Abstract
Biofilms are the primary cause of clinical bacterial infections and are impervious to typical amounts of antibiotics, necessitating very high doses for elimination. Therefore, it is imperative to have suitable methods for characterization to develop novel methods of treatment that can complement or replace existing approaches using significantly lower doses of antibiotics. This review presents some of the current developments in microsystems for characterization and sensing of bacterial biofilms. Initially, we review current standards for studying biofilms that are based on invasive and destructive end-point biofilm characterization. Additionally, biofilm formation and growth is extremely sensitive to various growth and environmental parameters that cause large variability in biofilms between repeated experiments, making it very difficult to compare experimental repeats and characterize the temporal characteristics of these organisms. To address these challenges, recent developments in the field have moved toward systems and miniature devices that can aid in the non-invasive characterization of bacterial biofilms. Our review focuses on several types of microsystems for biofilm evaluation including optical, electrochemical, and mechanical systems. This review will show how these devices can lead to better understanding of the physiology and function of these communities of bacteria, which can eventually lead to the development of novel treatments that do not rely on high-dosage antibiotics.
Keywords: Biofilm, Microsystems, Sensors, Microfluidics
1. Introduction
Bacterial biofilms are a major cause of concern in the clinical setting. They are the primary cause of infections, commonly forming on medical devices such as implants and catheters, as well as on respiratory tract surfaces and teeth [1,2]. It is estimated that 65% of all bacterial infections involve biofilms [3]. The high mutation rates and horizontal exchange of genetic material in biofilms promote antibiotic tolerance mechanisms and result in high resistance to antibiotics [1,[4], [5], [6], [7], [8]]. It is estimated that biofilms require 500 – 5000× higher doses of antibiotics for treatment as compared to freely floating planktonic bacteria [1,7,9]. The use of such high doses - significantly higher than the minimum inhibitory concentration (MIC) - to treat medical device-associated infections in patients is practically impossible due to adverse side effects such as renal failure and more importantly due to the emergence of antibiotic resistant strains [8]. Antimicrobial resistance is a major threat to health, disrupting our ability to treat a range of infections [10]. In 2019, the World Health Organization (WHO) declared antimicrobial resistance as one of the major ten threats to global health [11,12]. Hence, there is an urgent need to study and develop alternate treatment methodologies that eliminate the need for such excessive antibiotic doses. However, this aim requires effective methods to study and characterize biofilms.
While biofilms have been studied for decades [13,14], much is still unknown. Over the last few decades, a number of in vitro models have been developed to not only understand the biology of biofilms, but also to study the effect of biofilm response to external stimuli such as change in pH or exposure to antimicrobials [8,[15], [16], [17], [18], [19], [20]]. However, standard procedures are yet to be established for the characterization and study of biofilms. Moreover, the variability inherent to biological systems warrants the need for highly parallel study designs to ensure reliability, something that many current systems do not adequately address. Biofilms grown in vitro show a high degree of growth variability both between and within platforms [21,22], preventing reliable comparison of treated biofilms to their controls. Additionally, biofilm characterization and evaluation often rely on bulky external quantification equipment or laborious protocols that label components of the biofilm and destroy the biofilm itself [23,24]. Furthermore, maintaining the consistency of macroscale protocols is a challenge that adds to the variability of biofilms. Therefore, there is an urgent need to develop scalable and reliable systems that can noninvasively quantify and characterize biofilms. Considering the broad variations of the real environmental settings where biofilms form (e.g. implanted devices, surgical tools, etc.), it is also essential to develop different characterization methods in parallel to address the varying clinical needs.
Here, we briefly review the aforementioned challenges and conventional macroscale systems, and then proceed to give an in-depth overview of novel microtechnological approaches. Microscale devices and lab-on-a-chip (LOC) sensing platforms have been considered as an ideal solution to address this multi-faceted problem. Microfluidics provides several advantages including ease of fabrication, low reagent volumes and cost, tight environmental control, and high throughput; the implementation of microfluidic devices for biofilm studies has been discussed in detail by Greener et al. [25]. Their review provides an in-depth view of PDMS microchannel devices and the conventional metrology tools that these devices rely on. Our work touches upon these microfluidic devices for biofilm characterization with more emphasis on the microfluidics-integrable microsensing technologies [[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]] that can be used to evaluate biofilms in real-time for label-free, continuous, and noninvasive characterization of biofilm properties. This review aims to present some of the in vitro microsystems developed over the past few years for biofilm testing and treatment evaluation. First, we review macro-scale systems that have gained recognition as standards for biofilm testing, followed by different types of microsystems that aspire to mimic their macro-scale counterparts in portable and point-of-care settings, while reducing reagent and resource costs and improving biofilm monitoring. In particular, we review sensor-less microfluidic systems followed by the ones combined with optical, electrochemical, and mechanical monitoring systems.
2. Macro- and micro-scale biofilm reactors
In developing the field of biofilm science, various new techniques and formats to evaluate biofilms have been employed by researchers through the years, during which macroscale biofilm reactors have gained a reputation as the standard for growth and evaluation of biofilms. Broadly, these reactors can be divided into static reactors and flow cells. Static reactors allow for growth of biofilms in an environment where the media is replenished only periodically. As a result, nutrients can become locally depleted, and waste and planktonic cells can accumulate. The most common format for growing biofilms in a static environment utilizes microwell plates. Microwell plate readers are a staple in every microbiology and biotechnology laboratory. Furthermore, automated systems for filling and mixing reagents in microwells exist that allow for easy biofilm handling and experimentation.
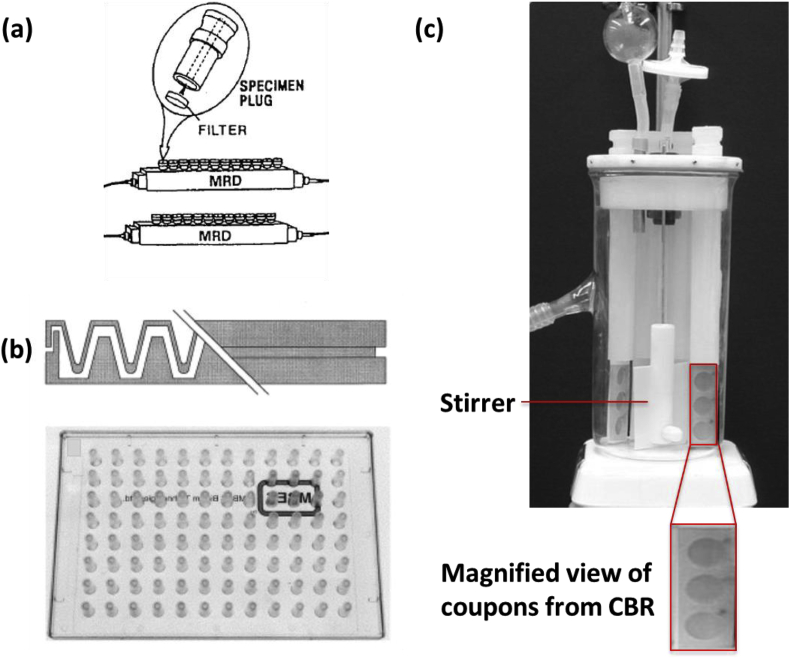
As opposed to static reactors, flow cells provide a continuous supply of nutrients to the biofilm. These platforms also aid in removal of waste and any planktonic cells that disperse from the biofilm, an added advantage over static systems. Flow cells typically have one inlet, one outlet, and one channel throughout which biofilms are grown. The biofilms grown in these devices can be monitored continuously using a microscope, provided the device is mounted on a transparent substrate. Some of the commonly used flow reactors are presented in Fig. 1 and are discussed in detail below.
Fig. 1.
(a) Schematic of two parallel modified Robbin’s devices, showing a specimen set of plugs on which the biofilm is allowed to grow. Reproduced with permission from Ref. [19]. (b) Cross-sectional (top) and top view (bottom) of a Calgary biofilm device. The figure on the top shows the pegs on which the biofilm grows as the channels below are used to flow nutrients. The bottom figure illustrates the pegs in a 12x8 array compatible with a 96-well plate. Reproduced with permission from Ref. [15]. (c) Photograph of a CDC biofilm reactor (CBR). Reproduced with permission from Ref. [59].
The modified Robbin’s device is a commonly used method for evaluating biofilms. The device, shown in Fig. 1a, consists of a main channel that contains multiple specimen plugs onto which biofilms can be grown in a flow environment with constant shear. After growth, these plugs can be removed and the biofilm on the plugs can be subjected to various experiments [19]. Although the biofilms are grown in parallel, their analysis remains serial in this configuration.
The Calgary biofilm device combines insertion capability with traditional microwell plate technology and the ability to apply shear stress through flow as provided by the modified Robbin’s device [15]. The device has two components, a pegged lid and a bottom with channels, shown in Fig. 1b. The pegs on the lid can be positioned over the channels or fitted into a traditional 96-well plate. The flow of the liquid in the channels can be directed around the pegs by placing the device on a rocking table. The biofilms grown on the pegs experience a constant amount of shear due to the flow of the fluid. They can be removed and analyzed individually, as in the case of the Robbin’s device, or the pegged lid can be inserted in a microwell plate filled with different antibiotics or new treatments that require testing. A major drawback of these two macroscale devices is that imaging biofilms on the pegs or specimen plugs using either confocal microscopy or scanning electron microscopy requires that each peg or plug be individually broken off of the lid and manipulated.
The CDC biofilm reactor (CBR) allows for the growth of biofilms under moderate to high fluid shear stress [59]. The reactor, shown in Fig. 1c, incorporates 24 removable biofilm growth surfaces (coupons) for sampling and analysis, and consists of a 1l glass vessel with an outlet positioned to provide about 350 ml of working fluid capacity. The polyethylene lid supports 8 independent removable rods that can each house 3 coupons, an inlet port, and a gas exchange port. The entire device is usually placed on a digitally controlled stir plate to provide constant rotation of the stir bar at a designated speed for controlling the amount of applied fluid shear. The CBR is used as a flow cell, i.e. a continuous-flow stirred-tank reactor, by constantly pumping fresh media into and out of the reactor. A disadvantage of this device is that it is bulky, and all 24 coupons must be subjected to the same treatment. Hence testing different treatments needs to be done separately, which contributes to an increase in experimental variation and means the CBR requires larger volumes than either microwell plates or microscale devices to test the same number of conditions. Furthermore, the biofilms grown in macroscale systems are usually analyzed using end-point analysis, which destroys the biofilm while only providing a single data point.
Alternatively, microscale systems are capable of addressing many of the disadvantages and measurement challenges of their macroscale counterparts. The use of microfluidics allows for greater ease in controlling the fluidic environment and integration of microfabricated sensors or micropatterned growth substrates [25,60,61]. A mm-scale flow through system developed by Tolker-Nielsen et al. for studying biofilm growth using microscopy overcomes some challenges associated with macroscale approaches, allowing non-destructive and continuous analysis [62]. However, this system still requires the relatively larger sample and reagent volumes. In comparison to the macroscale platforms, microfluidic systems can be designed to require smaller sample and reagent volumes, frequently on the order of nanoliters. In the recent past, several standard macroscale methods, routinely used for the analysis of biomolecules such as electrophoresis and PCR, have been successfully miniaturized into microscale systems [63,64]. While larger samples of bacterial cultures are easy to obtain, it is often difficult to obtain larger volumes of reagents, antibiotics, or new drugs under research. The following section discusses the advantages of microsystems over their macro-counterparts in more detail and delves into the various microsystems currently under development for biofilm studies.
3. Microsystems for biofilm studies
While the simplest microfluidic systems, whether they are single channel or chamber, are miniature versions of existing macroscale platforms such as flow cells or microwell plates, complex fluid-handling architectures can be integrated with arrays of channels or chambers to enable multi-experiment capabilities within a single device. On-chip pumps can provide tunable flow of solutions throughout a device whereas integrated valves, such as Quake valves which use pressurized gas to control valve orientation [[65], [66], [67]], can direct this flow to designated locations on demand.
Another significant advantage of using microfluidic systems is that they can be integrated with microfabricated sensors. These microscale LOC systems provide numerous advantages in biomedical research and clinical diagnostics, and can be a valuable tool in investigating novel therapies. They enable functional integration with other technologies, leading to portability and high-throughput usage. These translational systems hold the potential to improve the resolution, regulation, sensitivity, and flexibility over more traditional approaches. In summary, these devices can provide a dense array of sensors at the micro-scale to drastically reduce the necessary sample volumes, reproduce flow conditions similar to in vivo environments, and enable real-time and non-destructive biofilm analysis. These are critical elements to both biological testing for drug discovery and biofilm detection systems [[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58],68].
By leveraging these advantages of microsystems, more recent biofilm research is providing new insight on the various properties of biofilms in ways that are impossible with classical macroscale systems such as the Robbin’s device or microwell plate. This includes studies on the development of antibiotic resistance, biofilm growth characterization, and the role of intercellular communication [[69], [70], [71], [72], [73], [74]]. Furthermore, it has been suggested that microdevices will reduce the necessary analysis time from days to the order of 2–4 h with more accurate recognition of specific biological targets [75,76].
Although most biofilm studies utilize direct measurement of biofilm thickness with microscopy and image analysis, very few studies demonstrate real-time, continuous, non-invasive monitoring of biofilms. While microsystems have leveraged the properties of microfluidics to create environments more difficult to obtain with traditional biofilm reactors, only a few real-time integrated microsystems have been developed for biofilm sensing, characterization, and treatment. While integrated systems relying on the optical properties of biofilm are the most common, microsensor approaches utilizing the electrochemical or mechanical properties of biofilm have also shown promise. The development and validation of such microsystems against traditional methods will allow for easy detection of biofilm formation and evaluation of new treatments for prevention and removal in real-time.
3.1. Sensor-less microfluidic platforms
There have been numerous sensor-less microfluidic flow cells developed to understand and design complex synthetic biology circuits to study the effects of various growth parameters such as pH, flow rates, and temperature on biofilms and biofilm growth. Since these bioreactors are not equipped with real-time sensing capabilities, traditional methods of biofilm study like confocal microscopy, colony forming units (CFU), or crystal violet staining assays were employed for biofilm characterization.
Microfluidic platforms can help minimize the impact of the inherent variability of biofilm growth by designing platforms capable of multiple biofilm characterization experiments, including controls, in parallel on the same device [77]. This reduces inter-device variability, and along with the advantage of high-throughput experimentation, saves time and resources for reproducible biofilm studies.
Hong et al. report the development of a multi-channel microfluidic device to study a biofilm circuit that utilizes two dispersal proteins along with a population-driven quorum-sensing switch [78]. The microfluidic device consists of a diffusive mixer for cells and media, and eight microchambers where biofilm growth is imaged using confocal microscopy and COMSTAT. The device is made from PDMS with pneumatic elements to control microvalves, and it allows for the isolation of different biofilm samples and careful control of media to each of them. Within this microfluidic device, the authors show the displacement of the initial biofilm using a second disperser species, which is then removed using a chemically induced switch. This was the first demonstration within a fluidic device where cells that have been engineered to be able to displace an existing biofilm are then removed on command, allowing one to control multi-species biofilm formation. The same group also developed a modified cell mixer layer of the microfluidic flow cell device for investigating bacterial biofilm formation and organization in response to different concentrations of soluble signals [72]. They demonstrate the utility of the flow cell by studying the effect of 7-hydroxyindole and isatin, either individually or in combination at varying concentrations, on biofilm development of pathogenic E. coli. The authors suggest that this microfluidic model could be used towards a fundamental understanding and characterization of events leading to bacterial attachment to surfaces and of various chemicals that influence biofilm formation or inhibition. Connell et al. also probed how bacterial communication and colony formation drives antibiotic resistance by confining them in microscale ‘lobster traps’ made of photo-cross-linked-protein that is permeable to nutrients, waste products, and other biomolecules [79]. These structures were capable of capturing single cells and growing them into microcolonies, which begin to display resistance to antibiotics with as few as 150 cells.
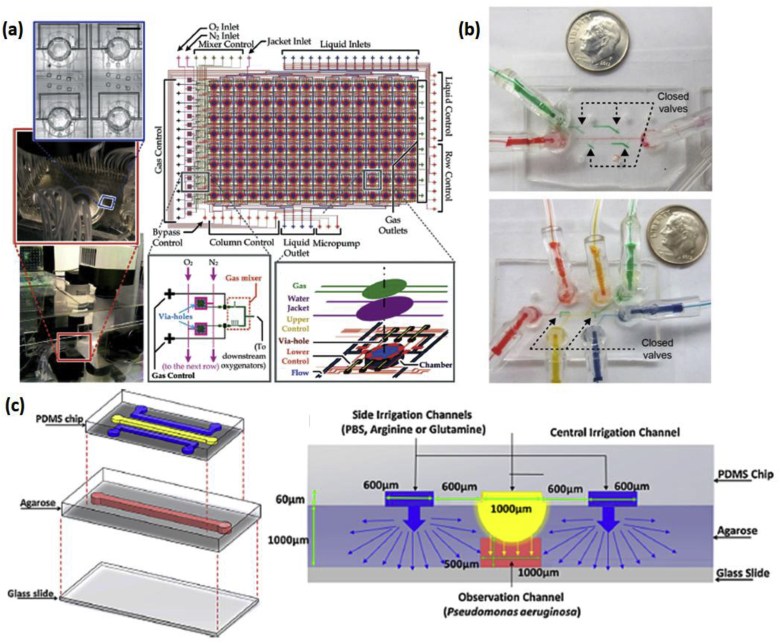
Lam et al. recently reported the development of a high-throughput microfluidic ‘artificial teeth’ device for quantitatively evaluating several biochemical factors in the growth and development of dental biofilms [80]. This device, shown in Fig. 2a, has 128 separate 1 mm diameter growth chambers, each of which can have its own controlled microenvironment. The specific parameters that can be manipulated per chamber include bacterial species, media composition, flow rate, and dissolved oxygen concentration. This controlled environment was achieved via layering PDMS microchannels with the pneumatic control channel on top, followed by an oxygenation channel, water jackets to minimize evaporation, upper and lower flow control channels, media flow channels, and culture chambers. Finally, an array of valves, micromixers, and micropumps allow these channels to control the individual microenvironment of each chamber, implementing 8 different dissolved gas conditions at a given time. Skolimowski et al. developed a microfluidic approach for controlling oxygen gradients for creating a cystic fibrosis model with Pseudomonas aeruginosa. The oxygen gradients in the PDMS microchannels were regulated simply by altering the flow rate, which changed the ratio of advective and diffusive transport [81,82].
Fig. 2.
(a) (Left) Fabricated microfluidic ‘artificial teeth’ device placed on the automated microfluidic control platform. (Right-top) Design layout of 128-chamber artificial teeth chip. (Right-bottom) Sketch of multiple structural layers in a culture region and design of the gas micromixer. Reproduced with permission from Ref. [80]. (b) Photographs of assembled devices with green water filling actuated control channels. (Top) Device in biofilm growth orientation, with side channels blocked by closed valves. (Bottom) Device in biofilm sectioning orientation, with side channels open and central channel sectioned by closed valves. Reproduced with permission from Ref. [84] (c) (Left) Schematic of the microfluidic 3D gradient-generating wound model used for quantifying the interaction between Pseudomonas aeruginosa and chemoeffector-releasing surfaces. (Right) Illustration of the mechanism of gradient generation (cross-sectional view of device). Reproduced with permission from Ref. [85]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Our group has developed a biofilm segmentation platform to perform multiple studies, with an integrated control, on a single biofilm. The authors leverage advantages of microfluidics to develop a system in which biofilms are formed and sectioned, allowing parallel assays on multiple sections of one biofilm. The device shown in Fig. 2b has two modes of operation. During biofilm growth mode, the device allows for media to flow along the length of the center channel while the side valves remain closed (Fig. 2b-top), enabling growth of a single uniform biofilm. In the treatment testing mode, the side valves are opened and the center valves are closed, allowing for segmentation of the center channel into multiple sections. Each section can then be independently treated with different treatments, thereby allowing for multiple experiments to be run in parallel (Fig. 2b-bottom). Using one of the channels as an integrated control helps to accurately compare the multiple experiments in the run, thereby eliminating inter-experimental variations.
There are also microfluidic systems reported that have been combined with macroscale detection techniques other than confocal microscopy. Hua et al. reported a microfluidic reactor that is compatible with time-of-flight secondary ion mass spectrometry (ToF SIMS) for spatial imaging of the chemical makeup of the biofilm [83]. The platform, or System for Analysis at the Liquid/Vacuum Interface (SALVI), allows the direct imaging of liquids with vacuum-based instruments and techniques and can be used under both ambient and vacuum conditions for imaging while being simultaneously compatible with confocal microscopy and ToF SIMS. SALVI consists of a PDMS microchannel fully enclosed in a 100 nm SiN membrane, with holes drilled in the SiN using the primary ion beam of the ToF SIMS as detection windows. This system was also used to examine the heterogeneity at different points along the microchannel using 2D and 3D chemical mapping of biofilms.
Other microfluidic systems have aided the development of new methodologies to study various biofilm properties, such as viscosity, antibiotic susceptibility, and mechanical stiffness. Paquet-Mercier et al. demonstrated a new method to monitor biofilm viscosity using multi-channel microfluidics [86]. The device is a simple PDMS/glass device and biofilms were tracked optically to determine how the biofilm segments moved temporally along the channel. A tracking algorithm and imageJ were used to analyze each image for tracking, and a two-phase viscous flow model was used to determine dynamic viscosity of biofilm at different phases of the experiment. The viscosity was based on the velocity of the segment and its thickness. Shin et al. reported the use of a similar single channel microfluidic device to examine the susceptibility of Pseudomonas aeruginosa to a combination of Tobramycin and sodium dodecyl sulphate (SDS) as verified using confocal microscopy [87]. The impact of varying hydrodynamic conditions has also been explored in microfluidic systems. Lee et al. explored the impact of wall shear stress and flow velocity on a biofilm in a straight, single channel [88]. The impact of shear stress within a microfluidic device and on biofilm formation is explored in more detail by Salta et al. The channels in their device consisted of four separate sections, each with a subsequently smaller height, in order to generate four different hydrodynamic shear stress conditions. They utilized this platform to study the attachment and formation of marine biofilm [89]. Serioli et al. developed centrifugal microfluidic devices which do not rely on external pumps to drive flow have been used to study the impact of shear on Pseudomonas aeruginosa with low sample volumes and increased ease of use. The Lab on a Disc platform simply uses a spinning motor to create the desired flow conditions within the device [90].
Microfluidics has also helped researchers develop models that come close to mimicking different microenvironments. For example, Wright et al. reported a microfluidic system that mimics the wound microenvironment in order to study polymicrobial biofilms [85]. The microfluidic device consists of a top layer of PDMS, a middle layer of agarose, and a bottom glass slide (Fig. 2c). The PDMS layer has three channels, two of which are used to introduce chemoeffectors. These compounds induce the chemical wound environment via a diffusion gradient through the agarose, to an observation channel where the biofilm is grown. The nutrients often have to diffuse through a collagen matrix in a wound environment. Since the agarose allows diffusion while the PDMS does not, it serves to recreate the physical wound environment. The authors also fabricated a single-inlet microfluidic device with 25-μm diameter microposts which mimics the topography found in wound environments. The third PDMS channel introduces chemoeffectors directly to the biofilm channel through the ceiling. An advantage the authors emphasize is the compatibility of their system with high resolution imaging techniques. Terry et al. also presented a microfluidic wound model, consisting of a simple Y-channel coated in collagen to help recapitulate a wound microenvironment. This platform was utilized to evaluate the efficacy of antimicrobials against Methicillin-resistant Staphylococcus pseudintermedius biofilm [91]. Glass and PDMS microfluidic systems have also been utilized to study biofilm dynamics on osmosis membranes nondestructively using confocal laser scanning microscopy [92].
In addition to these custom-fabricated systems, the commercially available BioFlux microfluidic device has emerged as a tool for studying biofilm susceptibility to antimicrobial compounds. The system uses pneumatic pressure to drive flow across a microfluidic channel, and a microscope images the resulting biofilm in the microchannel. The device is designed similarly to microwell plates, with 24 fresh media wells and 24 spent media wells connected to 24 microfluidic channels. Benoit et al. utilized this system to evaluate the efficacy of an array of anti-microbial compounds, and Kristensen et al. utilized this system to evaluate the impact of osteopontin on biofilm adhesion [93,94].
The microfluidic systems presented here (summarized in Table 1) demonstrate the viability of these devices to create complex microenvironments, thereby enabling multi-parameter biofilm studies. While integration of these devices with sensitive, low-power, real-time monitoring techniques remains a challenge, the recent progress made in this field is suggestive of the urgent need for new tools to understand and treat these difficult-to-eradicate infections.
Table 1.
Summarization of the characteristics of the microsystems presented.
| Microsystem Type | Bacterial Species Detected | Detection Method | Duration of Experiment | Interval between consecutive data points | Ref |
|---|---|---|---|---|---|
| Sensor-less Microfluidic Systems | E. coli | Fluorescence | 62 h | 2 h | [72,78] |
|
Streptococci spp, F. nucleatum |
Fluorescence | 7 d | 1 d | [80] | |
| E. coli | Fluorescence | 24 h | End-point | [84] | |
|
P. aeruginosa, E. coli |
Fluorescence | 72 h | Real-time | [85] | |
| P. aeruginosa | Fluorescence | 24 h | End-point | [87] | |
| Pseudomonas spp | Optical Density | 80 h | Real-time | [86] | |
| Shewanella spp | ToF-SIMS | 6 d | End-point | [83] | |
| S. pseudintermedius | Fluorescence | 24 h | End-point | [91] | |
| P. aeruginosa | Fluorescence | 4 d | End-point | [81,82] | |
| C. marina | Confocal Laser Scanning Microscopy | 210 min | End-point | [89] | |
| Optical Systems | S. mutans | Confocal Reflection Microscopy | 72 h | 12 h | [98] |
| E. coli | Synchrotron Radiation Fourier Transform Infrared Spectroscopy | 10 h | Real-time | [99] | |
|
C. albicans, C. tropicalis, C. parasilosis |
Brillouin Spectroscopy, Raman Spectroscopy | 72 h | End-point | [104] | |
| G. sulfurreducens | Electrochemical Surface Plasmon Resonance | 24 h | Real-time | [100,106] | |
| P. mirabilis | Fiber Optic Evanescent Wave Spectroscopy | 4 h | Real-time | [103] | |
| R. palustris | Fiber Optic Evanescent Wave Spectroscopy | 250 h | 4 h | [101] | |
| E. coli | Optical Density | 24 h | Real-time | [102] | |
| Electrochemical Systems |
S. mutans, S. aureus, S. epidermidis |
Impedance | 24 h | 1 h | [115] |
| S. oneidensis | Electrochemical Impedance Spectroscopy | 17 h | 30 min | [97] | |
| E. coli, Salmonella | Electrochemical Impedance Spectroscopy | 48 h | 3 h | [112] | |
|
P. aeruginosa, S. maltophilia |
Impedance, Amperometry | 12 d | Real-time | [118] | |
| S. aureus | Impedance | 24 h | Real-time | [119] | |
| E. coli | Impedance | 24 h | 12 h | [128] | |
|
S. epidermidis, S. aureus |
Electrochemical Impedance Spectroscopy | 20 h | 30 min | [129] | |
| P. aeruginosa | Impedance | 200 h | Real-time | [130] | |
| E. coli | Impedance | 48 h | Real-time | [124] | |
| E. coli | Impedance | 48 h | Real-time | [121] | |
|
P. stutzeri, S. epidermidis |
Electrochemical Impedance Spectroscopy | 2 h | 5 min | [136] | |
| S. epidermidis | Cyclic Voltammetry, Differential Pulse Voltammetry | 65 h | 15 min | [122] | |
| P. aeruginosa | Square Wave Voltammetry | 24 h | End-point | [123] | |
| Mechanical Systems | P. aeruginosa | Quartz Crystal Microbalance | 6 d | Real-time | [142] |
| S. mutans | Quartz Crystal Microbalance- Dissipation | 20 h | Real-time | [144] | |
| P. aeruginosa | Quartz Crystal Microbalance- Dissipation | 1 h | 5 min | [145] | |
| S. salivarius | Quartz Crystal Microbalance- Dissipation | 4 h | Real-time | [146] | |
| S. epidermidis | Quartz Crystal Microbalance- Dissipation | 140 min | Real-time | [147] | |
| P. aeruginosa | Quartz Tuning Fork Resonance | 72 h | Real-time | [148,149] | |
|
E. coli, P. aeruginosa |
Surface Acoustic Wave | 48 h | Real-time | [68,154,155] |
3.2. Optical microsystems
Traditional biofilm studies rely on standard optical techniques such as confocal microscopy, crystal violet staining, or SEMs. Multitudes of studies have been performed on various biofilms using these techniques [23,73,[95], [96], [97]]. However, these labeling procedures are inherently destructive to the biofilm, as a result of which only end-point measurements are obtainable.
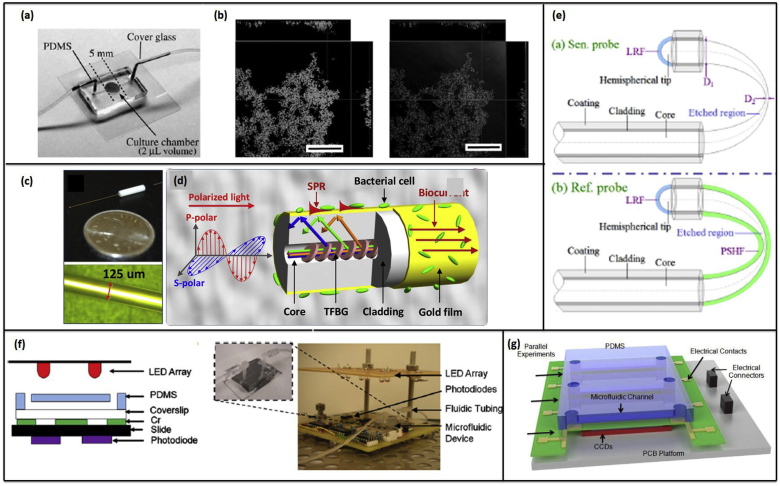
In recent years, other optical techniques that allow for real-time, non-invasive detection of biofilms have been developed. For example, Yawata et al. report a non-destructive label-free biofilm formation monitoring system using an image analysis technique based on a modified confocal reflection microscopy (CRM) in a single channel PDMS/glass microfluidic device shown in Fig. 3a [98]. To overcome the challenge of image saturation when using CRM, the authors modified the technique to allow for continuous manual adjustment of the detector gain while scanning along the Z-axis to maintain the signal intensity of cells at a constant level. This modification of CRM with continuous gain adjustment was termed Continuous Optimizing CRM (COCRM). Using this technique, successful visualization of Streptococcus mutans biofilm growth was obtained every 12 h over a 60-h growth period (Fig. 3b).
Fig. 3.
(a) A microfluidic device used for biofilm studies in Ref. [98]. (b) Orthometric views of a S. mutans NBRC13955 biofilm acquired by SYTO9 (left), and COCRM (right). (a) and (b) Reproduced with permission from Ref. [98]. (c) Photographs of plasmonic fiber-optic sensor for in situ biofilm monitoring. (d) The enlargement of the bioelectrochemical cell, showing the configuration of a gold-coated TFBG sensor probe with polarization of light oriented for SPR excitation. (c) and (d) reproduced with permission from Ref. [100]. Copyright 2016 American Chemical Society. (e) Schematics of the fiber-optic sensor and reference probes (LRF: light reflective film). Reproduced with permission from Ref. [101]. (f) Cross-sectional schematic of the microfluidic platform. (Left) The microfluidic channel molded in the PDMS layer is positioned on top of patterned measurement windows and aligned to external optical components. (Right) Photograph of microfluidic device for optical density monitoring of biofilm (inset) integrated with fluidic components, and positioned over photodiodes and under LEDs. Reproduced with permission from Ref. [73]. (g) Schematic of the microfluidic biofilm observation, analysis and treatment (Micro-BOAT) platform. The platform is capable of performing six experiments in parallel on a single chip. Real-time biofilm monitoring is achieved via the measurement of biofilm OD using charge-coupled devices (CCD) and a tuned light emitting diode (LED) source (not shown). Reproduced with permission from Ref. [102]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Imaging at long, 12-h intervals yields poor temporal resolution of biofilm formation and growth. However, real-time chemical imaging of bacterial activities can facilitate a more comprehensive understanding of the dynamics of biofilm structures and functions. This has been demonstrated using synchrotron radiation-based Fourier transform infrared (SR-FTIR) spectromicroscopy in an open-channel microfluidic system by Holman et al. and others [99,103]. This imaging technique has been known to yield high spatial resolution and label-free vibrational signatures of chemical bonds in biomolecules, but has been restricted in use due to the water in biofilms, shown to hinder SR-FTIR sensitivity. This was overcome by Holman, who developed a simple open-channel microfluidic system with hydrophilic DRIE-etched microstructures which can circumvent the water-absorption barrier for chemical imaging of the developmental dynamics of bacterial biofilms with a spatial resolution of several micrometers. To image the biochemical properties and the distribution of bacterial activity before and after antibiotic application, the entire view field of the biofilm was divided into equal-sized squares before raster scanning, collecting full SR-FTIR spectra at each position, following which the scans were processed using different algorithms. Recently, Mattana et al. reported using optical methods to generate maps of the bulk chemical and mechanical properties of biofilm on the microscale. Biofilm matrix stiffness was determined using Brillouin microspectroscopy and the composition was mapped using Raman microspectroscopy. Candida biofilms were grown for 72 h and dried for one week before being introduced into the custom microscope setup for imaging, which collects Brilluoin spectra using a multi-pass tandem Fabry-Perot interferometer and Raman spectra with a dispersive monochromator. While these were done as end-point measurements, the authors report that this approach could be used to probe these properties of biofilms in situ [104].
Surface plasmon resonance (SPR) has been demonstrated as a means to characterize biofilm physiology optically. A PDMS microchannel in combination with a gold sensing surface on a glass prism enables real-time and label-free dissemination of the attachment and growth dynamics of Escherichia coli and Pseudomonas aeruginosa biofilms [105]. The change in the resonance peak of the light reflected from the gold sensing surface arises from a change in refractive index attributable to the attached biofilm biomass. SPR offers non-destructive and continuous monitoring over a larger surface area than many other optical techniques, up to 1 cm2. Fiber optical methods of detecting biofilms have also been reported. One group reports a fiber-optic sensing system to detect the formation of electroactive biofilms via electrochemical surface plasmon resonance (EC-SPR), where biofilm formation on the fiber surface leads to a measureable shift in the refractive index [100]. Here, the optical fiber consists of a tilted fiber Bragg grating (TFBG), coated in a nano-gold film, which acts as the working electrode. The gold-coating offers simultaneous electrochemical and optical SPR-based measurements of biofilm in hard to reach environments. Hu et al. also report a similar electrochemical fiber optic sensor for biofilm detection on the surface of the sensor as shown in Fig. 3c–d [106].
Simpler fiber optical microsystems for biofilm detection have also been studied. Zhong et al. reported a gold coated fiber optic sensor for detection of biofilm growth in bioreactors via fiber-optic evanescence wave spectroscopy [101]. The fibers have a U-shaped chemically etched region, which was used as the exposed sensing region; the etch into the unclad fiber sensing region served to increase the evanescent field intensities. Each sensor contains an uncovered sensing probe, as well as a parallel reference probe coated in a porous polyimide-silica film (Fig. 3e). The reference probe measures the light transmission through the liquid, while the sensing probe measures the transmission through both the liquid and the bacterial biofilm. The sensing probe was able to measure a significant percent decrease in transmission intensity when biofilm formed, both with and without flow. Phillip-Chandy et al. presented a similar plastic optical fiber with its cladding removed over a sensitized length that measures the growth of biofilms in a closed loop water process system by evanescent field attenuation and intensity modulation [107]. The sensor detects biofilm build-up at the fiber surface by means of refractive index modulation. The authors showed that the increase in refractive index from the water to the biofilm reduces the intensity of light propagating in the fiber and attenuates the high order modes.
Although the aforementioned methods provide high sensitivity, they require extensive data collection and processing. Thus, simpler methods of imaging that require less or almost no data processing have been suggested. Previous work conducted in our group showed that biofilm thickness can be determined using simple off-the shelf optoelectronic devices, such as photodiodes or CCD arrays, shown in Fig. 3f–g [73,102]. Here, the optical density gives a direct measure of the biofilm thickness, while the CCD array functions in deriving the spatiotemporal thickness of the biofilms along the length of the channel. Each of the optical microsystems discussed here are summarized in Table 1.
3.3. Electrochemical microsystems
Electrochemical biosensors are a well-studied class of sensors and are the first widely successfully commercialized biosensors [108]. These are broadly divided into three types according to the operating principle governing their method of measurement: impedimetric/non-faradaic and faradaic (potentiometric, amperometric) transducers.
Impedimetric Microsystems: Impedance based techniques have been used as a method of transduction for detecting and quantifying bacteria. Specifically, impedance microbiology (IM) has been used for decades to detect the presence of microorganisms in samples in the food industry, environment, health care, etc. In IM, the change in impedance is measured using a pair of electrodes that is submerged in the culture medium. To detect bacterial growth in real-time, the relative or absolute change in conductance, impedance or capacitance of the solution are measured at a given temperature. While classical impedance microbiology uses either direct or indirect measurement techniques for measuring the impedance change of the media, several studies have found that the total impedance change during bacterial growth consists of two components that can be measured at different frequency ranges: (i) impedance change contributed by the media and (ii) impedance change contributed by the electrode/electrolyte interface, also known as the electrochemical double layer (EDL) impedance. This electrochemical impedance measurement is generally a non-faradaic process that does not involve charge transfer at the electrode surface, which contrasts with the faradaic – amperometric or potentiometric - approaches discussed in the next section. The EDL impedance dominates at lower frequencies (typically < 10 kHz), while the growth medium impedance becomes more dominant at frequencies above 10 kHz. A simple equivalent circuit model, in which a resistor, Rs, is in series with two EDL capacitors, each of value Cdl, can be used to understand the frequency dependence of both impedances on the overall impedance. The impedance Z of the circuit can be mathematically expressed as equation (2.1) below:
| (2.1) |
where f is the frequency, Rs is the solution resistance and Cdl is the EDL capacitance at the electrodes.
Similarly, interdigitated microelectrodes (IDEs) - a conventional two-electrode system configuration at miniaturized scales - have been used for sensing biological samples via impedance [75,[109], [110], [111]]. The equivalent circuit model, mathematically expressed in (2.1), remains valid for the case of the IDEs. However, the frequency ranges over which the EDL capacitance and the medium capacitance are dominant may change with the electrode spacing and width. Yang et al. demonstrated the use of IDEs for sensing bacterial growth; specifically, the growth induced a 30% change in the EDL capacitance with almost no change in the medium capacitance (-0.58%) [111]. In this respect, IDE-based systems can be advantageous over conventional electrode systems in that the IDE can measure the change in the EDL capacitance to monitor bacterial growth.
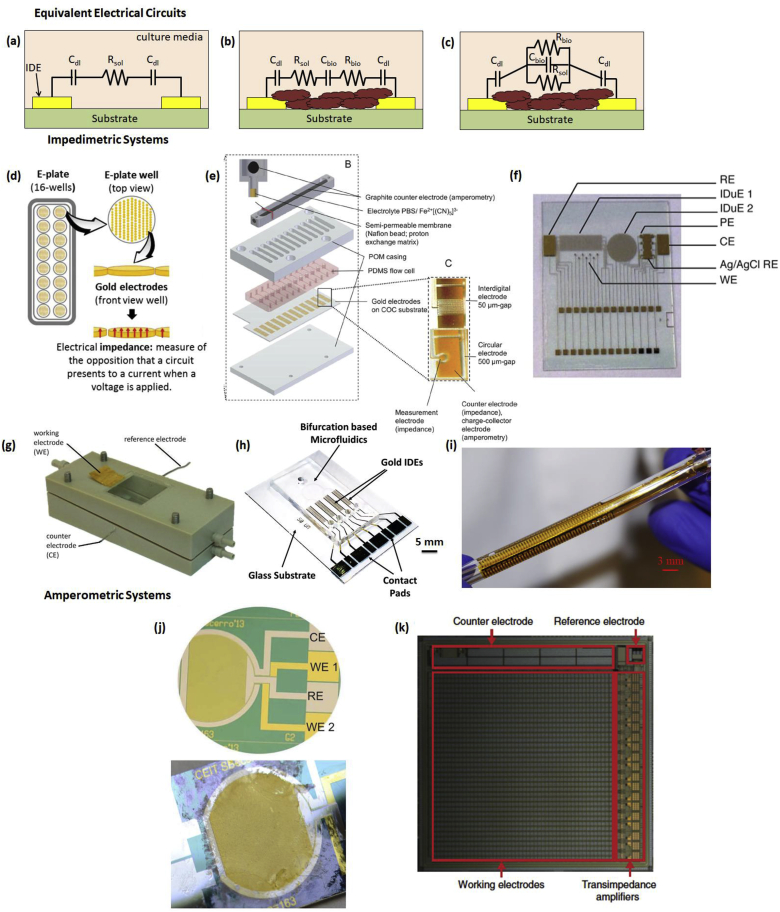
In biofilms, both the cells and the ECM within the biofilm serve as dielectric materials and thereby are responsible for its electrochemical impedance that varies with time, composition, or metabolic state of the biofilm. Hence, bacterial biofilms grown on the surface of microelectrodes can be modeled as an electrical circuit. One such equivalent electrical model is presented in Fig. 4a–c. Fig. 4a presents the electrical model of a sterile culture media that does not contain any bacteria. Fig. 4b–c presents a simplified series and equivalent parallel electrical model for when biofilm and ECM grow between the two electrodes. In the circuit, the parameters represent the following: Cdl is the EDL capacitance, Rsol is the resistance of the media without bacterial cells, and Cbio and Rbio are the capacitance and resistance of the biofilm, respectively. When bacterial metabolism causes a change in the first two parameters, the impedimetric response of the culture changes proportionally [70].
Fig. 4.
Cross-section schematic of electrical circuit model of a pair of interdigitated electrodes (IDE). (a) Circuit model for sterile culture media before inoculation with bacterial cells. (b) Equivalent series and (c) parallel circuit models after biofilm and ECM formation. (d) Standard E-plates (16-wells) and magnification of one of the wells coated with gold microelectrodes. Reproduced with permission from Ref. [115]. (e) A 12-flow channel unit consists of an amperometric counter electrode and the fluidically independent microfluidic flow channels sealed by a substrate with planar gold-electrode structures. Different electrode designs were used depending on the application including circular electrodes with a gap of 500 μm and interdigitated electrodes with gaps of 15–100 μm. Reproduced with permission from Ref. [118]. (f) Multi-parametric chip module containing the different sensors. The chip contains two IDEs, rectangular (IDE 1) and circular (IDE 2). The electrodes for the measurement of dissolved oxygen are the reference electrode (RE), the working electrode (WE) and the counter electrode (CE). The punctual electrodes (PE) for the potentiometric measures were prepared to be selective for Na+ and K+. For the pH measurement, an additional iridium oxide layer is electrodeposited on the electrode selected. For robustness purposes, the PE are replicated. The reference electrode for potentiometric measures of Na+, K+, and pH is the silver/silver chloride electrode (Ag/AgCl RE). Reproduced with permission from Ref. [119]. (g) Microfluidic flow cell with 3-electrode setup to perform EIS. Reproduced with permission from Ref. [97]. (h) Photograph f the multi-channel microfluidic device with IDE electrodes for biofilm sensing and treatment using the bioelectric effect. Reproduced with permission from Ref. [120]. Copyright 2017 American Chemical Society. (i) Photograph of flexible impedance sensor consisting of gold IDEs on polyimide film interfaced with a section of a silicone urinary catheter. Reproduced with permission from Ref. [121]. (j) Photograph of the thin-film electrochemical biosensor before and after a biofilm growth experiment. Reproduced with permission from Ref. [122] (k) Optical micrograph of the electrochemical camera chip with integrated electrodes and amplifiers highlighted. Chip is 1 cm x 1 cm. Reproduced with permission from Ref. [123]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The magnitude of the impedance of the three electrical circuits shown in Fig. 4a–c can be calculated using equations (2.2), (2.3), (2.4) listed below. Paredes et al. calculated the numerical values of the various parameters by fitting these equations to experimental data obtained for Staphylococcus epidermis biofilms [70].
| (2.2) |
| (2.3) |
| (2.4) |
By fitting these or other equivalent models to the experimental data, specific electrical parameters of the system can be tracked and used to accurately sense the onset of biofilm growth, as well as the growth over time. For example, Liu et al. utilized an IDE based impedance sensor and an equivalent circuit model to track changes in the resistance and capacitance of E. coli and Salmonella biofilms over time [112]. The resistive and capacitive components of E. coli growth have also been monitored using microelectrode arrays [113].
The advantages of using impedance based microsensor systems, including a reduction in sample volume, low resistance, low power requirement, high signal-to-noise ratio, and the rapid attainment of a steady state, make impedimetric techniques one of the most common methods for biofilm detection and characterization [114]. Gutierrez et al. used microelectrodes fabricated within the wells of microtiter E-plates to impedimetrically monitor the growth of Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus mutans biofilms and their response to anti-biofilm compounds and bacteriophages (Fig. 4d) [115]. Similarly, Tubia et al. employed an IDE based microsensor to detect fungal biofilm growth [116]. Chabowski et al. utilized an IDE based impedance system fabricated using printed circuit board and low temperature co-fired ceramics technology to measure the impedance characteristics of Pseudomonas aeruginosa biofilms [117]. Stöckl et al. employed a 3-electrode setup - which is a more stable electrode system for impedance measurements with separated reference and counter electrodes - to determine the bacterial adhesion properties of Shewanella oneidensis biofilms using electrochemical impedance spectroscopy (EIS) [97]. The flow cell and electrode setup is shown in Fig. 4g; this device allows parallel confocal laser scanning microscopy in addition to the EIS for biofilm characterization.
More recently, our group developed a microfluidic multi-channel device based on the principles of bifurcation that allowed for spatial segmentation of biofilms with the ability to perform multiple experiments on the same chip (Fig. 4h) [120,124]. The sensor-treatment system is comprised of IDE electrodes that detect biofilms using traditional impedance measurements and performs treatment using the bioelectric effect (BE) [125]. In studies performed with this device, the BE treatment was applied based on the state of the biofilm, accessed by comparing the measured impedance values with a user-set impedance change threshold. Thus, this system allowed for continuous real-time monitoring of biofilm growth, while simultaneously being programmed to administer treatment when necessary.
More sophisticated impedimetric systems with capabilities to monitor more than one parameter simultaneously have also been reported. Bruchmann et al. reported a multi-parametric sensor, presented in Fig. 4e, that uses both non-faradaic (EIS) and faradaic (amperometric current) measurements (discussed in the following subsection) within a multi-channel microfluidic platform to measure biofilm formation and activity, respectively [120]. Here, gold IDEs or circular electrodes were used to detect Stenotrophomonas maltophilia and Pseudomonas aueruginosa biofilms, as well as complex mixed population biofilm formation, exoenzymatic activity, and their responses to disinfectant and anti-biofilm reagents, thus making this sensor a qualified tool for assessing biofilm formation in specific environments. However, a large experimental variation between biofilms was observed. Similarly, the sensor developed by Estrada-Leypon et al. used multiple sets of electrodes not only to detect biofilm formation and activity, but also to measure dissolved oxygen, and Na+, K+, and pH levels within the biofilm [119]. This was achieved by using two sets of IDEs along with punctual electrodes (Fig. 4f). Carminati et al. utilized an impedimetric microsensor for measuring biofilm formation along with sensors for pH, conductivity, and temperature for pipe water quality monitoring [126]. Furthermore, Tubia et al. demonstrated that IDE-based impedimetric sensors functionalized with antibodies that could be utilized for detecting specific biofilm, in this case B. bruxellensis [127]. Moreover, other studies that employ microfluidic platforms with IDEs or parallel electrodes for detecting biofilm formation have been performed [[128], [129], [130], [131]].
Impedimetric biofilm monitoring techniques have been adapted to flexible substrates for detection in complex environments [121,132]. In particular, our group has developed an IDE-based impedance sensor on a flexible polyimide substrate for integration with the cylindrical inner lumen of a catheter, where biofilms routinely form (Fig. 4i) [121]. The decrease in impedance of the flexible IDE system corresponds to an increase in biofilm biomass. Furthermore, the same electrodes were used to implement BE treatment in the cylindrical environment. The flexible nature of this platform represents a significant step towards implementation of impedance microsensors on a wide array of vulnerable surfaces with high degrees of geometric complexity. An additional approach developed in our group to conform impedance microsensors to geometrically complex vulnerable surfaces utilized the surface of a urinary catheter as the electrode substrate. The electrodes were directly plated on the catheter in a two-electrode impedance sensing configuration using a 3D-printed insert for patterning to detect E. coli biofilm [133].
Commercially available tools have also been implemented for electrochemical biofilm characterization. Mira et al. utilized the xCELLigence system, which comprises microelectrodes for impedance monitoring embedded within a 96-well plate, to study oral biofilms [134]. Turolla et al. utilized commercially available DropSense IDEs for impedance monitoring of water system biofilms [135].
Potentiometric and Amperometric Microsystems: Faradaic electrochemical techniques including potentiometric and amperometric methods have also been employed for real-time biofilm sensing. These types of measurements allow monitoring of faradaic current generated by the reduction and oxidation of a redox species in contact with a solid electrode. It has been shown that during the first steps of bacterial adhesion there is a charge transfer between the cells and the substrate [136,137]. Bacterial cells generate a variety of molecules (e.g. pyocyanin, phenazine-1-carboxylic acid, etc) that possess electrochemically active groups that can react with the free electrons of the surface. Electrochemical techniques enable tracking of this behavior, making it possible to study them, and hence, to detect bacterial presence at the initial stages of adhesion and biofilm formation.
Beccero et al. achieved this by developing a thin-film sensor (Fig. 4j) that was designed for cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements [122]. The authors use a four-microelectrode configuration, which comprises of two gold working electrodes along with a platinum counter electrode and a platinum pseudo-reference electrode. This configuration provided higher sensitivity, a lower ohmic drop, and faster achievement of a steady-state current than a two-electrode configuration [138,139]. Staphylococcus epidermidis biofilm presence was detected within 2 h after initial inoculation using CV and 1 h after with DPV. An increase in both the current signal and the three recorded redox peaks were observed proportionally to biofilm growth. Additionally, the CV and DPV measurements yielded information about the specific stage of growth of the biofilm, indicated by different oxidation and reduction peaks, along with shifts in the overall current value. Similarly, Fysun et al. used CV and square-wave voltammetry to assess Paenibacillus polymyxa attachment [140].
Other researchers use electrochemical sensing to spatially monitor the chemical distribution in biofilms, which can help characterize the biochemical processes and regulation involved in cellular community development. Bellin et al. used an electrochemical camera chip capable of simultaneous spatial imaging of multiple redox-active phenazine metabolites, which are associated with biofilm activity and colonization, via square wave voltammetry [123]. The chip, shown in Fig. 4k, consists of multiplexed electrodes that enable potential-sweep based electrochemical imaging of whole Pseudomonas aeruginosa biofilms. Using both wild-type and mutant biofilms, the authors were able to confirm the spatial location of different phenazine metabolites. Additionally, this method lends itself to detecting unanticipated compounds.
Thus, electrochemical biosensors are a promising class of microsystems that lend themselves to miniaturization, require low power, and are extremely sensitive to small changes in the environment. These advantages make this class of sensors a serious candidate for real-time biofilm detection in medical devices or other systems. The electrochemical sensing systems presented here are summarized in Table 1.
3.4. Mechanical microsystems
Microsystems that utilize the inherent mechanical properties of the device to measure the mass loading onto the device surface have also been developed for accurate biofilm sensing in real-time. These microsystems typically employ a thin-film piezoelectric material which can provide an electrical response corresponding to the amount of mass loading. The most commonly researched mass loading sensors include the quartz crystal microbalance (QCM), quartz tuning fork oscillators, and surface acoustic wave sensors.
The QCM is a sensitive technique used extensively to study the solid-liquid interface. QCM measurements are based on a shift of the quartz crystal’s resonant frequency due to interactions with solution components [141]. QCMs have also been used to study and characterize biofilm growth and removal in real-time. Reipa et al. used the QCM in conjunction with optical techniques to monitor the long-term (6 days) growth of biofilms in a reactor [142]. The authors utilized a parameter, the ratio of change in resistance to change in frequency, which reflects changes in the viscoelastic properties of the biofilm, to monitor its growth and adaptation to low nutrient environments in real-time. In parallel, the optical technique of white light reflectance off the surface of the QCM gold electrode was used to determine the biofilm thickness. Similarly, Castro et al. used a QCM to analyze the viscoelastic properties of Staphylococcus epidermidis and Escherichia coli, identifying an increase in shear modulus as a distinctive characteristic in biofilm formation [143].
QCMs have been combined with dissipation monitoring, known as QCM-D, to continuously monitor in situ bacterial cell attachment and growth of biofilms [144]. Using this method, Schofield et al. showed that biofilms formed under continuous flow had greater biomass and were more viscoelastic, or softer, than biofilms that were seeded without flow, followed by growth under flow. The energy losses represented by the increases in the dissipative factor (D) indicated an increase in ‘softness’ of the attached cells. Additionally, the ratio of the dissipative factor to frequency or D/f was used to provide information of how viscoelasticity changed per unit mass. These studies allowed not only for the detection of biofilm monitoring during the various stages of growth and treatment with antibiotics or antibacterial agents, but also the continuous monitoring of the properties of cells while establishing that flow conditions over cells on the surface is important in creating biofilms with greater complexity and stability. More recently, this technique has been used to assess cell-surface interactions as well [145]. Markus et al. combined this technology with a fluorescent microscope and camera to monitor kinetics of cell adhesion over time. Two different surfaces, silica (SiO2) and polyvinylidene fluoride (PVDF), which are hydrophilic and hydrophobic, respectively, were used to test the role of surface wettability on biofilm formation [145]. Olsson et al. studied the adhesion of a series of Streptococcus salivarius mutants, each possessing various surface appendages of known lengths, as a function of time using QCM-D. The experimental results were used to further understand and interpret the frequency change and dissipation signal due to the complex interactions within the 250 nm between the substratum and the bacterial cell surface [146]. The same group also showed that while only bacterial attachment to the quartz crystal microbalance shifts the frequency in the positive direction, ECM-generating strains of bacteria that form biofilms elicit a frequency shift in the negative direction [147].
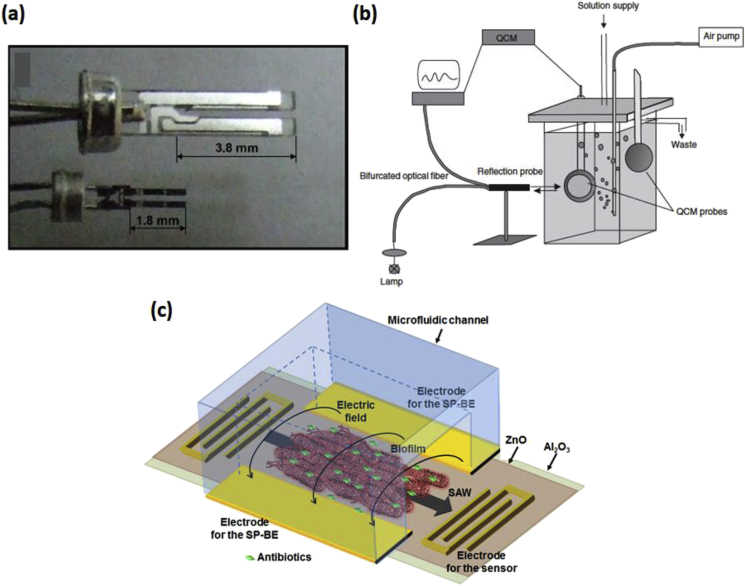
Waszczuk et al. developed disposable piezoelectric quartz tuning fork mass sensors, shown in Fig. 5a to evaluate bacterial biofilm growth [148]. The authors demonstrate the use of this low frequency quartz tuning fork sensor to detect the dynamics of the various phases of the Pseudomonas aeruginosa biofilm. Unlike QCMs, the effective mass of molecules adhering to a quartz tuning fork surface is not equal to real mass, and is only a fraction of the real mass. However, the real mass of bacterial biofilm grown on the tuning fork can be estimated using the tuning fork resonant frequency shift. The authors thus validate the use of the tuning fork as a crystal oscillator which experiences a change in frequency due to the change in mass resulting from biofilm growth on the sensing area. The same group demonstrated the use of quartz tuning forks to detect the viscosity and density of biofilms, and report the development of a quartz tuning fork ring-down system (QTFRS), based on the modulated excitation signal technique to provide the quartz tuning fork excitation, for detecting the formation of Pseudomonas aeruginosa biofilm in situ [149]. The authors use the QTFRS oscillation results for reference liquids to calibrate the device so that unknown densities and viscosities could be determined. This system was also used to successfully measure viscosity of the biofilm when subjected to different concentrations of the antibiotic ciprofloxacin at distinct incubation times.
Fig. 5.
(a) Photograph of the quartz tuning fork (QTF) of two different lengths. Reproduced with permission from Ref. [139]. (b) Schematic of the quartz crystal microbalance (QCM) setup coupled with the optical monitoring system. Reproduced with permission from Ref. [133]. (c) Schematic of a surface acoustic wave (SAW) sensor passivated with Al2O3 and integrated with BE treatment capabilities. Reproduced with permission from Ref. [145].
Surface acoustic wave (SAW) devices are an alternative platform to the QCM and quartz tuning fork, with the advantages of easier integration into array systems, simpler miniaturization, and significantly increased sensitivity due to the surface-guided nature of the mechanical wave and a higher operating frequency [[150], [151], [152]]. Berkenpas et al. developed a shear horizontal SAW device with stable temperature control and high frequency phase measurements for bacterial detection [153]. Escherichia coli bacteria were cultured and applied to the antibody-coated sensing surfaces, and the transmission coefficient phase of the biosensor was monitored continuously using a network analyzer.
Previous work conducted by our group also demonstrated the sensitive real-time detection of biofilm growth using a SAW sensor that is integrated with the BE treatment system [68,154,155]. A schematic of the SAW sensor integrated with electrodes for the BE treatment application is shown in Fig. 5c. Real-time detection of biofilms is achieved by measuring the resonant frequency shift of the SAW system, which is a function of the total biomass adhered to the surface of the sensor and the change in viscoelasticity. As both biofilm growth and BE treatment cause a change in the adhered biomass, they can be measured in real-time by monitoring the resonant frequency of the system. Each of the mechanical microsystems presented in this work are summarized in Table 1.
4. Conclusion
The rise in super-bugs and antibiotic-resistant infections due to biofilms over the last few years has created a global health challenge. This has necessitated the need for efficient tools and methods to characterize and evaluate these organisms, towards not only developing sensitive methods to detect these infections in situ, but also conceiving new antibiotic-free therapies. While macroscale systems currently exist to study biofilms, they primarily only provide end-point characterization, which is typically invasive in nature and thus destroys the biofilm. On the other hand, micro -devices/-systems have significant advantages over their macro-counterparts, the most important aspect being the ability to detect in a highly sensitive and non-invasive manner, and characterize biofilms and their properties in real-time while maintaining a controlled environment. These systems also aid in the development and testing of new therapies for biofilm treatment by reducing reagent and resource costs. These platforms have the potential to help standardize basic biofilm research and establish universal protocols for treatment and device development. In addition, efforts are being made to improve portability and ease of use of existing devices, making point of care the long-term primary goal. Integrating these microsystems for characterization in situ on medical implants and other affected surfaces remains a challenge towards this aim.
Acknowledgements
This work was supported in part by the U.S. National Science Foundation under Grant ECCS1809436.
References
- 1.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. May 21, 1999 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Høiby N., Ciofu O., Johansen H.K., Song Z.J., Moser C., Jensen P.Ø., Molin S., Givskov M., Tolker-Nielsen T., Bjarnsholt T. The clinical impact of bacterial biofilms. Int J Oral Sci. 2011;3:55. doi: 10.4248/IJOS11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potera C. Forging a link between biofilms and disease. Science. March 19, 1999 1999;283:1837–1839. doi: 10.1126/science.283.5409.1837. [DOI] [PubMed] [Google Scholar]
- 4.Flemming H.C., Wingender J., Szewzyk U., Steinberg P., Rice S.A., Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 08/11/online 2016;14:563. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 5.Fux C.A., Stoodley P., Shirtliff M., Costerton J.W. Antimicrobial drug resistance. Springer; 2009. The functional resistance of bacterial biofilms; pp. 121–131. [Google Scholar]
- 6.Richards J.J., Melander C. Controlling bacterial biofilms. Chembiochem. 2009;10:2287–2294. doi: 10.1002/cbic.200900317. [DOI] [PubMed] [Google Scholar]
- 7.Anwar H., Dasgupta M.K., Costerton J.W. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Chemother. 1990;34:2043. doi: 10.1128/aac.34.11.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson M.E., Ceri H., Morck D.W., Buret A.G., Read R.R. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res. 2002;66:86–92. [PMC free article] [PubMed] [Google Scholar]
- 9.Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . World Health Organization; Geneva, Switzerland: 2018. WHO report on surveillance of antibiotic consumption: 2016-2018. [Google Scholar]
- 11.World Health Organization . World Health Organization; Geneva, Switzerland: 2019. Ten threats to global health in 2019. [Google Scholar]
- 12.Chatzopoulou M., Reynolds L. The role of antimicrobial restrictions in bacterial resistance control: a systematic literature review. J Hosp Infect. September 19, 2019 doi: 10.1016/j.jhin.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Donlan R.M. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7:277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donlan R.M. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceri H., Olson M.E., Stremick C., Read R.R., Morck D., Buret A. The calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. June 1, 1999;37:1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crusz S.A., Popat R., Rybtke M.T., Cámara M., Givskov M., Tolker-Nielsen T., Diggle S.P., Williams P. Bursting the bubble on bacterial biofilms: a flow cell methodology. Biofouling. 2012;28:835–842. doi: 10.1080/08927014.2012.716044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore B.F., Hamill T.M., Jones D.S., Gorman S.P. Validation of the CDC biofilm reactor as a dynamic model for assessment of encrustation formation on urological device materials. J Biomed Mater Res B Appl Biomater. 2010;93:128–140. doi: 10.1002/jbm.b.31567. [DOI] [PubMed] [Google Scholar]
- 18.Williams D.L., Woodbury K.L., Haymond B.S., Parker A.E., Bloebaum R.D. A modified CDC biofilm reactor to produce mature biofilms on the surface of PEEK membranes for an in vivo animal model application. Curr Microbiol. 2011;62:1657–1663. doi: 10.1007/s00284-011-9908-2. [DOI] [PubMed] [Google Scholar]
- 19.Kharazmi A., Giwercman B., Høiby N. Robbins device in biofilm research. Methods Enzymol. 1998;310:207–215. doi: 10.1016/s0076-6879(99)10018-1. [DOI] [PubMed] [Google Scholar]
- 20.Azeredo J., Azevedo N.F., Briandet R., Cerca N., Coenye T., Costa A.R., Desvaux M., Di Bonaventura G., Hébraud M., Jaglic Z., Kačániová M., Knøchel S., Lourenço A., Mergulhão F., Meyer R.L., Nychas G., Simões M., Tresse O., Sternberg C. Critical review on biofilm methods. Crit Rev Microbiol. 2017/05/04 2017;43:313–351. doi: 10.1080/1040841X.2016.1208146. [DOI] [PubMed] [Google Scholar]
- 21.Heydorn A., Ersbøll B.K., Hentzer M., Parsek M.R., Givskov M., Molin S. Experimental reproducibility in flow-chamber biofilms. Microbiology. 2000;146:2409–2415. doi: 10.1099/00221287-146-10-2409. [DOI] [PubMed] [Google Scholar]
- 22.Roeselers G., Zippel B., Staal M., Van Loosdrecht M., Muyzer G. On the reproducibility of microcosm experiments–different community composition in parallel phototrophic biofilm microcosms. FEMS Microbiol Ecol. 2006;58:169–178. doi: 10.1111/j.1574-6941.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 23.Palmer R.J., Jr., Sternberg C. Modern microscopy in biofilm research: confocal microscopy and other approaches. Curr Opin Biotechnol. 1999;10:263–268. doi: 10.1016/S0958-1669(99)80046-9. [DOI] [PubMed] [Google Scholar]
- 24.Hannig C., Follo M., Hellwig E., Al-Ahmad A. Visualization of adherent micro-organisms using different techniques. J Med Microbiol. Jan 2010;59:1–7. doi: 10.1099/jmm.0.015420-0. [DOI] [PubMed] [Google Scholar]
- 25.Pousti M., Zarabadi M.P., Amirdehi M.A., Paquet-Mercier F., Greener J. Microfluidic bioanalytical flow cells for biofilm studies: a review. Analyst. January 7, 2019;144:68–86. doi: 10.1039/c8an01526k. [DOI] [PubMed] [Google Scholar]
- 26.Hong J., Edel J.B., Demello A.J. Micro-and nanofluidic systems for high-throughput biological screening. Drug Discov Today. 2009;14:134–146. doi: 10.1016/j.drudis.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Yang W., Woolley A.T. Integrated multiprocess microfluidic systems for automating analysis. J Assoc Lab Autom. 2010;15:198–209. doi: 10.1016/j.jala.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craighead H. Future lab-on-a-chip technologies for interrogating individual molecules. Nature. 2006;442:387–393. doi: 10.1038/nature05061. [DOI] [PubMed] [Google Scholar]
- 29.Dittrich P.S., Manz A. Lab-on-a-chip: microfluidics in drug discovery. Nat Rev Drug Discov. 2006;5:210–218. doi: 10.1038/nrd1985. [DOI] [PubMed] [Google Scholar]
- 30.Dutse S.W., Yusof N.A. Microfluidics-based lab-on-chip systems in DNA-based biosensing: an overview. Sensors. 2011;11:5754–5768. doi: 10.3390/s110605754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figeys D., Pinto D. Lab-on-a-chip: a revolution in biological and medical sciences. Anal Chem. 2000;72 doi: 10.1021/ac002800y. 330 A-335 A. [DOI] [PubMed] [Google Scholar]
- 32.Ghanim M., Abdullah M. Integrating amperometric detection with electrophoresis microchip devices for biochemical assays: recent developments. Talanta. 2011;85:28–34. doi: 10.1016/j.talanta.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 33.Haeberle S., Zengerle R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip. 2007;7:1094–1110. doi: 10.1039/b706364b. [DOI] [PubMed] [Google Scholar]
- 34.Jiang H., Weng X., Li D. Microfluidic whole-blood immunoassays. Microfluid Nanofluidics. 2011;10:941–964. [Google Scholar]
- 35.Pollack M.G., Pamula V.K., Srinivasan V., Eckhardt A.E. Applications of electrowetting-based digital microfluidics in clinical diagnostics. Expert Rev Mol Diagn. 2011;11:393–407. doi: 10.1586/erm.11.22. [DOI] [PubMed] [Google Scholar]
- 36.Trietsch S., Hankemeier T., Van der Linden H. Lab-on-a-chip technologies for massive parallel data generation in the life sciences: a review. Chemometr Intell Lab Syst. 2011;108:64–75. [Google Scholar]
- 37.Uhlen M., Svahn H.A. Affinity reagents for lab on chips. Lab Chip. 2011;11:1417–1419. doi: 10.1039/c1lc90005f. [DOI] [PubMed] [Google Scholar]
- 38.Mir M., Homs A., Samitier J. Integrated electrochemical DNA biosensors for lab-on-a-chip devices. Electrophoresis. 2009;30:3386–3397. doi: 10.1002/elps.200900319. [DOI] [PubMed] [Google Scholar]
- 39.Mark D., Haeberle S., Roth G., von Stetten F., Zengerle R. Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chem Soc Rev. 2010;39:1153–1182. doi: 10.1039/b820557b. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues Ribeiro Teles F.S., Pires de Távora Tavira L.A., Pina da Fonseca L.J. Biosensors as rapid diagnostic tests for tropical diseases. Crit Rev Clin Lab Sci. 2010;47:139–169. doi: 10.3109/10408363.2010.518405. [DOI] [PubMed] [Google Scholar]
- 41.Rosen Y., Gurman P. MEMS and microfluidics for diagnostics devices. Curr Pharmaceut Biotechnol. 2010;11:366–375. doi: 10.2174/138920110791233316. [DOI] [PubMed] [Google Scholar]
- 42.Lin C.-C., Wang J.-H., Wu H.-W., Lee G.-B. Microfluidic immunoassays. J Assoc Lab Autom. 2010;15:253–274. [Google Scholar]
- 43.Focke M., Kosse D., Müller C., Reinecke H., Zengerle R., von Stetten F. Lab-on-a-Foil: microfluidics on thin and flexible films. Lab Chip. 2010;10:1365–1386. doi: 10.1039/c001195a. [DOI] [PubMed] [Google Scholar]
- 44.Varghese S.S., Zhu Y., Davis T.J., Trowell S.C. FRET for lab-on-a-chip devices—current trends and future prospects. Lab Chip. 2010;10:1355–1364. doi: 10.1039/b924271f. [DOI] [PubMed] [Google Scholar]
- 45.Liu K.-K., Wu R.-G., Chuang Y.-J., Khoo H.S., Huang S.-H., Tseng F.-G. Microfluidic systems for biosensing. Sensors. 2010;10:6623–6661. doi: 10.3390/s100706623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta K., Kim D.-H., Ellison D., Smith C., Kundu A., Tuan J., Suh K.-Y., Levchenko A. Lab-on-a-chip devices as an emerging platform for stem cell biology. Lab Chip. 2010;10:2019–2031. doi: 10.1039/c004689b. [DOI] [PubMed] [Google Scholar]
- 47.Huo D.-Q., Liu Z., Hou C.-J., Yang J., Luo X.-G., Fa H.-B., Dong J.-L., Zhang Y.-C., Zhnag G.-P., Li J.-J. Recent advances on optical detection methods and techniques for cell-based microfluidic systems. Chin J Anal Chem. 2010;38:1357–1365. [Google Scholar]
- 48.Wlodkowic D., Cooper J.M. Tumors on chips: oncology meets microfluidics. Curr Opin Chem Biol. 2010;14:556–567. doi: 10.1016/j.cbpa.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 49.Simon E. Biological and chemical sensors for cancer diagnosis. Meas Sci Technol. 2010;21:112002. [Google Scholar]
- 50.Didar T.F., Tabrizian M. Adhesion based detection, sorting and enrichment of cells in microfluidic Lab-on-Chip devices. Lab Chip. 2010;10:3043–3053. doi: 10.1039/c0lc00130a. [DOI] [PubMed] [Google Scholar]
- 51.Koev S., Dykstra P., Luo X., Rubloff G., Bentley W., Payne G., Ghodssi R. Chitosan: an integrative biomaterial for lab-on-a-chip devices. Lab Chip. 2010;10:3026–3042. doi: 10.1039/c0lc00047g. [DOI] [PubMed] [Google Scholar]
- 52.Lim Y.C., Kouzani A.Z., Duan W. Lab-on-a-chip: a component view. Microsyst Technol. 2010/12/01 2010;16:1995–2015. [Google Scholar]
- 53.Hrnčiřík P., Náhlík J. Novel micro-scale analytical devices for on-line bioprocess monitoring: a review. Chem Biochem Eng Q. 2010;24:489–500. [Google Scholar]
- 54.Weddemann A., Albon C., Auge A., Wittbracht F., Hedwig P., Akemeier D., Rott K., Meißner D., Jutzi P., Hütten A. How to design magneto-based total analysis systems for biomedical applications. Biosens Bioelectron. 2010;26:1152–1163. doi: 10.1016/j.bios.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 55.Webster A., Greenman J., Haswell S.J. Development of microfluidic devices for biomedical and clinical application. J Chem Technol Biotechnol. 2011;86:10–17. [Google Scholar]
- 56.Mohammed M.-I., Desmulliez M.P. Lab-on-a-chip based immunosensor principles and technologies for the detection of cardiac biomarkers: a review. Lab Chip. 2011;11:569–595. doi: 10.1039/c0lc00204f. [DOI] [PubMed] [Google Scholar]
- 57.Jang A., Zou Z., Lee K.K., Ahn C.H., Bishop P.L. State-of-the-art lab chip sensors for environmental water monitoring. Meas Sci Technol. 2011;22 [Google Scholar]
- 58.Sharma H., Nguyen D., Chen A., Lew V., Khine M. Unconventional low-cost fabrication and patterning techniques for point of care diagnostics. Ann Biomed Eng. 2011;39:1313–1327. doi: 10.1007/s10439-010-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goeres D.M., Loetterle L.R., Hamilton M.A., Murga R., Kirby D.W., Donlan R.M. Statistical assessment of a laboratory method for growing biofilms. Microbiology. March 1, 2005;151:757–762. doi: 10.1099/mic.0.27709-0. [DOI] [PubMed] [Google Scholar]
- 60.Jing G., Polaczyk A., Oerther D.B., Papautsky I. Development of a microfluidic biosensor for detection of environmental mycobacteria. Sens Actuators B Chem. 2007;123:614–621. [Google Scholar]
- 61.Richter L., Stepper C., Mak A., Reinthaler A., Heer R., Kast M., Brückl H., Ertl P. Development of a microfluidic biochip for online monitoring of fungal biofilm dynamics. Lab Chip. 2007;7:1723–1731. doi: 10.1039/b708236c. [DOI] [PubMed] [Google Scholar]
- 62.Tolker-Nielsen T., Sternberg C. Growing and analyzing biofilms in flow chambers. Curr Protoc Microbiol. May 2011;Chapter 1 doi: 10.1002/9780471729259.mc01b02s21. Unit 1B 2. [DOI] [PubMed] [Google Scholar]
- 63.Zhang C., Xu J., Ma W., Zheng W. PCR microfluidic devices for DNA amplification. Biotechnol Adv. 2006;24:243–284. doi: 10.1016/j.biotechadv.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Jesús-Pérez N.M., Lapizco-Encinas B.H. Dielectrophoretic monitoring of microorganisms in environmental applications. Electrophoresis. 2011;32:2331–2357. doi: 10.1002/elps.201100107. [DOI] [PubMed] [Google Scholar]
- 65.Melin J., Quake S.R. Microfluidic large-scale integration: the evolution of design rules for biological automation. Annu Rev Biophys Biomol Struct. 2007;36:213–231. doi: 10.1146/annurev.biophys.36.040306.132646. [DOI] [PubMed] [Google Scholar]
- 66.Studer V., Hang G., Pandolfi A., Ortiz M., Anderson W.F., Quake S.R. Scaling properties of a low-actuation pressure microfluidic valve. J Appl Phys. 2004;95:393–398. [Google Scholar]
- 67.Thorsen T., Maerkl S.J., Quake S.R. Microfluidic large-scale integration. Science. 2002;298:580–584. doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- 68.Kim Y.W., Sardari S.E., Meyer M.T., Iliadis A.A., Wu H.C., Bentley W.E., Ghodssi R. An ALD aluminum oxide passivated Surface Acoustic Wave sensor for early biofilm detection. Sens Actuators B Chem. 2012;163:136–145. [Google Scholar]
- 69.Kim K.P., Kim Y.-G., Choi C.-H., Kim H.-E., Lee S.-H., Chang W.-S., Lee C.-S. In situ monitoring of antibiotic susceptibility of bacterial biofilms in a microfluidic device. Lab Chip. 2010;10:3296–3299. doi: 10.1039/c0lc00154f. [DOI] [PubMed] [Google Scholar]
- 70.Paredes J., Becerro S., Arana S. Label-free interdigitated microelectrode based biosensors for bacterial biofilm growth monitoring using Petri dishes. J Microbiol Methods. 2014;100:77–83. doi: 10.1016/j.mimet.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 71.Janakiraman V., Englert D., Jayaraman A., Baskaran H. Modeling growth and quorum sensing in biofilms grown in microfluidic chambers. Ann Biomed Eng. 2009/06/01 2009;37:1206–1216. doi: 10.1007/s10439-009-9671-8. [DOI] [PubMed] [Google Scholar]
- 72.Kim J., Hegde M., Kim S.H., Wood T.K., Jayaraman A. A microfluidic device for high throughput bacterial biofilm studies. Lab Chip. 2012;12:1157–1163. doi: 10.1039/c2lc20800h. [DOI] [PubMed] [Google Scholar]
- 73.Meyer M.T., Roy V., Bentley W.E., Ghodssi R. Development and validation of a microfluidic reactor for biofilm monitoring via optical methods. J Micromech Microeng. 2011;21 [Google Scholar]
- 74.Roy V., Meyer M.T., Smith J.A.I., Gamby S., Sintim H.O., Ghodssi R., Bentley W.E. AI-2 analogs and antibiotics: a synergistic approach to reduce bacterial biofilms. Appl Microbiol Biotechnol. 2013;97:2627–2638. doi: 10.1007/s00253-012-4404-6. [DOI] [PubMed] [Google Scholar]
- 75.Radke S.M., Alocilja E.C. Design and fabrication of a microimpedance biosensor for bacterial detection. Sensors Journal, IEEE. 2004;4:434–440. [Google Scholar]
- 76.Brady R.A., Leid J.G., Camper A.K., Costerton J.W., Shirtliff M.E. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun. 2006;74:3415–3426. doi: 10.1128/IAI.00392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meyer M.T. Ph.D., Department of Bioengineering, University of Maryland; College Park, MD: 2014. Design and implementation of microfluidic systems for bacterial biofilm monitoring and manipulation. [Google Scholar]
- 78.Hong S.H., Hegde M., Kim J., Wang X., Jayaraman A., Wood T.K. Synthetic quorum-sensing circuit to control consortial biofilm formation and dispersal in a microfluidic device. Nat Commun. 2012;3:613. doi: 10.1038/ncomms1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Connell J.L., Wessel A.K., Parsek M.R., Ellington A.D., Whiteley M., Shear J.B. Probing prokaryotic social behaviors with bacterial "lobster traps. mBio. Sep-Oct, 2010;1 doi: 10.1128/mBio.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lam R.H., Cui X., Guo W., Thorsen T. High-throughput dental biofilm growth analysis for multiparametric microenvironmental biochemical conditions using microfluidics. Lab Chip. 2016;16:1652–1662. doi: 10.1039/c6lc00072j. [DOI] [PubMed] [Google Scholar]
- 81.Skolimowski M., Nielsen M.W., Emneus J., Molin S., Taboryski R., Sternberg C., Dufva M., Geschke O. Microfluidic dissolved oxygen gradient generator biochip as a useful tool in bacterial biofilm studies. Lab Chip. 2010;10:2162–2169. doi: 10.1039/c003558k. [DOI] [PubMed] [Google Scholar]
- 82.Skolimowski M., Nielsen M.W., Abeille F., Skafte-Pedersen P., Sabourin D., Fercher A., Papkovsky D., Molin S., Taboryski R., Sternberg C., Dufva M., Geschke O., Emnéus J. Modular microfluidic system as a model of cystic fibrosis airways. Biomicrofluidics. 2012;6 doi: 10.1063/1.4742911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hua X., Marshall M.J., Xiong Y., Ma X., Zhou Y., Tucker A.E., Zhu Z., Liu S., Yu X.-Y. Two-dimensional and three-dimensional dynamic imaging of live biofilms in a microchannel by time-of-flight secondary ion mass spectrometry. Biomicrofluidics. 2015;9 doi: 10.1063/1.4919807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer M.T., Subramanian S., Kim Y.W., Ben-Yoav H., Gnerlich M., Bentley W.E., Ghodssi R. Multi-depth valved microfluidics for biofilm segmentation. J Micromech Microeng. 2015;25 [Google Scholar]
- 85.Wright E., Neethirajan S., Weng X. Microfluidic wound model for studying the behaviors of Pseudomonas aeruginosa in polymicrobial biofilms. Biotechnol Bioeng. 2015;112:2351–2359. doi: 10.1002/bit.25651. [DOI] [PubMed] [Google Scholar]
- 86.Paquet-Mercier F., Gashti M.P., Bellavance J., Taghavi S.M., Greener J. Through thick and thin: a microfluidic approach for continuous measurements of biofilm viscosity and the effect of ionic strength. Lab Chip. 2016;16:4710–4717. doi: 10.1039/c6lc01101b. [DOI] [PubMed] [Google Scholar]
- 87.Shin S., Ahmed I., Hwang J., Seo Y., Lee E., Moon S., Hong J.W. A microfluidic approach to investigating a synergistic effect of Tobramycin and sodium dodecyl sulfate on Pseudomonas aeruginosa biofilms. Anal Sci. 2016;32:67–73. doi: 10.2116/analsci.32.67. [DOI] [PubMed] [Google Scholar]
- 88.Lee J.-H., Kaplan J.B., Lee W.Y. Microfluidic devices for studying growth and detachment of Staphylococcus epidermidis biofilms. Biomed Microdevices. August 01 2008;10:489–498. doi: 10.1007/s10544-007-9157-0. [DOI] [PubMed] [Google Scholar]
- 89.Salta M., Capretto L., Carugo D., Wharton J.A., Stokes K.R. Life under flow: a novel microfluidic device for the assessment of anti-biofilm technologies. Biomicrofluidics. 2013;7 doi: 10.1063/1.4850796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serioli L.S., Giaele, Laksafoss Trygvi Z., Haagensen Janus A.J., Sorensen Mads P., Molin Soren, Johansen Helle K., Zor Kinga, Boisen Anja. 44th international conference on micro and NanoEngineering, copenhagen, Denmark. 2018. Centrifugal microfluidic platform for optical monitoring and treatment of biofilms. [Google Scholar]
- 91.Terry J., Neethirajan S. A novel microfluidic wound model for testing antimicrobial agents against Staphylococcus pseudintermedius biofilms. J Nanobiotechnol. 2014;12(1) doi: 10.1186/1477-3155-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mukherjee M., Menon N.V., Liu X., Kang Y.J., Cao B. Confocal laser scanning microscopy-compatible microfluidic membrane flow cell as a nondestructive tool for studying biofouling dynamics on forward osmosis membranes. Environ Sci Technol Lett. Aug. 2016;3:303–309. [Google Scholar]
- 93.Benoit M.R., Conant C.G., Ionescu-Zanetti C., Schwartz M., Matin A. New device for high-throughput viability screening of flow biofilms. Appl Environ Microbiol. July 1, 2010;76:4136–4142. doi: 10.1128/AEM.03065-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kristensen M.F., Zeng G., Neu T.R., Meyer R.L., Baelum V., Schlafer S. Osteopontin adsorption to Gram-positive cells reduces adhesion forces and attachment to surfaces under flow. J Oral Microbiol. 2017/01/01 2017;9:1379826. doi: 10.1080/20002297.2017.1379826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swope K.L., Flickinger M.C. The use of confocal scanning laser microscopy and other tools to characterize Escherichia coli in a high-cell-density synthetic biofilm. Biotechnol Bioeng. 1996;52:340–356. doi: 10.1002/(SICI)1097-0290(19961020)52:2<340::AID-BIT14>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 96.Subramanian S., Gerasopoulos K., Guo M., Sintim H.O., Bentley W.E., Ghodssi R. Autoinducer-2 analogs and electric fields - an antibiotic-free bacterial biofilm combination treatment. Biomed Microdevices. 2016;18:1–12. doi: 10.1007/s10544-016-0120-9. [DOI] [PubMed] [Google Scholar]
- 97.Stoeckl M., Schlegel C., Sydow A., Holtmann D., Ulber R., Mangold K.-M. Membrane separated flow cell for parallelized electrochemical impedance spectroscopy and confocal laser scanning microscopy to characterize electro-active microorganisms. Electrochim Acta. 2016;220:444–452. [Google Scholar]
- 98.Yawata Y., Toda K., Setoyama E., Fukuda J., Suzuki H., Uchiyama H., Nomura N. Monitoring biofilm development in a microfluidic device using modified confocal reflection microscopy. J Biosci Bioeng. 2010;110:377–380. doi: 10.1016/j.jbiosc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 99.Holman H.-Y.N., Miles R., Hao Z., Wozei E., Anderson L.M., Yang H. Real-time chemical imaging of bacterial activity in biofilms using open-channel microfluidics and synchrotron FTIR spectromicroscopy. Anal Chem. 2009;81:8564–8570. doi: 10.1021/ac9015424. [DOI] [PubMed] [Google Scholar]
- 100.Yuan Y., Guo T., Qiu X., Tang J., Huang Y., Zhuang L., Zhou S., Li Z., Guan B.-O., Zhang X. Electrochemical surface plasmon resonance fiber-optic sensor: in situ detection of electroactive biofilms. Anal Chem. 2016;88:7609–7616. doi: 10.1021/acs.analchem.6b01314. [DOI] [PubMed] [Google Scholar]
- 101.Zhong N., Zhao M., Li Y. U-shaped, double-tapered, fiber-optic sensor for effective biofilm growth monitoring. Biomed Opt Express. 2016;7:335–351. doi: 10.1364/BOE.7.000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim Y.W., Mosteller M.P., Subramanian S., Meyer M.T., Bentley W.E., Ghodssi R. An optical microfluidic platform for spatiotemporal biofilm treatment monitoring. J Micromech Microeng. 2016;26 [Google Scholar]
- 103.Keirsse J., Boussard-Pledel C., Loreal O., Sire O., Bureau B., Leroyer P., Turlin B., Lucas J. IR optical fiber sensor for biomedical applications. Vib Spectrosc. 2003;32:23–32. [Google Scholar]
- 104.Mattana S., Alunni Cardinali M., Caponi S., Casagrande Pierantoni D., Corte L., Roscini L., Cardinali G., Fioretto D. High-contrast Brillouin and Raman micro-spectroscopy for simultaneous mechanical and chemical investigation of microbial biofilms. Biophys Chem. 2017/10/01/2017;229:123–129. doi: 10.1016/j.bpc.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 105.Abadian P.N., Tandogan N., Jamieson J.J., Goluch E.D. Using surface plasmon resonance imaging to study bacterial biofilms. Biomicrofluidics. Mar 2014;8 doi: 10.1063/1.4867739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu W., Qiu X., Zhang X., Zhang Z., Tang J., Yuan Y., Guan B.-O., Guo T. Asia-pacific optical sensors conference. 2016. I n-situ detection of electroactive biofilms using an electrochemical surface Plasmon resonance fiber-optic sensor. W4A. 61. [DOI] [PubMed] [Google Scholar]
- 107.Philip-Chandy R., Scully P.J., Eldridge P., Kadim H., Grapin M.G., Jonca M.G., D’Ambrosio M.G., Colin F. An optical fiber sensor for biofilm measurement using intensity modulation and image analysis. IEEE J Sel Top Quantum Electron. 2000;6:764–772. [Google Scholar]
- 108.Clark L., Lyons C. Development of the first glucose enzyme electrode that rely on a thin layer of glucose oxidase on an oxygen electrode. Ann N Y Acad Sci. 1962;102:29. [Google Scholar]
- 109.Van Gerwen P., Laureyn W., Laureys W., Huyberechts G., Op De Beeck M., Baert K., Suls J., Sansen W., Jacobs P., Hermans L., Mertens R. Nanoscaled interdigitated electrode arrays for biochemical sensors. Sens Actuators B Chem. 1998;49:73–80. [Google Scholar]
- 110.Gómez R., Bashir R., Bhunia A.K. Microscale electronic detection of bacterial metabolism. Sens Actuators B Chem. 2002;86:198–208. [Google Scholar]
- 111.Yang L., Li Y., Griffis C.L., Johnson M.G. Interdigitated microelectrode (IME) impedance sensor for the detection of viable Salmonella typhimurium. Biosens Bioelectron. 2004;19:1139–1147. doi: 10.1016/j.bios.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 112.Liu L., Xu Y., Cui F., Xia Y., Chen L., Mou X., Lv J. Monitoring of bacteria biofilms forming process by in-situ impedimetric biosensor chip. Biosens Bioelectron. Jul 30 2018;112:86–92. doi: 10.1016/j.bios.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 113.Goikoetxea E., Routkevitch D., de Weerdt A., Green J.J., Steenackers H., Braeken D. Impedimetric fingerprinting and structural analysis of isogenic E. coli biofilms using multielectrode arrays. Sens Actuators B Chem. Jun 15 2018;263:319–326. [Google Scholar]
- 114.Yang L., Bashir R. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol Adv. 2008;26:135–150. doi: 10.1016/j.biotechadv.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 115.Gutiérrez D., Hidalgo-Cantabrana C., Rodríguez A., García P., Ruas-Madiedo P. Monitoring in real time the formation and removal of biofilms from clinical related pathogens using an impedance-based technology. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tubia I., Paredes J., Perez-Lorenzo E., Arana S. Brettanomyces bruxellensis growth detection using interdigitated microelectrode based sensors by means of impedance analysis. Sens Actuators A Phys. Jan 1 2018;269:175–181. [Google Scholar]
- 117.Chabowski K., Junka A.F., Piasecki T., Nowak D., Nitsch K., Smutnicka D., Bartoszewicz M., Moczala M., Szymczyk P. Impedance sensors made in pcb and ltcc technologies for monitoring growth and degradation of pseudomonal biofilm. Metrol Meas Syst. Jun 17 2017;24:369–380. [Google Scholar]
- 118.Bruchmann J., Sachsenheimer K., Rapp B.E., Schwartz T. Multi-channel microfluidic biosensor platform applied for online monitoring and screening of biofilm formation and activity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Estrada-Leypon O., Moya A., Guimera A., Gabriel G., Agut M., Sanchez B., Borros S. Simultaneous monitoring of Staphylococcus aureus growth in a multi-parametric microfluidic platform using microscopy and impedance spectroscopy. Bioelectrochemistry. 2015;105:56–64. doi: 10.1016/j.bioelechem.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 120.Subramanian S., Tolstaya E.I., Winkler T.E., Bentley W.E., Ghodssi R. An integrated microsystem for real-time detection and threshold-activated treatment of bacterial biofilms. ACS Appl Mater Interfaces. Sep 20 2017;9:31362–31371. doi: 10.1021/acsami.7b04828. [DOI] [PubMed] [Google Scholar]
- 121.Huiszoon R.C., Subramanian S., Rajasekaran P.R., Beardslee L.A., Bentley W.E., Ghodssi R. Flexible platform for in situ impedimetric detection and bioelectric effect treatment of Escherichia coli biofilms. IEEE (Inst Electr Electron Eng) Trans Biomed Eng. May 2019;66:1337–1345. doi: 10.1109/TBME.2018.2872896. [DOI] [PubMed] [Google Scholar]
- 122.Becerro S., Paredes J., Mujika M., Lorenzo E.P., Arana S. Electrochemical real-time analysis of bacterial biofilm adhesion and development by means of thin-film biosensors. IEEE Sens J. 2016;16:1856–1864. [Google Scholar]
- 123.Bellin D.L., Sakhtah H., Zhang Y., Price-Whelan A., Dietrich L.E., Shepard K.L. Electrochemical camera chip for simultaneous imaging of multiple metabolites in biofilms. Nat Commun. 2016;7 doi: 10.1038/ncomms10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Subramanian S. Ph.D. , Department of Electrical and Computer Engineering, University of Maryland; College Park: 2016. Integrated threshold-activated feedback microsystem for real-time characterization, sensing and treatment of bacterial biofilms. [Google Scholar]
- 125.Costerton J.W., Ellis B., Lam K., Johnson F., Khoury A.E. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother. 1994;38:2803–2809. doi: 10.1128/aac.38.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carminati M., Ferrari G., Sampietro M. Emerging miniaturized technologies for airborne particulate matter pervasive monitoring. Measurement. Apr 2017;101:250–256. [Google Scholar]
- 127.Tubia I., Paredes J., Perez-Lorenzo E., Arana S. Antibody biosensors for spoilage yeast detection based on impedance spectroscopy. Biosens Bioelectron. Apr 15 2018;102:432–438. doi: 10.1016/j.bios.2017.11.057. [DOI] [PubMed] [Google Scholar]
- 128.Zikmund A., Ripka P., Krasny L., Judl T., Jahoda D. 3rd international conference on biomedical engineering and informatics. BMEI); 2010. Biofilm detection by the impedance method; pp. 1432–1434. [Google Scholar]
- 129.Paredes J., Becerro S., Arizti F., Aguinaga A., Del Pozo J.L., Arana S. Interdigitated microelectrode biosensor for bacterial biofilm growth monitoring by impedance spectroscopy technique in 96-well microtiter plates. Sens Actuators B Chem. 2013;178:663–670. [Google Scholar]
- 130.Muñoz-Berbel X., Muñoz F.J., Vigués N., Mas J. On-chip impedance measurements to monitor biofilm formation in the drinking water distribution network. Sens Actuators B Chem. 2006;118:129–134. [Google Scholar]
- 131.Zarabadi M.P., Paquet-Mercier F., Charette S.J., Greener J. Hydrodynamic effects on biofilms at the biointerface using a microfluidic electrochemical cell: case study of Pseudomonas sp. Langmuir. Feb 28 2017;33:2041–2049. doi: 10.1021/acs.langmuir.6b03889. [DOI] [PubMed] [Google Scholar]
- 132.Carminati L.M.M., turolla A., Pani G., Tizzoni M., Di Mauro M., Antonelli M. 2019 IEEE international symposium on circuits and systems (ISCAS), sapporo, Japan. 2019. Flexible impedance sensor for in-line monitoring of water and beverages. [Google Scholar]
- 133.Huiszoon R.C., Chu S., Beardslee L.A., Ramiah Rajasekaran P., Bentley W.E., Ghodssi R. The 20th international conference on solid-state sensors, actuators and microsystems. Transducers 2019; Berlin, Germany: 2019. In situ sensor electrode patterning on urinary catheters towards infection prevention; pp. 2193–2196. [Google Scholar]
- 134.Mira A., Buetas E., Rosier B., Mazurel D., Villanueva-Castellote A., Llena C., Ferrer M.D. Development of an in vitro system to study oral biofilms in real time through impedance technology: validation and potential applications. J Oral Microbiol. Jan 1 2019;11 doi: 10.1080/20002297.2019.1609838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Turolla A., Di Mauro M., Mezzera L., Antonelli M., Carminati M. Development of a miniaturized and selective impedance sensor for real-time slime monitoring in pipes and tanks. Sens Actuators B Chem. Feb 15 2019;281:288–295. [Google Scholar]
- 136.Bayoudh S., Othmane A., Ponsonnet L., Ouada H.B. Electrical detection and characterization of bacterial adhesion using electrochemical impedance spectroscopy-based flow chamber. Colloid Surf Physicochem Eng Asp. 2008;318:291–300. [Google Scholar]
- 137.Palmer J., Flint S., Brooks J. Bacterial cell attachment, the beginning of a biofilm. J Ind Microbiol Biotechnol. 2007;34:577–588. doi: 10.1007/s10295-007-0234-4. [DOI] [PubMed] [Google Scholar]
- 138.Min J., Baeumner A.J. Characterization and optimization of interdigitated ultramicroelectrode arrays as electrochemical biosensor transducers. Electroanalysis. 2004;16:724–729. [Google Scholar]
- 139.Rahimi M., Mikkelsen S.R. Cyclic biamperometry at micro-interdigitated electrodes. Anal Chem. 2011;83:7555–7559. doi: 10.1021/ac2012703. [DOI] [PubMed] [Google Scholar]
- 140.Olga Fysun S.K., Rauschnabel Johannes, Langowski Horst-Christian. Electrochemical detection of a P. polymyxa biofilm and CIP cleaning solutions by voltammetric microsensors. Engineering in Agriculture, Environment and Food. 2019;12:232–243. [Google Scholar]
- 141.Kanazawa K.K., Gordon J.G. The oscillation frequency of a quartz resonator in contact with liquid. Anal Chim Acta. 1985;175:99–105. [Google Scholar]
- 142.Reipa V., Almeida J., Cole K.D. Long-term monitoring of biofilm growth and disinfection using a quartz crystal microbalance and reflectance measurements. J Microbiol Methods. 2006;66:449–459. doi: 10.1016/j.mimet.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 143.Castro P., Elvira L., Maestre J.R., de Espinosa F.M. Study of the relation between the resonance behavior of thickness shear mode (TSM) sensors and the mechanical characteristics of biofilms. Sensors. Jun 2017;17 doi: 10.3390/s17061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schofield A.L., Rudd T.R., Martin D.S., Fernig D.G., Edwards C. Real-time monitoring of the development and stability of biofilms of Streptococcus mutans using the quartz crystal microbalance with dissipation monitoring. Biosens Bioelectron. 2007;23:407–413. doi: 10.1016/j.bios.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 145.Marcus I.M., Herzberg M., Walker S.L., Freger V. Pseudomonas aeruginosa attachment on QCM-D sensors: the role of cell and surface hydrophobicities. Langmuir. 2012;28:6396–6402. doi: 10.1021/la300333c. [DOI] [PubMed] [Google Scholar]
- 146.Olsson A.L.J., van der Mei H.C., Busscher H.J., Sharma P.K. Influence of cell surface appendages on the Bacterium−Substratum interface measured real-time using QCM-D. Langmuir. 2009/02/03 2009;25:1627–1632. doi: 10.1021/la803301q. [DOI] [PubMed] [Google Scholar]
- 147.A. L. J. Olsson, H. C. van der Mei, H. J. Busscher, and P. K. Sharma, "Acoustic sensing of the bacterium–substratum interface using QCM-D and the influence of extracellular polymeric substances," J Colloid Interface Sci, vol. 357, pp. 135-138, 5/1/2011. [DOI] [PubMed]
- 148.Waszczuk K., Gula G., Swiatkowski M., Olszewski J., Herwich W., Drulis-Kawa Z., Gutowicz J., Gotszalk T. Evaluation of Pseudomonas aeruginosa biofilm formation using piezoelectric tuning fork mass sensors. Sens Actuators B Chem. 2012;170:7–12. [Google Scholar]
- 149.Piasecki T., Guła G., Markwitz P., Waszczuk K., Gosiewska A., Drulis-Kawa Z., Gotszalk T. Autonomous system for in situ assay of antibiotic activity on bacterial biofilms using viscosity and density sensing quartz tuning forks. Procedia Engineering. 2016;168:745–748. [Google Scholar]
- 150.Ballantine D., Jr., White R.M., Martin S.J., Ricco A.J., Zellers E., Frye G., Wohltjen H. Academic press; 1996. Acoustic wave sensors: theory, design and physico-chemical applications. [Google Scholar]
- 151.Freudenberg J., Von Schickfus M., Hunklinger S. A SAW immunosensor for operation in liquid using a SiO 2 protective layer. Sens Actuators B Chem. 2001;76:147–151. [Google Scholar]
- 152.Gizeli E. Acoustic transducers. Biomolecular sensors. 2002:183. [Google Scholar]
- 153.Berkenpas E., Millard P., Da Cunha M.P. Detection of Escherichia coli O157: H7 with langasite pure shear horizontal surface acoustic wave sensors. Biosens Bioelectron. 2006;21:2255–2262. doi: 10.1016/j.bios.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 154.Kim Y.W., Meyer M.T., Berkovich A., Subramanian S., Iliadis A.A., Bentley W.E., Ghodssi R. A surface acoustic wave biofilm sensor integrated with A treatment method based on the bioelectric effect. Sens Actuators A Phys. 2016;238:140–149. [Google Scholar]
- 155.Kim Y.W. Ph.D., Department of Electrical and Computer Engineering, University of Maryland; College Park, MD: 2014. An integrated microsystem for biofilm detection and treatment. [Google Scholar]