Abstract
Wounds complicated by biofilms challenge even the best clinical care and can delay a return to duty for service members. A major component of treatment in wounded warriors includes infected wound management. Yet, all antibiotic therapy options have been optimized against planktonic bacteria, leaving an important gap in biofilm-related wound care. We tested the efficacy of a unique compound (CZ-01179) specifically synthesized to eradicate biofilms. CZ-01179 was formulated as the active agent in a hydrogel, and tested in vitro and in vivo in a pig excision wound model for its ability to treat and prevent biofilm-related wound infection caused by Acinetobacter baumannii. Data indicated that compared to a clinical standard—silver sulfadiazine—CZ-01179 was much more effective at eradicating biofilms of A. baumannii in vitro and up to 6 days faster at eradicating biofilms in vivo. CZ-01179 belongs to a broader class of newly-synthesized antibiofilm agents (referred to as CZ compounds) with reduced risk of resistance development, specific efficacy against biofilms, and promising formulation potential for clinical applications. Given its broad spectrum and biofilm-specific nature, CZ-01179 gel may be a promising agent to increase the pipeline of products to treat and prevent biofilm-related wound infections.
Keywords: Military wound care, Biofilm, Infection, Antibiofilm agent, Experimental models
Introduction
Biofilms can be one of the most complicating factors in wound healing; having prevalence rates between 60% and 100% in chronic wounds [1,2]. Biofilm-related infections are debilitating and disheartening to those who are affected as they can persist for months if not years, and far too often leave patients with a loss of hope. These difficult-to-treat wound types constitute a significant challenge in military and Veterans Affairs medical centers, as well as civilian healthcare facilities [1,3,4]. Following battlefield injury a majority of wounds contain at least one bacterial species, highlighting the prevalence of contamination and potential for infection to develop in wounded warriors [5]. Battlefield wounds affected by biofilms are significantly associated with persistence of wound infections [3], and complicate a spectrum of pathologies including decubitus ulcers, i.e. pressure sores [6]. Rehabilitation is often delayed in wounded warriors as infected ulcers or sores require treatment and healing before rehabilitation measures can begin or continue. For example, if chronic wounds are present, prosthetic socket fittings can be painful, leaving a patient wheelchair-bound or bed-ridden and altogether immobile.
More than 6 million Americans in the civilian sector are estimated to develop chronic, biofilm-impaired wounds each year [7]. The cost burden of treatment reaches tens of billions of dollars. Infected wound types comprise, but are not limited to, open wounds, infected surgical sites, diabetic foot ulcers, venous ulcers and other trauma-induced wounds. Current therapeutic measures include intravenous (IV) antibiotics, oral antibiotics and/or topical agents. One or more therapies may need to be administered for months at a time. Efficacy profiles and dosing regimens of traditional antibiotic therapies focus solely on planktonic (free-floating) bacteria [8,9]. There is no product, therapeutic or agent that is regulated and optimized for biofilm cases, despite the predominance of this phenotype in natural ecosystems, including human tissues [10,11].
The persistence and prevalence of biofilms in wounds can be directly related to the hallmarks of their physiology. These characteristics include the ability to destabilize cell-cell junctions in skin [12]. Furthermore, oxygen gradients and/or anoxic environments may form that can lead to an anaerobic state deep within the biofilm structure [13,14]. Cells in this microgeography of the biofilm have reduced metabolism, making them less susceptible to antibiotics. Despite improving knowledge of biofilm characteristics, clinical problems persist and current therapies specific to biofilm-related infections are limited. Novel antimicrobial technologies that target this phenotype may have the potential to improve wound-related outcomes.
A first-in-class series of antibiofilm antibiotic, referred to as CZ compounds (condensed from company name, CŪRZA), was synthesized to address this need [[15], [16], [17]]. This compound class was inspired by the long history of naturally-occurring antimicrobial peptides and aminosterols such as magainin [18] and squalamine [19]. Synthetic constructs that maintain antimicrobial properties similar to naturally-occurring compounds provide promising potential, yet they improve upon aspects such as scalability, manufacturability and avoid protease degradation.
During screening, CZ-01179 was identified and displayed broad spectrum activity against biofilms of methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa and Acinetobacter baumannii. Given its promising activity, a focused approach was taken to assess the in vitro and in vivo efficacy of CZ-01179 against A. baumannii in the planktonic and biofilm phenotype. A. baumannii is a common complicating organism in wounded warriors returning from current conflicts in Iraq and Afghanistan that is well-known for its biofilm forming nature [3,20,21]. Its multidrug-resistant characteristic has made it difficult to treat in injured soldiers, has led to delayed wound healing, and many other complications. Limited therapeutic options exist for this organism.
CZ-01179 was examined and optimized in vitro, and formulated for topical delivery for in vivo evaluation. It was hypothesized that when applied topically in an excision pig wound model, CZ-01179 would have the ability to treat and prevent wound infection caused by A. baumannii in both the planktonic and biofilm phenotypes. Current standards of care including IV (colistin/imipenem) and topical (silver sulfadiazine) therapies were also tested for comparison. It was further hypothesized that wounds inoculated with well-established biofilms would harbor more bacteria than those inoculated with planktonic bacteria.
Materials and methods
Supplies and reagents
General supplies were purchased from Fisher Scientific or Amazon (Hampton, NH). LIVE/DEAD™ BacLIGHT™ bacterial viability kit was purchased from ThermoFisher Scientific. The CDC biofilm reactor and parts from Biosurface Technologies (Bozeman, MT). HeliPLUG® absorbable collagen was from Integra-Miltex (York, PA). Tubing and peristaltic pumps from Cole Parmer (Vernon Hills, IL). Solvents, starting material and chemicals for CZ-01179 synthesis, and silver sulfadiazine (SSD) powder for in vitro analyses were form Sigma Aldrich (St. Louis, MO). SSD topical ointment (1%) was purchased through the University of Utah Pharmacy as were colistin, imipenem and heparin. A. baumannii ATCC BAA 1605 was purchased from the American Type Culture Collection (ATCC). A. baumannii CDC isolates were provided and tested by Curza Global, LLC. The ATCC isolate was the primary isolate (used in vivo) and was maintained on Columbia blood agar and passaged with overnight incubation at 37 °C. Sodium hyaluronate (HA; Research Grade HA15 M 1.01 MDa–1.8 MDa) was from Lifecore Biomedical (Chaska, MN). Vascular access ports (VAP), VAP catheters (7 French size x 36″) and accompanying Posi-Grip Huber point needles (22 gauge at ¾”) were from Norfolk Medical (Skokie, IL) and Access Technologies (a division of Norfolk Medical). VAPs were ordered in two sizes—ClearPort Medium or SwirlPort Max—with the SwirlPort Max being the better option for locating the device subdermally. Digital images were collected using a Nikon D90 camera.
Synthesis of CZ-01179
CZ-01179 (Fig. 1) was synthesized by the following method: to a stirring solution of a dicarbaldehyde (5’-(tert-butyl)-[1,1’:3′,1″-terphenyl]-4,4″-dicarbaldehyde: 2.12 g, 6.22 mmol, 1 eq.) in MeOH (100 mL) and DCE (25 mL) at 0 °C was added the diamine (N1-(3-aminopropyl)-N3-(2-ethylbutyl)propane-1,3-diamine: 3.61 g, 16.8 mmol, 2.7 eq.) portion wise over 20 min. The solution was stirred for 16 h NaBH4 (0.95 g, 24.9, 1 eq.) was added portion wise over 20 min and the reaction stirred for an additional 1 h. The solvent was evaporated, and the crude solid partitioned between EtOAc (500 ml) and 10% NaOH (250 ml). The NaOH phase was washed with EtOAc (500 ml), and the combined organics were dried over Na2SO4. Column chromatography can be performed using gradient conditions starting at (300:16:1 CH2Cl2:MeOH:NH4OH). The free base was acidified with HCl in MeOH (100 ml), and cooled to 0 °C for 1 h. The resulting precipitate was filtered and dried to afford the HCl salt as a white solid (25–52%). Recrystallization with H2O (solvent) and iPrOH (anti-solvent) delivered analytically pure material. 1H NMR (500 MHz, D2O) δ ppm 7.78–7.69 (m, 7H), 7.61 (bs, 4H), 4.38 (s, 4H), 3.26–3.20 (m, 16H), 3.01 (s, 4H), 2.17 (bs, 8H), 1.67 (bs, 2H), 1.38 (bs, 17H), 0.88 (s, 12H). 13C NMR (125 MHz, D2O) δ ppm 153.4, 141.8, 140.4, 130.4, 129.7, 127.8, 123.8, 122.9, 50.9, 50.9, 44.9, 44.6, 43.9, 37.6, 34.4, 30.5, 22.6, 22.4, 22.4, 9.4. IR (neat): 3334 (bs), 2963, 2766, 1457 (all s) cm−1. mp decomposition (180–184 °C). LRMS Calculated for C48H80N6 m/z 741.6 [M+H]+, Obsd. 370.7 [M+H]+/2.
Fig. 1.
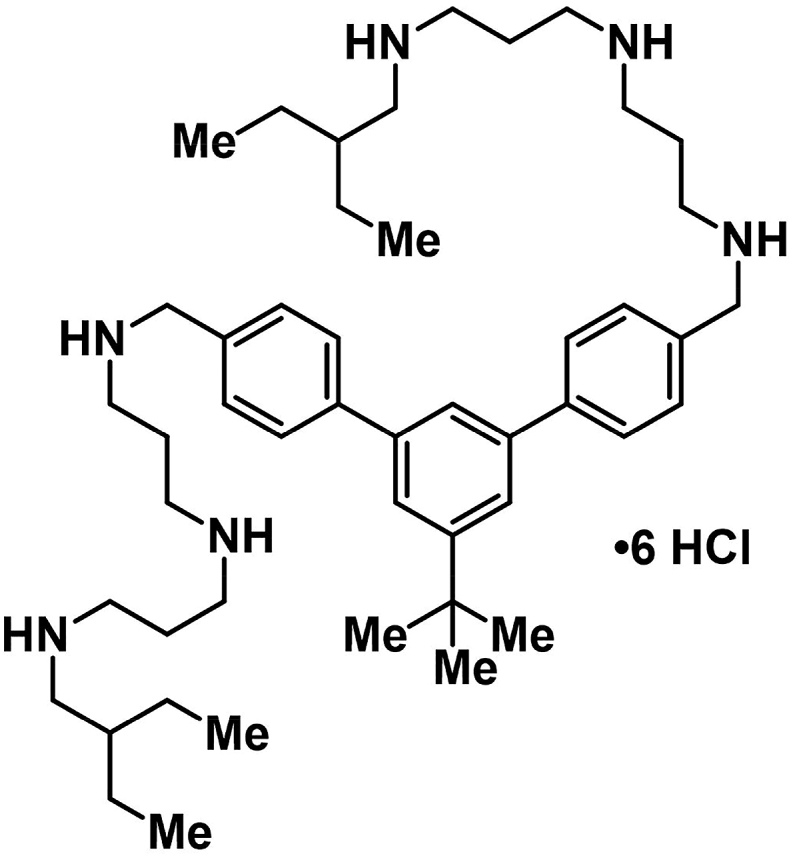
Structure of CZ-01179.
In vitro efficacy of CZ-01179
In vitro activity against A. baumannii was conducted in planktonic and biofilm phenotypes.
Planktonic efficacy
A modified protocol of the Clinical and Laboratory Standards Institute (CLSI) guideline M07 was used to determine minimum inhibitory concentration (MICs). In short, the tests were performed using a 96-well plate containing a two-fold serial dilution of antibiotics across the plate rows and identical replicates down each column. Specifically, ~1.5 mL of a stock concentration (256 μg/ml) of desired antibiotic was made in CAMHB. Separately, a 0.5 McFarland standard was suspended in PBS using a nephelometer and diluted 1:100 in CAMHB (concentration ~7.5 x 105 CFU/mL).
To set up the MIC, 50 μl of sterile CAMHB were first added to each well of columns 2–12 in the 96-well plate. Fifty μl of the stock antibiotic solution were then added to each well of columns 1 and 2. The antibiotic solution in column 2 was mixed thoroughly with the CAMHB and serially diluted 1:2 (50 μl removed from column 2 and added to column 3, etc.) down to column 11. Fifty μl of broth from each well of column 11 were discarded so as each well of the 96-well plate contained a volume of 50 μl at this point. Lastly, 50 μl of the 1:100 bacterial solution was added to each well of columns 2–12. This process resulted in a range of antibiotic testing from 64 μg/ml to 0.0625 μg/mL with column 1 being a negative control of growth (antibiotic only) and column 12 a positive control of growth (no antibiotic solution).
The 96-well plate was covered with a gas-permeable adhesive film and incubated 24 h at 37 °C. The concentration of antibiotic that inhibited pellet formation or turbidity was considered the MIC. MICs of CZ-01179, imipenem, colistin, and SSD were determined against the ATCC isolate and eleven CDC isolates (Table 1).
Table 1.
MIC values of the antibiotics tested against various A. baumannii isolates.
| A. baumannii ID | MIC (μg/ml) |
|||
|---|---|---|---|---|
| CZ-01179 | Imipenem | Colistin | SSD | |
| ATCC BAA-1605 | 4 | 32 | 0.5 | 2 |
| CDC-277 | 8 | >64 | 0.5 | 4 |
| CDC-278 | 8 | 64 | 0.5 | 2 |
| CDC-286 | 8 | >64 | 0.5 | 2 |
| CDC-296 | 4 | 64 | 0.25 | 4 |
| CDC-299 | 4 | 32 | 4 | 4 |
| CDC-301 | 4 | >64 | 0.25 | 2 |
| CDC-307 | 2 | 16 | 16 | 4 |
| CDC-308 | 1 | 16 | 16 | 4 |
| CDC-311 | 8 | 64 | 0.25 | >8 |
| CDC-312 | 2 | 1 | 0.25 | 4 |
| CDC-313 | 8 | 32 | 0.5 | 8 |
Biofilm efficacy
Biofilms were grown on polycarbonate coupons in a CDC biofilm reactor following a protocol modified from ASTM E3161-18. The reactor was inoculated after being assembled and autoclaved; a fresh overnight culture of A. baumannii was used to make a 0.5 McFarland standard of the bacterial isolate (~5 x 107 CFU/ml). One ml of the 0.5 McFarland solution was inoculated into 500 ml of brain heart infusion (BHI) broth in the CDC biofilm reactor. The reactor was placed on a hot plate set at 34 °C and a baffle rotation of 130 rpm for 24 h. After 24 h batch growth, a continuous flow of 10% BHI was flowed through the reactor at ~6.9 ml/min for an additional 168 h (7 d).
Following 192 h (8 d) of total growth, coupons were aseptically removed and placed into 2 ml of CAMHB that contained CZ-01179, colistin, imipenem, or a 1:1 combination of colistin:imipenem. All were tested at multiple concentrations—0.00125% (12.5 μg/ml), 0.0025% (25 μg/ml), 0.005% (50 μg/ml), 0.00625% (62.5 μg/ml), 0.0125% (125 μg/ml), 0.025% (250 μg/ml) 0.05% (500 μg/ml), 1.0% (10 mg/ml) and 2.0% (20 mg/ml)—in order to obtain a profile of in vitro efficacy. Biofilms were exposed to CZ-01179 for 24 h at 37 °C after which time coupons were vortexed for 1 min, sonicated at 42 kHz for 10 min and vortexed again for ~10 s. A 100 μl aliquot of broth was removed and plated using a 10-fold dilution series in order to quantify the CFU/coupon that remained. Testing was performed with n = 3 repeats per concentration. A baseline of growth was determined by quantifying n = 3 coupons/reactor immediately following growth.
Additional testing was performed with CZ-01179 formulated in a gel to confirm activity against biofilms. A CDC biofilm reactor was again used to grow biofilms for analysis. In this case biofilms were grown on absorbable collagen to more closely model a physiological substrate. Blank reactor arms were purchased from Biosurface Technologies and custom-modified to hold collagen plugs as published previously [22]. Specifically, four holes of 8.5 mm diameter each were drilled in the lower portion of a blank polypropylene holder. Collagen was aseptically removed from packaging and cut into coupons (1 cm diameter x 0.3 cm height) using a sterile scalpel. Coupons were sterilely loaded into modified reactor arms that had been autoclaved previously. Once assembled, the CDC biofilm reactor was inoculated and biofilms were grown as described.
CZ-01179 was formulated in a gel by combining the antibiotic powder in sterile PBS to a final concentration of 2% (20 mg/ml) and mixing thoroughly. HA powder was then added to a final concentration of 1.5% and mixed by shaking until dissolved. The formulation was allowed to gel at room temperature overnight (air bubbles dissipated). Approximately 1 ml of CZ-01179 gel was placed into a single well of a 12-well plate. A collagen plug was removed from the CDC biofilm reactor and placed on the gel. The collagen plug was covered with an additional ~1 ml of gel; making biofilms on collagen submerged in ~2 ml of gel. Samples were incubated 24 h at 37 °C, then quantified as described. Data were collected with n = 3 repeats and CZ-01179 was tested at both 1% and 2% concentrations.
Antibiofilm efficacy of SSD was also determined to compare CZ-01179 to an agent that is commonly used clinically. Biofilms were grown on polycarbonate as described and the efficacy of SSD was tested first in broth solution at concentrations of 0.05% (500 μg/ml) and 0.025% (250 μg/ml) following the procedures above. In addition to broth susceptibility testing, efficacy testing was also performed with clinically-relevant SSD cream (final concentration of 1% SSD) following the biofilm growth and 12-well plate testing methods outlined above.
In vivo efficacy of CZ—01179
In vivo efficacy testing of CZ-01179 gel was performed in a porcine excision wound model. All work was approved by the University of Utah Institutional Animal Care and Use Committee (IACUC) and Animal Care and Use Review Office (ACURO), a component of the US Army Office of Research Protections (ORP).
Animal acclimation and surgical procedure
Four Yorkeshire pigs were purchased from Innovative Livestock Solutions (Salt Lake City, UT) with weight of ~40–50 kg and were quarantined for ≥7 d. Positive reinforcement (e.g., Swedish Fish®, marshmallows, fruit) was provided while they were worked on—e.g., a back scratch. A custom-fit jacket was placed on pigs during quarantine to acclimate to the covering.
Pigs were fasted the night before surgery. Anesthesia used a combination of tiletamine-zolazepam (Telazol®; 4.4 mg/kg), Ketamine (2.2 mg/kg) and Xylazine (2.2 mg/kg). Pigs were intubated, given isoflurane inhalant at 0.5–5.0%, transported to a surgical suite, placed in sternal recumbency and clipped/razor-shaved of hair in the region where excision wounds would be created. Pigs were rotated to dorsal recumbency and the jugular vein area was sterilely prepped using alternating betadine/isopropyl alcohol. Once prepped, the site was sterilely draped and a VAP was implanted. A ventral midline incision was made to isolate the jugular vein, and a catheter was placed in the vein. A second incision was made on the dorsal side of the neck and a tunnel created along the subcutaneous space from the second incision to the jugular vein. The catheter was passed through the tunnel. A VAP was anchored subdermally in the dorsal neck space with non-absorbable suture (e.g., Proline). The catheter was connected to the VAP and secured in the jugular vein. Both incision sites were closed using absorbable suture (e.g., Vicryl). One pig (i.e., the one used for positive and negative control wounds) did not have a VAP.
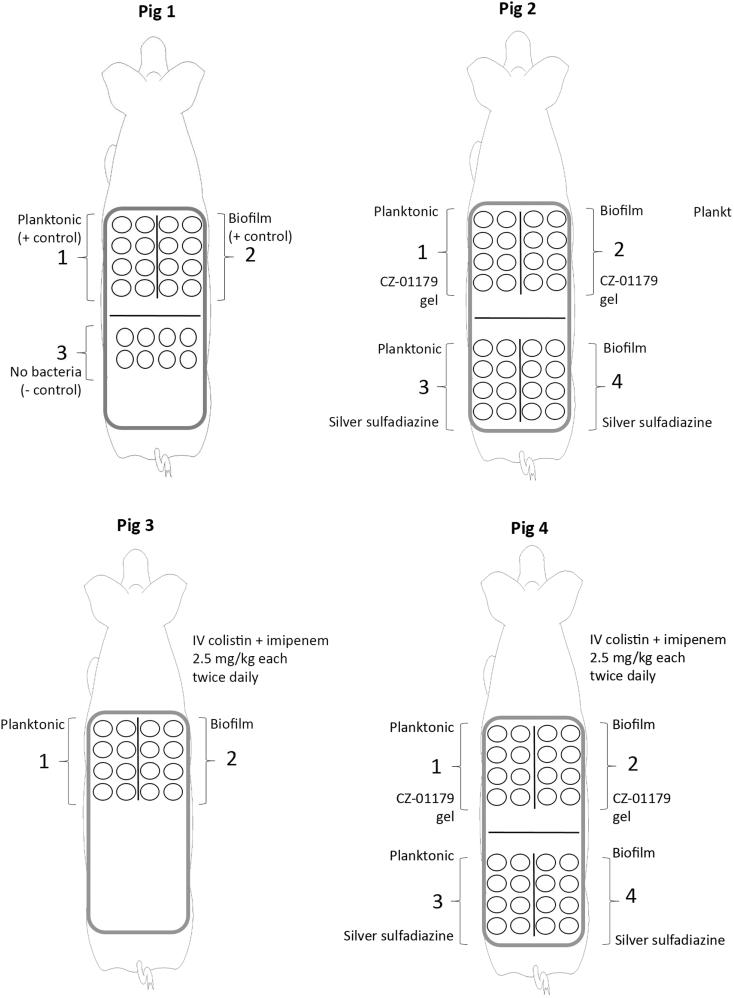
With a VAP in place, a pig was rotated to sternal recumbency. The back was sterilely prepped for surgery, then draped. Excision wounds were created using a 1 cm biopsy punch with a separation of approximately 2 cm between each wound. Wounds were organized into three or four sections with n = 8 wounds/section (see Fig. 2). Wound beds were treated with μl quantities of dilute epinephrine (1 mg/ml) as needed to reduce bleeding during wound creation. Sterile saline-soaked gauze was placed on excised wounds to maintain moisture as additional wounds were created. Once created, wounds were inoculated with bacteria (with the exception of negative control wounds) in either the planktonic or biofilm phenotype (see Fig. 2).
Fig. 2.
Schematic of each pig, inoculation patterns and antimicrobial treatments that were given. Wounds on the left flank of each pig were inoculated with planktonic bacteria and wounds on the right flank of each pig were inoculated with well-established biofilms. Wounds were divided into 2–4 sections on each pig back with n = 8 wounds/section.
Bacterial inoculation
For inoculation with planktonic bacteria, 2–3 colonies from a fresh overnight culture of A. baumannii were adjusted to a turbidity of 10% (~1 x 109 CFU/mL) in sterile PBS using a nephelometer. One hundred μl were pipetted into wound beds on the left flank of an animal (see Fig. 2). This resulted in an inoculum of ~1 × 108 CFU of planktonic bacteria/wound.
Biofilm inoculation was performed by first growing biofilms on absorbable collagen for a total of 192 h (8 d) as described and transported to the operating room in approved containers. Once the excision wounds were created, biofilm-containing collagen coupons were aseptically placed into wounds (one coupon/wound) on the right flank of an animal (see Fig. 2). Notably, a subset of collagen coupons from each reactor run were kept in the lab and quantified in order to obtain a baseline of biofilm growth. Following inoculation, tincture of benzoin was applied to the border of each wound section to help maintain bandage adherence. Wounds were bandaged with a non-stick Telfa pad and Tegaderm. A custom jacket was also placed to further protect bandaging. Pigs were recovered and allowed to eat and drink ad libitum.
All wounds were reinoculated with planktonic or biofilm bacteria once daily for 3 d following the surgical procedure (total of 4 inoculations). Multiple inoculations were found to result in delayed healing and increased infection signal in each wound set. To perform the reinoculations, planktonic bacteria were made fresh each day. Likewise, multiple biofilm reactors were set up 24 h apart such that wounds were reinoculated with biofilms that had been grown for a total of 8 d in each case.
Study design, antibiotic administration and bandage changes
The in vivo portion of this study was designed to determine the efficacy of CZ-01179 as a stand-alone topical gel product and as an adjunct therapy with clinically-relevant IV antibiotics. An additional objective was to compare the efficacy of CZ-01179 gel to SSD cream, a clinically-relevant product. As a general overview, wounds in Pig 1 served as positive and negative controls of infection (see Fig. 2). Wounds in Pig 2 were treated with topical CZ-01179 gel (2% active) or SSD cream (1% active; see Fig. 2). Pig 3 received IV antibiotics only (Fig. 2). Wounds in Pig 4 were treated with both topical products and IV antibiotics (Fig. 2).
All antibiotic therapies began on Day 5 following surgery. The delay between infection development and treatment was to model a scenario wherein a wound becomes infected over time. Specific antibiotic administration: in Pig 2–0.3 ml of CZ-01179 gel was applied to each wound in sections 1 & 2, 1 & 2, and ~0.3 ml of SSD cream applied to each wound in sections 3 & 4, 3 & 4 once daily for 14 d (see Fig. 2). In Pig 3, colistin and imipenem were administered IV (via the VAP) in combination with each at a dose of 2.5 mg/kg, twice daily for 14 d. These same regimens were followed for Pig 4 with both topical and IV antibiotics being administered in the same pig (see Fig. 2).
To maintain the VAPs in those pigs that had one, after it was initially implanted, it was locked with heparin solution (~5 mL with heparin at a concentration of 100 IU/ml). Following each use, it was flushed/locked with ~5 ml of heparin solution. When not in use, the VAP was flushed every 7–10 d with heparin solution.
Bandages were changed once daily on each pig. A trough/bucket was filled with feed and topped with treats for positive reinforcement. As the pig ate, the jacket and bandaging were aseptically removed. Digital pictures were taken of each wound section. A ruler was placed against the skin allowing for wound size measurements to be made. Culture swabs were collected of each wound (approximately twice weekly) to qualitatively confirm the presence of the inoculum, A. baumannii. Half of the wounds in each wound set were lightly debrided with sterile forceps and saline, whereas the other half remained undebrided. The rationale was to determine the influence that debridement would have on levels of bacteria in either the planktonic or biofilm phenotype. Following debridement or lack thereof, topical antibiotic therapy was applied. Wounds were bandaged once again and the jacket replaced. In pigs that received IV antibiotics, they were administered after the jacket was in place.
Topical and IV therapies were discontinued after 14 d of administration. Each pig was monitored to an endpoint of 28 d, leaving a gap of 9 d on the back end of the monitoring period without treatment on any of the wounds (bandage changes and image collection were still performed daily). This gap period was intentional to determine if infection would recur and/or if bacteria would recolonize the site.
Necropsy and microbiology
On Day 28 a pig was sedated initially (as above) and humanely euthanized. Bandages were aseptically removed to perform necropsy. Wounds were lightly debrided with sterile gauze and saline, culture swabs were taken of each wound site, plated on Columbia blood agar, and incubated overnight for semi-quantitative and morphological analysis. Digital images were collected. A 0.5 cm biopsy punch was then used to collect a tissue sample of each excised wound. Residual eschar, if present, was removed first, then a tissue punch collected. This reduced the risk of quantification of bacteria/biofilm that may have resided in eschar. To collect a tissue sample, the outer rim of a sterile biopsy punch was placed on the outer-most edge of the original wound margin. Each tissue sample was weighed, then placed in a tissue grinder tube that contained 1 ml of sterile saline. Tissue was ground for approximately 2 min. An aliquot of 100 μl was removed and plated using a 10-fold dilution series to quantify CFU/g of tissue.
Statistical analysis
Bacterial counts and wound measurements were compared between groups and sections using a one-way ANOVA analysis with alpha level at 0.05. Descriptive statistics and LSD Post-hoc analysis were used for interpretations. Data were analyzed in SPSS v17.0 software.
Results
In vitro analyses
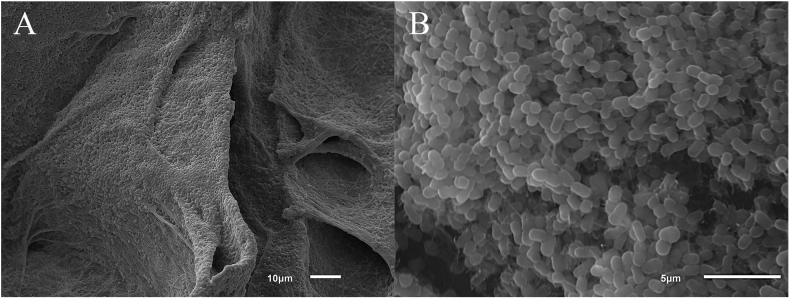
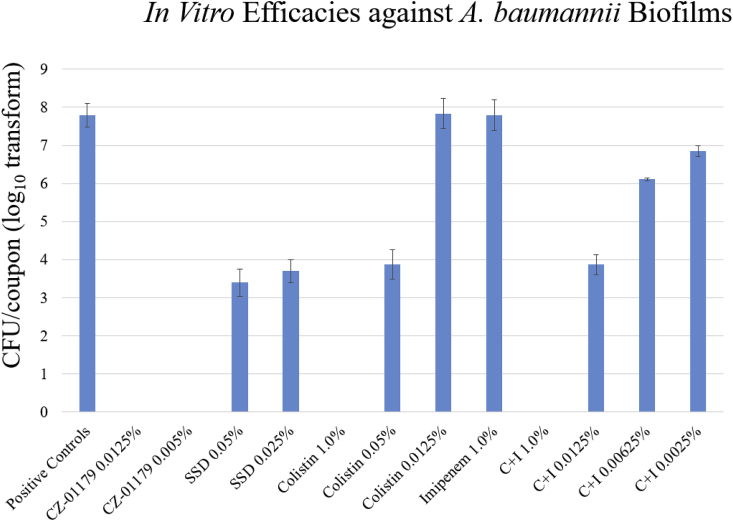
The MIC of CZ-01179 against A. baumannii isolates ranged from 1 to 8 μg/ml (Table 1). The majority of isolates were resistant to imipenem, whereas the majority were susceptible to colistin, and the MIC of SSD against the ATCC isolate was 2 μg/ml (Table 1). Baseline biofilm growth on polycarbonate coupons resulted in ~7.5 x 107 CFU/coupon. SEM images showed that biofilms of A. baumannii grew to maturity and formed three-dimensional sheet-like structures across the surface with notable extracellular polymeric substance (Fig. 3). When exposed to CZ-01179 in CAMHB, full eradication of biofilms was achieved at concentrations from 2% (20 mg/ml) down to 0.005% (50 μg/ml; see Fig. 4). When exposed to CZ-01179 at 0.0025% (25 μg/ml), there were ~4.8 x 103 CFU/coupon (~4 log10 reduction), and at 0.00125% (12.5 μg/ml) there were ~9.2 x 105 CFU/coupon (~2 log10 reduction). Biofilms exposed to SSD in CAMHB were not fully eradicated (see Fig. 4). At 0.025% (250 μg/ml) there were ~5 x 103 CFU/coupon (~4 log10 reduction) and at 0.05% (500 μg/ml) there were ~2.5 x 103 CFU/coupon (~4 log10 reduction).
Fig. 3.
Biofilms of A. baumannii ATCC BAA 1605. (A) Biofilm formation followed the contour of the collagen substrate. This image was selected to show the fibers of collagen onto which bacterial cells adhered. (B) Higher magnification image of the A. baumannii biofilm community with notable extracellular polymeric substance extending from cell to cell.
Fig. 4.
Representative outcomes of the in vitro efficacy testing with various antimicrobials against biofilms of A. baumannii on polycarbonate coupons.
Colistin fully eradicated biofilms at 1% (which isn’t a clinically-relevant dose, but was determined for comparison). Imipenem showed no reduction at 1%, and efficacies of these decreased with lowering concentrations (Fig. 4). The combination of the two improved biofilm reduction, but as clinically-relevant doses (e.g., 0.0025% or 25 μg/ml) were reached, efficacy was minimal (Fig. 4).
Baseline biofilm growth on collagen coupons resulted in ~5.8 x 107 CFU/coupon, which was similar to growth levels on polycarbonate. At 1% and 2% concentrations, CZ-01179 gel eradicated biofilms of A. baumannii completely. In contrast, when exposed to SSD cream (at 1%), biofilms were reduced to ~5.9 x 104 CFU/coupon (~3 log10 reduction from baseline controls). The in vitro outcomes supported advancement of CZ-01179 toward in vivo analysis.
In vivo analyses
Infection signal
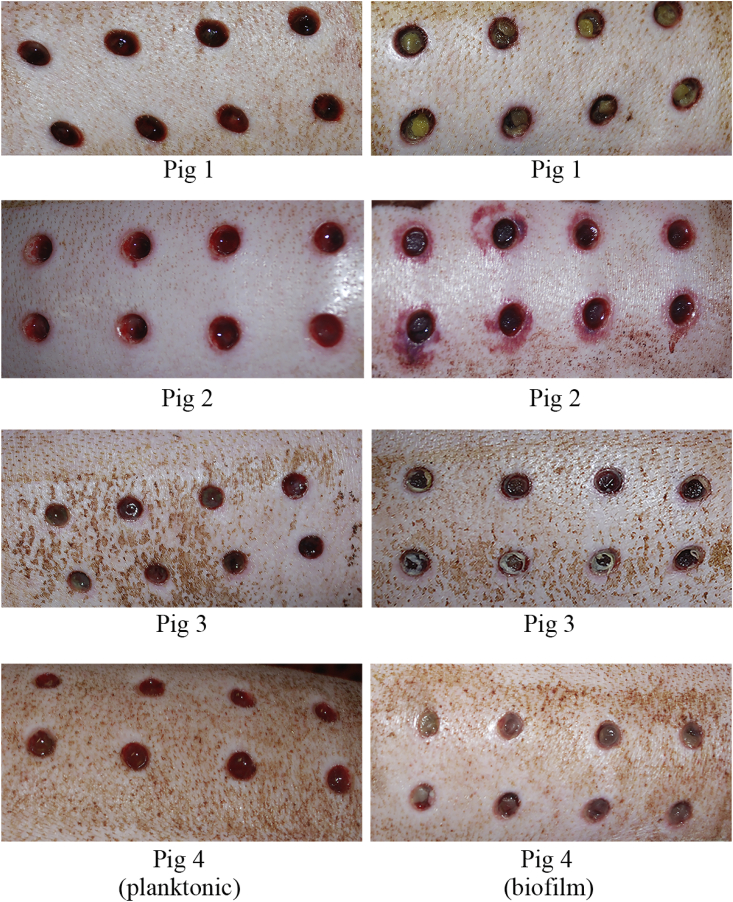
In all pigs, a modest signal of infection developed in each wound that was inoculated with bacteria of either phenotype. In the early stages of infection (2–3 d post-surgery), wounds inoculated with planktonic bacteria had moist, serous discharge with raised borders, redness and inflammation (Fig. 5). In contrast, wounds inoculated with biofilms had a dryer wound bed appearance, notable purulence with less serous discharge compared to planktonic wounds. In general, wounds inoculated with biofilms had slightly more pronounced irritation, redness and inflammation, in particular in Pig 2 (Fig. 5).
Fig. 5.
Representative images of infected wounds 3–4 d after surgery. Wounds inoculated with planktonic bacteria are shown in the left panel. Wounds inoculated with biofilms are shown in the right panel. In Pig 1, wounds are shown that had been lightly cleansed of discharge. The right panel shows inoculated wounds with fresh collagen plugs on which biofilms were grown. Pig 2 had noticeably more redness develop around wound borders with biofilm versus planktonic bacteria inocula. Wounds of Pigs 3 and 4 demonstrate noticeable purulence in biofilm wounds compared to planktonic wounds, which predominantly had serous discharge.
Infection resolution, wound closure and reepithelialization
In Pig 1 (control wounds) early clinical signs of infection resolution were observed in debrided planktonic and biofilm wounds by Day 10 and 8, respectively. Early granulation tissue and contraction were beginning by those times. Notably, despite early signs of infection resolution, wounds were still colonized, as will be discussed below. It wasn’t until Day 20 and beyond that all debrided positive control wounds were largely healed and had limited clinical signs of infection (Fig. 6). To try and define the point at which infection resolved in undebrided wounds would be inaccurate as the wound bed, granulation, reepithelialization and contraction levels could not be deciphered with confidence through the presence of eschar (Fig. 5). In negative control wounds, granulation tissue began to develop by Day 6. Reepithelialization and contraction were obvious by Day 10.
Fig. 6.
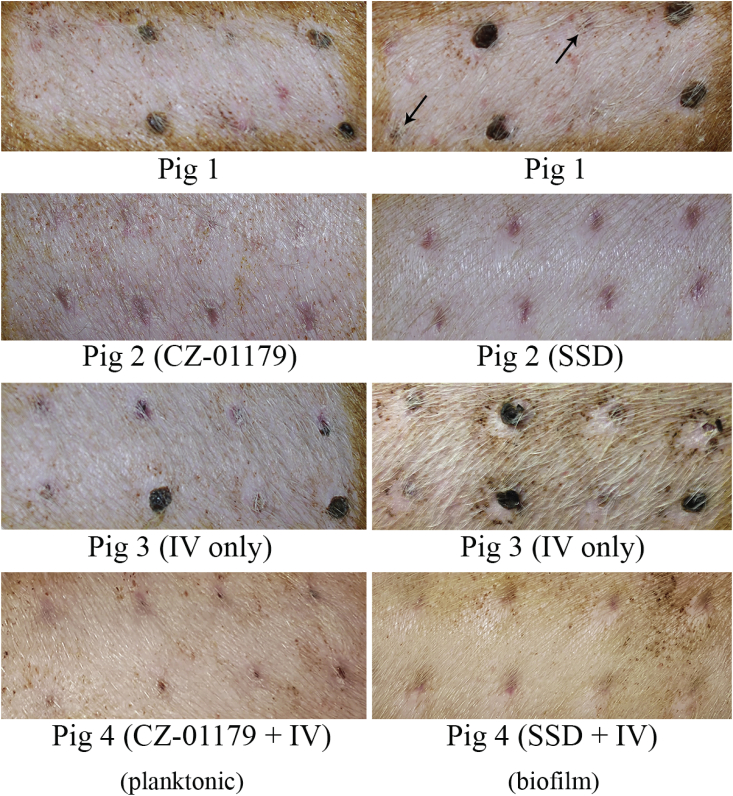
Wound outcomes by Day 24 with treatments indicated for each pig. Wounds that were inoculated with planktonic bacteria are shown in the panels on the left. Wounds inoculated with biofilms are shown in the panels on the right. Positive control wounds (Pig 1) that were undebrided had not yet healed fully with eschar, incomplete contraction and reepithelialization still occurring. Debrided wounds in Pig 1 were largely healed by Day 24, yet still showed signs of incomplete contraction and reepithelialization (see arrows). Representative wounds treated with CZ-01179 gel or SSD cream are shown for Pig 2. By Day 24, wounds were healed in Pig 2 with scarring present. Wounds in Pig 3 had not fully reepithelialized by Day 24 and were still contracting, in particular those that were undebrided. Wounds that were treated with both IV and topical antimicrobials (Pig 4) looked similar in nature to those in Pig 2.
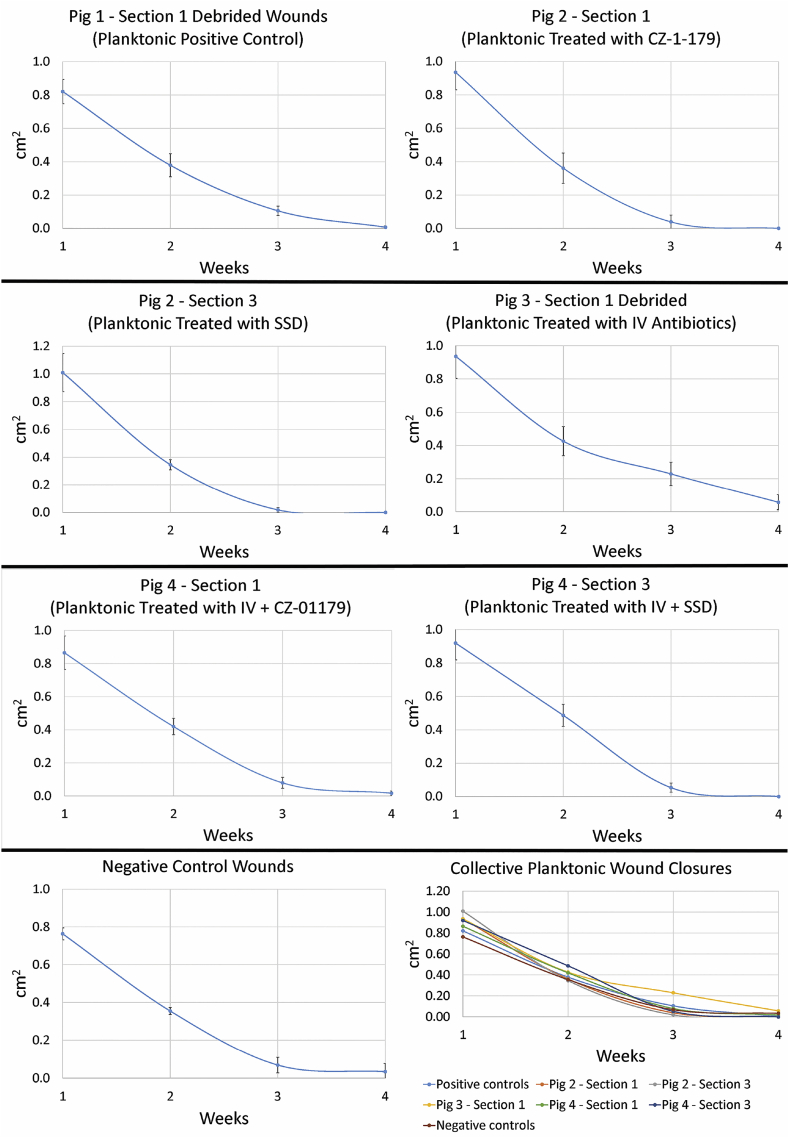
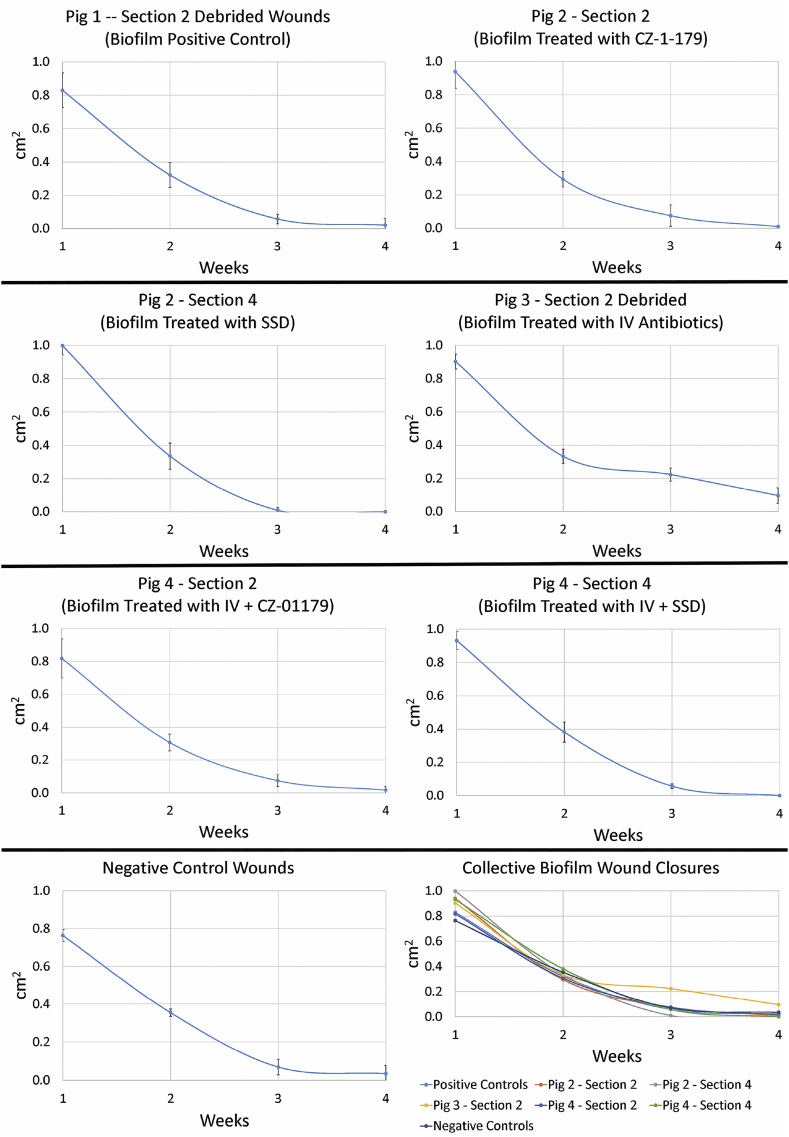
Wound measurements of Pig 1 were collected on debrided wounds only. Measurements of undebrided wounds would have been skewed due to presence of eschar. However, qualitative observation indicated that undebrided wounds, particularly those inoculated with biofilms, took noticeably longer to heal/reepithelialize, did not have a healthy appearance for up to three weeks and harbored more bacteria as shown by culture data (see below). Closure of debrided wounds progressed steadily until the 28-day (4-week) timepoint (Fig. 7, Fig. 8). Diameters of planktonic and biofilm wounds were no longer statistically significantly different by Week 3 or 4 (p = 0.07). Similarly, diameters of negative control wounds were not significantly different than positive controls by Week 3 or 4 (p = 0.06).
Fig. 7.
Measurements of planktonic bacteria-inoculated wounds over the course of the monitoring period. Each section of a pig back and its treatment regimen (see Fig. 2) is represented individually and in comparison on a collective graph. Data showed that wounds treated with IV antibiotics closed at the slowest rate. Wound diameters in Pigs 1, 2 & 4 varied slightly from Weeks 1–3, but were similar by the endpoint.
Fig. 8.
Measurements of biofilm-inoculated wounds over the course of the monitoring period. Each section of a pig back and its treatment regimen (see Fig. 2) is represented individually and in comparison on a collective graph. Similar to planktonic wounds, data showed that wounds treated with IV antibiotics closed at the slowest rate. Wound diameters in Pigs 1, 2 & 4 varied slightly from Weeks 1–3, but were similar by the endpoint.
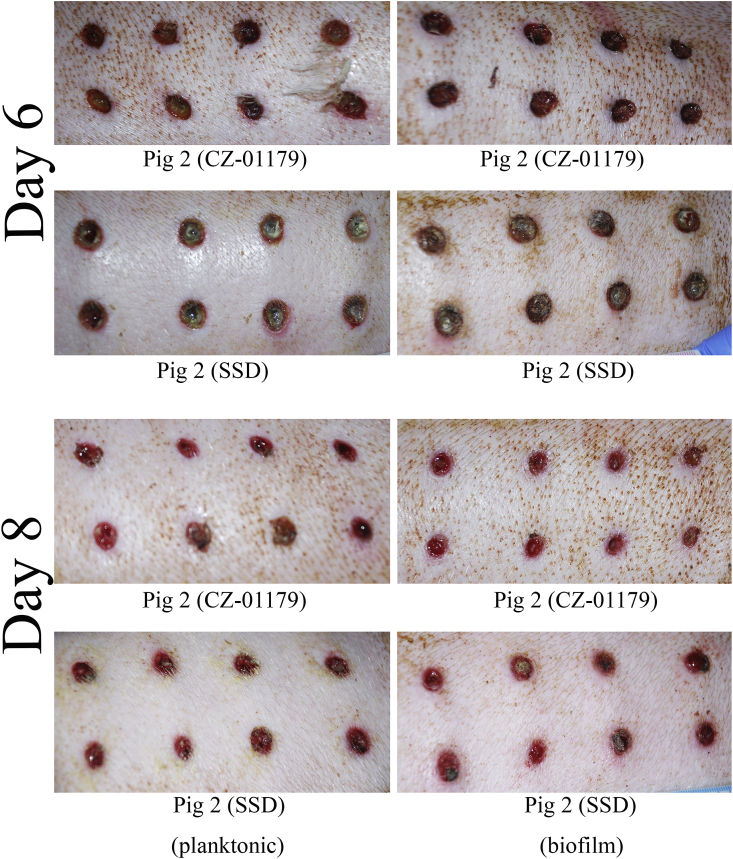
In Pig 2 (topical treatments only), wounds that were treated with CZ-01179 gel had mild redness around borders on Day 6 (24 h after the first application), but no pus or discharge (Fig. 9). Early granulation tissue was observed in planktonic and biofilm wounds. By Day 8 all CZ-01179-treated wounds had taken a noticeable shift toward healing (Fig. 9). Granulation tissue was abundant and contraction had advanced in all wounds. Wounds that were treated with SSD cream took roughly 1 d longer to clear infection (Fig. 9). Signs of infection were present in particular in planktonic wounds on Day 7 with pus, discharge and redness along borders. However, similar to CZ-01179 gel-treated wounds, the majority of wounds treated with SSD had taken a notable shift on Day 8 toward healing; granulation tissue and contraction were developing, and mild redness around borders was still present, but resolving (Fig. 9).
Fig. 9.
Wound progression between Day 6 and Day 8 with CZ-01179 or SSD treatment. Wounds treated with CZ-01179 began to resolve 1–2 days faster than those treated with SSD. By Day 6, wounds treated with CZ-01179 displayed notable contraction, granulation tissue, and little to no redness around borders whereas those treated with SSD still had pus, rednes,s and mild inflammation. By Day 8, biofilm-inoculated wounds treated with CZ-01179 appeared slightly healthier than planktonic counterparts, and had no redness around borders compared to biofilm-inoculated wounds treated with SSD.
The presence of topical gel or cream reduced eschar formation in wounds of Pig 2, thus all wound diameters were measured. All wounds reepithelialized almost fully (>90%) by Week 3 (Fig. 7, Fig. 8). Compared to other animals on study, Pig 2 wounds closed soonest (see Fig. 5, Fig. 6) and were the healthiest visually (Fig. 6, Fig. 9). By the endpoint, there were no statistically significant differences in diameters between wounds treated with CZ-01179 or SSD, or when compared to positive control wounds (p > 0.09 in all cases). Notably, CZ-01179 gel did not cause rash, necrosis or adversely affect healing, suggesting that cytotoxic effects were absent.
In Pig 3 (IV antibiotics only), clinical signs of infection in both planktonic and biofilm wounds that were debrided had resolved by Day 9. Granulation tissue and contraction had begun by Day 9 as well. Interestingly, wound closure stagnated during the period that IV antibiotics were administered, in particular in biofilm wounds (Fig. 7, Fig. 8). Wounds in Pig 3 had the largest diameters, were the slowest to close for both planktonic and biofilm wounds (Fig. 7, Fig. 8), and diameters were significantly different compared to all wounds in Pig 1, 2 and 4 by the endpoint (p < 0.008 in all cases).
In Pig 4 (IV + topical products), signs of infection in wounds that were treated with topical CZ-01179 resolved by Day 6. The beginning of wound contraction was notable by Day 7 in both planktonic and biofilm-inoculated wounds and as in Pig 2, by Day 8 healing was pronounced. In contrast, wounds that were treated with SSD had significant infection (i.e., pus, discharge, redness) on Day 6 and did not resolve until Day 10. Wound contraction was notable by Day 9 in planktonic wounds and notable in biofilm wounds by Day 10. Healing was obvious by Day 12. By the endpoint, the only significant difference in wound diameters of Pig 4 was between Pig 3 (p = 0.001) and negative control wounds (p = 0.007).
In summary, wounds treated with CZ-01179 gel were clear of infection 1–3 d sooner than wounds treated with SSD cream in planktonic or biofilm inoculated wounds. CZ-01179 also cleared signs of infection 3–4 d sooner than the host alone.
Culture data
As a general overview, culture data showed distinct differences between debrided and undebrided wounds in Pig 1. In Pig 3, bacteria were cultured in all wound types throughout the course of the study, which indicated that although infection resolved, bacteria still colonized the wounds. Topical products used in Pigs 2 and 4 kept wounds moist and debridement was largely unnecessary as little to no eschar formed. Nevertheless, data from Pigs 2 and 4 in this culture data section is presented as debrided versus undebrided wounds for ease of comparison. Culture data for debrided and undebrided wounds are presented in Table 2, Table 3, respectively.
Table 2.
Microbiological results of wounds that were debrided regularly.
| Pig # | Wound Section | Bacterial Phenotype | Treatments | Last Day that A. baumannii was Detected in At Least One Wound by Culture Swab | Log10 Transformed CFU/g Tissue at Necropsy (Day 28) |
|---|---|---|---|---|---|
| 1 | 1 | Planktonic | Positive controls | 28 | 0 |
| 2 | Biofilm | Positive controls | 28 | 5.8 ± 6.1 | |
| 3 | N/A | Negative controls | 0 | 0 | |
| 2 | 1 | Planktonic | CZ-01179 | 5 | 0 |
| 2 | Biofilm | CZ-01179 | 5 | 0 | |
| 3 | Planktonic | SSD | 5 | 0 | |
| 4 | Biofilm | SSD | 12 | 0 | |
| 3 | 1 | Planktonic | Colistin/imipenem (IV) | 28 | 2.4 ± 2.7 |
| 2 | Biofilm | Colistin/imipenem (IV) | 28 | 2.5 ± 2.8 | |
| 4 | 1 | Planktonic | CZ-01179 + colistin/imipenem (IV) | 17 | 0 |
| 2 | Biofilm | CZ-01179 + colistin/imipenem (IV) | 10 | 0 | |
| 3 | Planktonic | SSD + colistin/imipenem (IV) | 7 | 0 | |
| 4 | Biofilm | SSD + colistin/imipenem (IV) | 28 | 0 |
Table 3.
Microbiological results of wounds that were undebrided.
| Pig # | Wound Section | Bacterial Phenotype | Treatments | Last Day that A. baumannii was Detected in At Least One Wound | Log10 Transformed CFU/g Tissue at Necropsy (Day 28) |
|---|---|---|---|---|---|
| 1 | 1 | Planktonic | Positive controls | 28 | 4.5 ± 4.7 |
| 2 | Biofilm | Positive controls | 28 | 7.0 ± 7.3 | |
| 3 | N/A | Negative controls | 0 | 0 | |
| 2 | 1 | Planktonic | CZ-01179 | 5 | 0 |
| 2 | Biofilm | CZ-01179 | 5 | 0 | |
| 3 | Planktonic | SSD | 5 | 0 | |
| 4 | Biofilm | SSD | 5 | 0 | |
| 3 | 1 | Planktonic | Colistin/imipenem (IV) | 28 | 2.5 ± 2.4 |
| 2 | Biofilm | Colistin/imipenem (IV) | 28 | 2.5 ± 2.7 | |
| 4 | 1 | Planktonic | CZ-01179 + colistin/imipenem (IV) | 15 | 0 |
| 2 | Biofilm | CZ-01179 + colistin/imipenem (IV) | 15 | 0 | |
| 3 | Planktonic | SSD + colistin/imipenem (IV) | 17 | 0 | |
| 4 | Biofilm | SSD + colistin/imipenem (IV) | 17 | 0 |
In Pig 1, A. baumannii was identified in at least one positive control wound throughout the 28-day monitoring period (Table 2). However, the host immune system was largely able to eradicate planktonic bacteria in debrided wounds. Only one colony of A. baumannii was detected by swab culture at necropsy, whereas tissue samples were negative (Table 2). In contrast, wounds that had been inoculated with biofilms and that were debrided had greater than 105 CFU/g tissue at necropsy (Table 2). Undebrided wounds harbored more bacteria in both the biofilm and planktonic phenotype, with biofilm wounds having a higher bioburden (Table 3).
Culture swabs that were collected from Pig 2 (topical agents only) showed that on Day 6 post-surgery, CZ-01179 gel had eradicated the majority of A. baumannii in all wounds (Table 2, Table 3). A. baumannii was detected in 2/8 wounds that were inoculated with planktonic bacteria and 3/8 wounds that were inoculated with biofilms. Beyond Day 6, A. baumannii was no longer detected by culture swab in wounds that were treated with CZ-01179. Tissue samples collected at necropsy (Day 28) were also negative for growth (Table 2, Table 3). In the case of wounds in Pig 2 that were treated with topical SSD, on Day 6 post-surgery A. baumannii was cultured in 2/8 wounds that had been inoculated with planktonic bacteria, and 7/8 wounds that were inoculated with biofilms. After Day 12, A. baumannii was no longer detected by culture, and tissue samples collected at necropsy were also negative (Table 2, Table 3).
In Pig 3 (IV antibiotics only), A. baumannii was detected in all wounds throughout the 28-day monitoring period. Tissue samples collected at necropsy had approximately 102 CFU/g in all wounds inoculated with planktonic or biofilm bacteria (Table 2, Table 3).
Culture data from Pig 4 (IV + topical products) showed that on Day 6, none of the wounds treated with CZ-01179 had detectable A. baumannii. However, 10 d post-surgery a culture swab identified 3 colonies of A. baumannii in one of the biofilm-inoculated wounds, 14 d post-surgery cultures identified an additional few colonies in a second biofilm-inoculated wound, and 17 d post-surgery one wound that had been inoculated with planktonic bacteria identified a few colonies (see Table 2, Table 3). Tissue samples were negative for growth at necropsy (Table 2, Table 3). Wounds in Pig 4 that were treated with SSD all had notable growth on Day 6 post-surgery. On Day 10, one debrided wound that had been inoculated with biofilms had 2 colonies of growth, on Day 15 a single colony was identified in a wound that had been inoculated with planktonic bacteria, on Day 17 one colony was identified in a biofilm wound, and on Day 28 a single colony was identified in a biofilm wound (Table 2, Table 3). Tissue samples that were collected and quantified at necropsy showed no positive growth for A. baumannii.
ANOVA analysis showed that the number of bacteria in undebrided biofilm wounds of Pig 1 were significantly different than the number of bacteria in all other wound groups amongst all pigs (highest p = 0.001). No statistically significant differences were found in bacterial numbers between any other wound groups of pigs (lowest p = 0.79), but residual presence of pathogenic bacteria would be considered clinically significant.
Discussion
Chronic wounds adversely affect wounded warriors and the public in general [[1], [2], [3],23,24]. These wounds can develop following a variety of injuries or pathologies, and lead to significant healthcare costs. Currently, there are relatively few therapeutic options for A. baumannii. In addition, all antibiotics in clinical use have been tested, developed, optimized and regulated to manage infections caused by planktonic bacteria [8,9]. However, the biofilm phenotype predominates in natural ecosystems and persists in healthcare settings. BlastX™, a recently approved benzalkonium chloride (0.13%) and sodium citrate topical product, appears to be the only antimicrobial that specifically targets the biofilm phenotype, with emphasis on staphylococcal and Pseudomonas isolates. In this study, a novel antibiofilm agent, CZ-01179, was assessed for its in vitro activity against A. baumannii, as well as its potential to be formulated as the active agent in a topical gel to treat and prevent wound-related infection in an excision wound model.
The infection signal in young healthy pigs was mild (Fig. 4), but significant enough to assess outcome measures. Pig 1 was able to clear infection and rid wounds of planktonic bacteria naturally, in particular in debrided wounds (Table 2). However, wounds inoculated with well-established biofilms harbored more bacteria in both debrided and undebrided wounds (Table 2, Table 3). These results supported the hypothesis that wounds inoculated with well-established biofilms would harbor more bacteria, and indicated that there may be important differences to consider in wounds inoculated/contaminated with biofilms versus planktonic bacteria. Similar differences have been observed in sheep studies wherein biofilms were used as initial inocula [25,26].
In vivo data from Pig 2 indicated that CZ-01179 gel was effective. The gel maintained moist wound beds, reduced eschar formation, eradicated bacteria in both phenotypes and expedited closure. SSD cream performed similarly, but required slightly longer time intervals, in particular when used in combination with IV antibiotics, to eradicate bacteria. CZ-01179 gel did not lead to necrotic tissue or affect wound healing.
Wounds in Pig 3 treated with IV antibiotics struggled to heal fully. A two-week course of IV colistin/imipenem antibiotics failed to reduce planktonic bacteria in debrided wounds to a greater degree than positive control planktonic wounds that were debrided (Table 2). The antibiotics were successful at reducing bioburden to a greater degree in undebrided wounds and biofilm wounds (Table 2, Table 3). Nevertheless, the finding that bacteria in both the planktonic and biofilm phenotype were still present in wounds that were treated with IV antibiotics is important to consider. Although infection resolved, wounds were still colonized with bacteria and suggested that IV antibiotic therapy may not be sufficient to fully eradicate bacteria from a wound. Recurring infection can be a problem in wounds, and is a hallmark indicator of biofilm-related infection [[27], [28], [29]]. There is a rule of thumb, specifically for planktonic bacteria, that at a concentration of 105 CFU/g tissue, infection will develop [8,30]. In this case, IV antibiotics reduced planktonic bacteria to less than 105 CFU/g tissue, but in those wounds inoculated with well-established biofilms of A. baumannii, they were at concentrations greater than 105 CFU/g (Table 2, Table 3). Could residual bacteria in wounds contribute to recurring infection? Future work would be required to answer this question, and other bacterial species would need to be included, but these data may provide insight into clinical cases wherein IV antibiotics fail to eradicate biofilms to an acceptable level; an important consideration in wound management. Furthermore, the residual bacteria cultured in wounds of Pig 3 may provide an explanation as to why the wounds displayed a slower closure rate compared to other pigs/treatments (see Fig. 7, Fig. 8)—bacteria may have still been competing with the immune system.
When CZ-01179 was used in combination with IV antibiotics (Pig 4), bioburden was reduced completely within two weeks. A. baumannii was found at the endpoint in at least one wound treated with SSD/IV antibiotics. Although not tested directly, these outcomes indicated that CZ-01179 may work synergistically or additively with IV antibiotics.
At least four limitations will be addressed in future work. First, an n = 4 wounds (half debrided, half undebrided) was sufficient to assess initial efficacy profiles in Pigs 1 and 3, yet future work will be needed to increase the sample size. In addition, only one pig per treatment group was analyzed. Future work will involve an increase in pig numbers to provide increased robustness in data measures. Second, the design of this study was limited to assessing treatment in wounds that were already infected. In other scenarios, CZ-01179 could be assessed as a prophylactic approach to prevent infection from developing initially. Third, the pigs used in this study were young, healthy and were not immunocompromised. Work is currently being performed in a diabetic pig model to assess infection outcomes in an immunocompromised model. Fourth, only one bacterial isolate was examined in this study. Work is currently ongoing to assess CZ-01179 topical against MRSA and P. aeruginosa as monomicrobial and polymicrobial biofilms.
In summary, the topical products used alone and in combination were able to eradicate bacteria in both the planktonic and biofilm phenotypes more effectively than IV antibiotics alone. CZ-01179 gel reduced the bioburden of planktonic and biofilm bacteria up to 6 d faster than SSD cream, yet both were ultimately able to treat infection, assisted wound healing and did not adversely affect host tissue. Data indicated that CZ-01179 may be a promising agent for topical applications. In an era of reduced antibiotic efficacy, the development of a unique class of antibiofilm agent that is active against A. baumannii and other organisms is important. The addition of a topical therapy that can be used in conjunction with and improve standards of care is needed to improve current clinical limitations in the management of biofilm wound-related infections.
CRediT authorship contribution statement
Dustin L. Williams: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition. Brooke Kawaguchi: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Project administration. Nicholas B. Taylor: Methodology, Formal analysis, Investigation, Resources, Data curation. Gina Allyn: Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Project administration. Marissa A. Badham: Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing. Jeffery C. Rogers: Methodology, Formal analysis, Investigation, Data curation, Writing - review & editing. Brittany R. Peterson: Methodology, Formal analysis, Investigation, Resources, Data curation. Paul R. Sebahar: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Supervision, Project administration. Travis J. Haussener: Methodology, Formal analysis, Investigation, Resources, Data curation. Hariprasada Reddy Kanna Reddy: Methodology, Formal analysis, Investigation, Resources, Data curation. Brad M. Isaacson: Conceptualization, Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition. Paul F. Pasquina: Conceptualization, Methodology, Project administration, Funding acquisition. Ryan E. Looper: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
Authors DLW, PRS, TJH and REL have financial interest in Curza Global.
Acknowledgments and Funding Sources
The authors thank Seungah Goo, Lousili Cadenas Peniata, Mattias Nielsen, Richer Tyler Epperson, and the animal services and veterinary staff at the University of Utah for their technical assistance. This project was generously funded by the Center for Rehabilitation Sciences Research, award #HU0001-15-2-0003. Partial funding support was also received from Curza Global, LLC. Facilities, equipment and lab space at the Department of Veterans Affairs were also used to support the study. The opinions or assertions contained herein are the views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army, Department of Defense, Department of Veterans Affairs, or the United States government.
Abbreviations and Acronyms
- A. baumannii
Acinetobacter baumannii
- IV
intravenous
- MRSA
methicillin-resistant Staphylococcus aureus
- SSD
silver sulfadiazine
- ATCC
American type culture collection
- VAP
vascular access port
- CLSI
clinical laboratory and standards institute
- MIC
minimum inhibitory concentration
- CFU
colony forming units
- PBS
phosphate buffered saline
- CAMHB
cation adjusted Mueller-Hinton broth
- BHI
brain heart infusion
- CDC
Centers for Disease Control
- IACUC
Institutional care and use committee
References
- 1.Malone M., Bjarnsholt T., McBain A.J., James G.A., Stoodley P., Leaper D. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care. 2017;26(1):20–25. doi: 10.12968/jowc.2017.26.1.20. [DOI] [PubMed] [Google Scholar]
- 2.James G.A., Swogger E., Wolcott R., Pulcini E., Secor P., Sestrich J. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 3.Akers K.S., Mende K., Cheatle K.A., Zera W.C., Yu X., Beckius M.L. Biofilms and persistent wound infections in United States military trauma patients: a case-control analysis. BMC Infect Dis. 2014;14:190. doi: 10.1186/1471-2334-14-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez C., Mende K., Beckius M., Akers K., Romano D., Wenke J. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013;13:47. doi: 10.1186/1471-2334-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray C.K. Epidemiology of infections associated with combat-related injuries in Iraq and Afghanistan. J Trauma. 2008;64:S232–S238. doi: 10.1097/TA.0b013e318163c3f5. [DOI] [PubMed] [Google Scholar]
- 6.Percival S.L., McCarty S.M., Lipsky B. Biofilms and wounds: an overview of the evidence. Adv Wound Care. 2015;4(7):373–381. doi: 10.1089/wound.2014.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen C.K., Gordillo G.M., Roy S., Kirsner R., Lambert L., Hunt T.K. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowler P.G. The 105 bacterial growth guideline: reassessing its clinical relevance in wound healing. Ostomy/Wound Manag. 2003;49:44–53. [PubMed] [Google Scholar]
- 9.Williams D.L., Costerton J.W. Using biofilms as initial inocula in animal models of biofilm-related infections. J Biomed Mater Res B. 2011;100(4):1163–1169. doi: 10.1002/jbm.b.31979. [DOI] [PubMed] [Google Scholar]
- 10.Costerton J.W. The predominance of biofilms in natural and engineered ecosystems. In: Costerton J.W., editor. The biofilm primer. Springer; Heidelberg: 2007. pp. 5–13. [Google Scholar]
- 11.Costerton J.W., Geesey G.G., Cheng K.J. How bacteria stick. Sci Am. 1978;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 12.Golovkine G., Reboud E., Huber P. Pseudomonas aeruginosa takes a multi-target approach to achieve junction breach. Frontiers in cellular and infection microbiology. 2017;7:532. doi: 10.3389/fcimb.2017.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borriello G., Werner E., Roe F., Kim A.M., Ehrlich G.D., Stewart P.S. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother. 2004;48(7):2659–2664. doi: 10.1128/AAC.48.7.2659-2664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walters M.C., Roe F., Bugnicourt A., Franklin M.J., Stewart P.S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother. 2003;47(1):317–323. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haussener T.J., Sebahar P.R., Reddy H.R.K., Williams D.L., Looper R.E. A practical synthesis of N-alkyl- and N,N′-dialkyl-polyamines. Tetrahedron Lett. 2016;57(26):2845–2848. [Google Scholar]
- 16.Ashton N.N., Allyn G., Porter S.T., Haussener T.J., Sebahar P.R., Looper R.E. In vitro testing of a first-in-class tri-alkylnorspermidine-biaryl antibiotic in an anti-biofilm silicone coating. Acta Biomater. 2019;93:25–35. doi: 10.1016/j.actbio.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Williams D.L., Epperson R.T., Ashton N.N., Taylor N.B., Kawaguchi B., Olsen R.E. In vivo analysis of a first-in-class tri-alkyl norspermidine-biaryl antibiotic in an active release coating to reduce the risk of implant-related infection. Acta Biomater. 2019 doi: 10.1016/j.actbio.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 18.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore K.S., Wehrli S., Roder H., Rogers M., Forrest J.N., McCrimmon D. Squalamine: an aminosterol antibiotic from the shark. Proc Natl Acad Sci Unit States Am. 1993;90(4):1354–1358. doi: 10.1073/pnas.90.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallo S.F., Weitao T. Insights into acinetobacter war-wound infections, biofilms, and control. Adv Skin Wound Care. 2010;23(4):169–174. doi: 10.1097/01.ASW.0000363527.08501.a3. [DOI] [PubMed] [Google Scholar]
- 21.Hujer K.M., Hujer A.M., Hulten E.A., Bajaksouzian S., Adams J.M., Donskey C.J. Analysis of antibiotic resistance genes in multidrug-resistant acinetobacter sp. isolates from military and civilian patients treated at the walter reed Army medical center. Antmicrob Agents Chemother. 2006;50(12):4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams D.L., Smith S.R., Peterson B.R., Allyn G., Cadenas L., Epperson R.T. Growth substrate may influence biofilm susceptibility to antibiotics. PloS One. 2019;14(3) doi: 10.1371/journal.pone.0206774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirketerp-Møller K., Jensen P., Fazli M., Madsen K., Pedersen J., Moser C. Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol. 2008;46(8):2717–2722. doi: 10.1128/JCM.00501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray C.K., Roop S.A., Hospenthal D.R., Dooley D.P., Wenner K., Hammock J. Bacteriology of war wounds at the time of injury. Mil Med. 2006;171:826–829. doi: 10.7205/milmed.171.9.826. [DOI] [PubMed] [Google Scholar]
- 25.Williams D.L., Haymond B.S., Beck J.P., Savage P.B., Chaudhary V., Epperson R.T. In vivo efficacy of a silicone - cationic steroid antimicrobial coating to prevent implant-related infection. Biomaterials. 2012;33(33):8641–8656. doi: 10.1016/j.biomaterials.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams D.L., Haymond B.S., Woodbury K.L., Beck J.P., Moore D.E., Epperson R.T. Experimental model of biofilm implant-related osteomyelitis to test combination biomaterials using biofilms as initial inocula. J Biomed Mater Res A. 2012;100(7):1888–1900. doi: 10.1002/jbm.a.34123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrie T., Nelligan J., Costerton J. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982;66:1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- 28.Gristina A.G., Costerton J.W. Bacteria-laden biofilms: a hazard to orthopedic prostheses. Infect Surg. 1984;3:655–662. [Google Scholar]
- 29.Saye D.E. Recurring and antimicrobial-resistant infections:considering the potential role of biofilms in clinical practice. Ostomy/Wound Manag. 2007;53(4):46–48. 50, 2 passim. [PubMed] [Google Scholar]
- 30.Krizek T.J., Robson M.C., Kho E. Bacterial growth and skin graft survival. Surg Forum. 1967;18:518. [Google Scholar]