Since the recognition of SARS-CoV-2 virus in December 2019 there have been more than seventy-two million cases and greater than 1.6 million deaths globally, as well as more than 300,000 deaths in the United States attributable to COVID-19 [1]. COVID-19 has had a dramatic impact on modern global society and will be a major part of the collective global biosphere for the foreseeable future. Similar to the response to other modern-era infectious disease outbreaks (Human Immunodeficiency Virus, H1N1 Influenza, Ebola virus, and Zika virus) vaccination is a potent mitigation strategy being aggressively pursued against SARS-CoV-2. The strategic approach to vaccine development has been outlined by global scientific leaders [2]. As the approach unfolds for the development, testing, and implementation, pregnant women represent an important albeit scientifically complex population(s) that warrants consideration and responsible inclusion throughout the entire process. The complexity of pregnancy should be viewed as an opportunity to generate much needed evidence through responsible inclusion of these women in research, rather than a barrier to progress and reason for unjust exclusion, which has been the norm for decades.
For many respiratory infectious diseases, pregnant women and neonates are two high-risk populations that suffer disproportionate rates of morbidity and mortality. The cumulative data thus far suggest that pregnant women are at a higher risk for serious morbidities from COVID-19, albeit more modest than other pathogens, such as 2009 H1N1 Influenza. These heightened morbidities are noted in terms of an increased need for intensive care, mechanical ventilation and death among symptomatic pregnant women, as well as suggestions of increased rates of preterm birth [3], [4]. For many decades the powerful concept of passive immunization of the neonate via maternal infection or immunization and transplacental passage of protective antibody into the fetal/neonatal circulation has been recognized, with protection afforded against tetanus, smallpox, influenza, and pertussis, among other pathogens [5]. Thus, maternal vaccination can protect the mother, the fetus, and the infant. As one example, influenza vaccination during pregnancy decreases the risk of severe disease in the mother, which positively impacts the fetus by reducing the risk of preterm birth and/or pregnancy loss, as well as providing protection for the infant during the first few months of life [5]. Therefore, a single intervention offers powerful protection for two susceptible individuals who are at increased risk of a disease and its consequences.
There is an important need to demonstrate safety of vaccine products specifically during pregnancy. Moreover, given the distinct physiologies and susceptibilities of pregnancy, response to vaccination may differ from that of the general population (although this has not generally been noted to date). Consequently, optimal public health programming and clinical use requires pregnancy-specific data. Evidence from research evaluations of vaccines in pregnancy may increase vaccine confidence in pregnant women and their obstetric providers so that women themselves are protected, as well as facilitate efforts to achieve herd immunity among the entire population. These efforts will help also to decrease household transmissions among children potentially too young to receive vaccination, as vaccine becomes available for public use. Additionally, as prioritization schemata have been designed for novel coronavirus vaccines, it is apparent that reproductive-age and pregnant women represent a significant proportion of the health-care workforce that are at top priority for receipt. Pregnancy-specific data will help to ensure that those at highest risk have access to vaccine. Encouragingly, some pharmaceutical manufacturers, working in collaboration with the National Institutes of Health, are beginning to share their plans in designing and sponsoring pregnancy-specific trials. This is a welcome development and in line with a United States Lawmakers’ requests written near the outset of the pandemic [6].
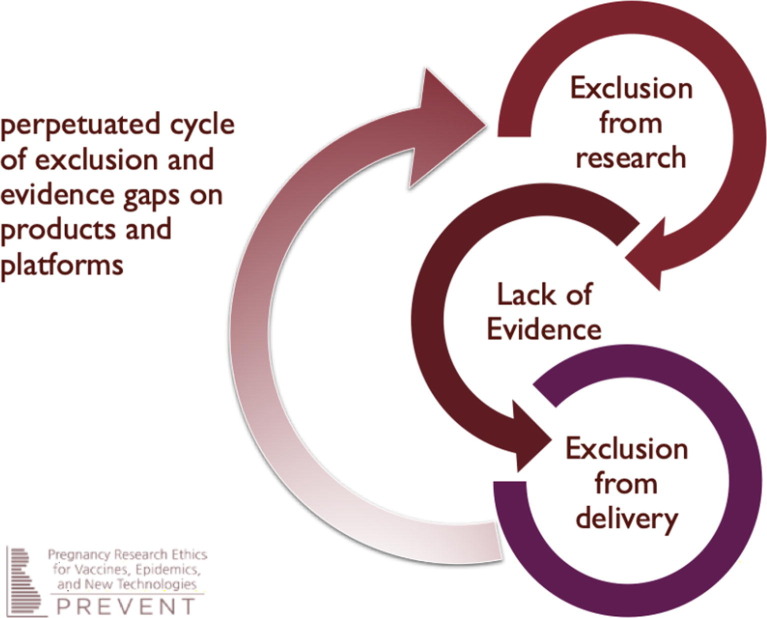
While there is a clear need to include pregnant women in COVID-19 vaccine research efforts, there are barriers to gathering needed data and ensuring that this population is protected in the epidemic response. Pregnant women have been historically excluded from vaccine trials, except those that were carried out for 2009 H1N1 influenza vaccines (due in large part to years of use of seasonal influenza vaccines in pregnancy), pertussis vaccines, and most recently vaccines expressly developed for maternal immunization. Without timely and robust evidence about safety and efficacy during pregnancy, pregnant women have previously been denied opportunities to receive vaccines that would have protected them and their offspring in numerous epidemics. When pregnant women are neither expressly considered nor prioritized in early efforts to develop vaccines, they are in turn excluded from participating in research and the generation of evidence, which then results in exclusion from vaccine delivery programs. This perpetuated cycle of exclusion (Fig. 1 ) is profoundly unjust and deeply problematic. As a recent important example, exclusion of pregnant women from earlier Ebola vaccine trials in 2015–2016 meant that suboptimal data were available only from the small number of inadvertent exposures during pregnancy. This in turn led to the exclusion of pregnant women from subsequent Ebola vaccine trial and deployment activities in 2018–2019, despite clear signals that Ebola-related outcomes were worse during pregnancy, including devastating consequences for the woman and fetus [7]. The global medical and research establishment can and should do better with the response to COVID-19.
Fig. 1.
Cycle of exclusion [8].
If this pattern persists in the context of COVID-19, we risk a double injustice: (1) pregnant women would be unfairly denied opportunities to participate in COVID-19 vaccine studies that may directly benefit them and/or their offspring and (2) as the response continues, all pregnant women, their providers, and health policymakers would have to make unnecessarily difficult decisions because of inadequate evidence about vaccine use in pregnancy, leading to overall less vaccine use and its afforded protections in this population. These inequitable outcomes are not, however, inevitable. Global efforts to change the status quo have been underway in recent years. Among the many global efforts in this space, the PREVENT Working Group [8] issued 22 specific recommendations in 2019 to promote equity for pregnant women and their offspring in epidemic vaccine development and response. Below, we highlight a few key points from the PREVENT report’s recommendations on the inclusion of pregnant women in vaccine studies.
Pregnant women should have opportunities to enroll in COVID-19 vaccine studies whenever the prospect of benefit outweighs the risks to themselves, their offspring, or both. Trials of a multitude of candidate SARS-CoV2 vaccines are progressing rapidly with many efficacy trials underway (or recently completed). These trials are anticipated to be large and enroll thousands of participants [9]. There is an urgent need to proactively plan for appropriate evaluation of vaccine candidates in pregnancy, with attention to which trials meet ethical standards for fair inclusion based on risk–benefit assessment, approaches to generate needed evidence on pregnancy-specific indicators and outcomes, and ensuring compliance with regulatory prerequisites.
Ethical standards generally consider pregnant women eligible for enrollment when there is a reasonable judgment that research participation is likely to be at least as beneficial to the pregnant woman and the fetus as alternatives to participation. Applied to the context of COVID-19 vaccine trials, this would entail an assessment of the prospect of benefits and risks from receiving an investigational vaccine as compared to the risk of exposure to community-acquired infection and progression to severe COVID-19 disease amidst available treatment options—taking into account that the benefits and risks in both scenarios apply across the maternal-fetal dyad. Determining whether the prospect of benefit outweighs the risk in these and other trials depends on several factors, including characteristics of vaccine candidates as well as the epidemiological context in which trials are conducted (8, table 1 specifically). When the full range of harms and risks to pregnant women and their neonates of contracting SARS-CoV-2 virus is considered, this criterion is likely to be satisfied for many vaccine candidates. Consideration of the background risks of infection and severe disease as part of the risk–benefit assessment may be particularly relevant for the subset of pregnant frontline and essential workers, who face an unavoidable increased risk of infection and disease.
When the prospect of benefit exceeds the risks, enough pregnant women should be recruited to allow an assessment of safety and immunogenicity to gather as much evidence as possible using standardized outcome measures [10]. Clinical development plans and study protocols may adopt a range of approaches for collecting data from pregnant women, including the conduct of parallel or companion studies to the main efficacy trial, or through a sub-study of the main trial.
In addition, it should be anticipated that some of the women of childbearing potential who participate in these large efficacy trials will become pregnant within a relevant window following immunization. It is important to know now whether sponsors of current vaccine trials (and for forthcoming trials soon to launch) have protocols in place to capture data on immunogenicity and pregnancy-specific indicators of safety that can be systematically collected from these individuals [10]. Again, where benefit exceeds risks, women who become pregnant while participating in a trial should be given the opportunity to receive all doses in a vaccine series.
Because certain non-clinical studies are often a prerequisite for including pregnant women in trials, such as developmental and reproductive toxicology studies, investigators and developers should coordinate with national regulatory authorities to determine what will be required and initiate required non-clinical studies for promising candidates, depending on vaccine platforms used and historical experience with these platforms. Doing so as possible will allow for timely participation of pregnant women in appropriate future trials.
In conclusion, there are many compelling scientific, public health, and ethical reasons pregnant women need to be considered and included in vaccine investigations and eventual use in our global efforts to mitigate COVID-19. The current pandemic presents a critical opportunity to correct the current practice of exclusion and the paradoxical harms such an approach produces. Professional societies such as the American College of Obstetricians & Gynecologists have recently recommended that authorized COVID-19 vaccines should not be withheld from pregnant individuals who are otherwise eligible to be immunized [11]. This was based on a situational-appropriate decision by both the FDA and the ACIP to use permissive language for this population given the lack of safety signals from ongoing animal studies nor a strong biologic plausibility of harm from the newer mRNA vaccine platforms. While this is an appropriate and welcome decision and direction, much more needs to be done to generate an equitable evidence base in pregnancy [11]. The time to do better is now.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Coronavirus Resource Center. Available at: www.coronovirus.jhu.edu. Retrieved December 15, 2020.
- 2.Corey L., Mascola J.R., Fauci A.S., Collins F.S. A strategic approach to COVID-19 Vaccine R&D. Science. 2020 doi: 10.1126/science.abc5313. [DOI] [PubMed] [Google Scholar]
- 3.Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. https://www.bmj.com/content/370/bmj.m3320. [DOI] [PMC free article] [PubMed]
- 4.Zambrano L.D., Ellington S., Strid P., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status — United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omer S.B. Maternal immunization. N Engl J Med. 2017;376(13):1256–1267. doi: 10.1056/NEJMra1509044. [DOI] [PubMed] [Google Scholar]
- 6.Warren E, Murray P. United States Senate. https://www.warren.senate.gov/oversight/letters/senators-warren-and-murray-urge-nih-and-fda-to-incorporate-the-needs-of-pregnant-people-and-other-underrepresented-populations-as-companies-develop-vaccines-and-therapeutics-for-covid-19. Retrieved September23, 2020.
- 7.Beigi R. Emerging infectious diseases in pregnancy. Obstet Gynecol. 2017;129(5):896–906. doi: 10.1097/AOG.0000000000001978. [DOI] [PubMed] [Google Scholar]
- 8.Krubiner CB, Faden RR, Karron RA, Little MO, Lyerly AD, Abramson JS, et al. PREVENT Working Group. Pregnant women & vaccines against emerging epidemic threats: ethics guidance for preparedness, research, and response. Vaccine. 2021;39:85–120. [DOI] [PMC free article] [PubMed]
- 9.WHO R&D Blueprint. Novel Coronavirus: An international randomised trial of candidate vaccines against COVID-19. April 19 2020. https://www.who.int/blueprint/priority-diseases/key-action/WHOCOVID-2019_SolidarityVaccineTrial_ExpandedOutline_19April_Web.pdf?ua=1.
- 10.7. Bonhoeffer J, Kochhar S, Hirschfeld S, Heath PT, Jones CE, Bauwens J, et al. GAIA project participants. Global alignment of immunization safety assessment in pregnancy - the GAIA project. Vaccine. 2016;34(49):5993–97. doi:10.1016/j.vaccine.2016.07.006. Epub 2016 Oct 14.PMID: 27751641. [DOI] [PubMed]
- 11.Vaccinating Pregnant and Lactating Patients Against COVID-19, Practice Advisory. Available at: https://www.acog.org/en/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-Pregnant-and-Lactating-Patients-Against-COVID-19. Retrieved December 15, 2020.