-020-00254-8 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball A., Hatfield K.M., Arons M., James A., Taylor J., Spicer K. Asymptomatic and presymptomatic SARS-COV-2 infections in residents of a long-term Care Skilled Nursing Facility—King County, Washington, March 2020. Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. DOI: https://doi.org/10.15585/mmwr.mm6913e1external icon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X., Wang M., Qin C., Tan L., Ran L., Chen D. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Wu J., Wu F., Guo D., Chen L., Fang Z. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55(6):327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. An outbreak of NCIP (2019-nCoV) infection in China—Wuhan, Hubei Province, 2019–2020. China CDC Wkly. 2020;2(5):79–80. [PMC free article] [PubMed] [Google Scholar]
- Li Y.K., Peng S., Li L.-Q., Wang Q., Ping W., Zhang N. Clinical and transmission characteristics of Covid-19–a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci. 2020;40(2):295–300. doi: 10.1007/s11596-020-2176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjolein F.Q. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael T.M. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C.G., Ma W. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Pub Health. 2020;5(9):e475–83. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.H., Drew D.A., Joshi A.D., Guo C.-G., Ma W., Mehta R.S. Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. medRxiv. 2020;5(9):E475–E483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran L., Chen X., Wang Y., Wu W., Zhang L., Tan X. Risk factors of healthcare workers with corona virus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020;71(16):2218–2221. doi: 10.1093/cid/ciaa287. PMID: 32179890; PMCID: PMC7184482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C.B., Buiting A., Bleeker-Rovers C., Diederen B., Hooiveld M., Friesema I. Rapid assessment of regional SARS-CoV-2 community transmission through a convenience sample of healthcare workers, the Netherlands, March 2020. Euro Surveill. 2020;25(12):2000334. doi: 10.2807/1560-7917.ES.2020.25.12.2000334. PMID: 32234115; PMCID: PMC7118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Izquierdo M., Valero-Ubierna M.D.C., Martínez-Diz S., Fernández-García M.Á, Martín-Romero D.T., Maldonado-Rodríguez F. Clinical factors, preventive behaviours and temporal outcomes associated with COVID-19 infection in health professionals at a Spanish hospital. Int J Environ Res Public Health. 2020;17(12):1–13. doi: 10.3390/ijerph17124305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [Internet]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020;2(8):1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwierzeck V., König J.C., Kühn J., Mellmann A., Correa-Martínez C.L., Omran H. First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric dialysis unit. Clin Infect Dis. 2020;(April) doi: 10.1093/cid/ciaa491. ciaa491, Epub ahead of print. PMID: 32337584; PMCID: PMC7197625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. HWE-phenomenon. Indian J Occup Environ Med. 2009;13(2):77–79. doi: 10.4103/0019-5278.55123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalhoub S., Al-Hameed F., Mandourah Y., Balkhy H.H., Al-Omari A., al Mekhlafi G.A. Critically ill healthcare workers with the middle east respiratory syndrome (MERS): a multicenter study. PLoS One. 2018;13(11):1–12. doi: 10.1371/journal.pone.0206831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. Erratum in: BMJ. 2016 Jul 21;354:i4086. PMID: 25555855. [DOI] [PubMed] [Google Scholar]
- Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–617. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostmann A., Bradley J., Bousema T., Yiek W.-K., Holwerda M., Bleeker-Rovers C. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Euro Surveill. 2020;25(16):2000508. doi: 10.2807/1560-7917.ES.2020.25.16.2000508. PMID: 32347200; PMCID: PMC7189649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X.-S., Wang X.-R., Zhang J.-C., Yang W.-B., Ma W.-L., Yang B.-H. A cluster of health care workers with COVID-19 pneumonia caused by SARS-CoV-2. J Microbiol Immunol Infect. 2020;(April) doi: 10.1016/j.jmii.2020.04.013. S1684-1182(20)30107-9 Epub ahead of print. PMID: 32359943; PMCID: PMC7185000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2015. Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003.https://www.who.int/csr/sars/country/table2004_04_21/en/ [Internet] Available from: [Google Scholar]
- Xiao J., Fang M., Chen Q., He B. SARS, MERS and COVID-19 among healthcare workers: a narrative review. J Infect Public Health. 2020;13(6):843–848. doi: 10.1016/j.jiph.2020.05.019. Epub 2020 May 27. PMID: 32493671; PMCID: PMC7250777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Mo P., Xiao Y., Zhao O., Zhang Y., Wang F. Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Euro Surveill. 2020;25(March (10)) doi: 10.2807/1560-7917.ES.2020.25.10.2000191. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.10.2000191 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan M. Death from Covid-19 of 23 health care workers in China. N Engl J Med. 2020;382(23):2267–2268. doi: 10.1056/NEJMc2005696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)-China, 2020. CCDC Weekly. 2020;41(2):113–122. [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Wang X., Zhou C., Liu Q., Li S., Sun Q. Analysis of the infection status of the health care workers in Wuhan during the COVID-19 outbreak: a cross-sectional study. Clin Infect Dis. 2020;71(16):2109–2113. doi: 10.1093/cid/ciaa588. PMID: 32409825; PMCID: PMC7239233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.