Abstract
Being responsible for delayed wound healing, the presence of biofilms in infected wounds leads to chronic, and difficult to treat infections. One of the reasons why antimicrobial treatment often fails to cure biofilm infections is the reduced penetration rate of antibiotics through dense biofilms. Strategies that have the ability to somehow interfere with the integrity of biofilms and allowing a better penetration of drugs are highly sought after. A promising new approach is the use of laser-induced vapor nanobubbles (VNB), of which it was recently demonstrated that it can substantially enhance the penetration of antibiotics into biofilms, resulting in a marked improvement of the killing efficiency. In this study, we examined if treatment of biofilms with laser-induced vapor nanobubbles (VNB) can enhance the potency of antimicrobials which are commonly used to treat wound infections, including povidone-iodine, chlorhexidine, benzalkonium chloride, cetrimonium bromide and mupirocin. Our investigations were performed on Pseudomonas aeruginosa and Staphylococcus aureus biofilms, which are often implicated in chronic wound infections. Pre-treatment of biofilms with laser-induced VNB did enhance the killing efficiency of those antimicrobials which experience a diffusion barrier in the biofilms, while this was not the case for those compounds for which there is no diffusion barrier. The magnitude of the enhanced potency was in most cases similar to the enhancement that was obtained when the biofilms were completely disrupted by vortexing and sonication. These results show that laser-induced VNB are indeed a very efficient way to enhance drug penetration deep into biofilms, and pave the way towards clinical translation of this novel approach for treatment of wound infections.
Keywords: Vapor nanobubbles, Laser treatment, Biofilms, Wound infections, Disinfectants, Diffusion barrier
Introduction
Chronic wounds such as diabetes wounds, pressure ulcers, and venous ulcers, affect a substantial fraction of the world population [1]. It is estimated that in the United States alone, 6.5 million people suffer from chronic wounds [2]. Due to increasing health care costs, aging population and epidemic rise of obese and diabetic people, the burden of chronic wounds to the healthcare system is believed to increase even further in the nearby future [1]. Bacterial infections can significantly delay wound healing [3] and microbial biofilms are increasingly recognized as an important reason for treatment failure [4,5]. Therefore, it is important to direct wound care towards diagnosis and eradication of these biofilms in order to achieve better clinical outcomes [6].
The biofilm lifestyle offers protection to bacterial cells against antimicrobials due to a complex interplay of multiple factors, including reduced metabolic activity, heterogeneity of microenvironments, presence of persister cells and reduced penetration of antimicrobials [7,8]. In the present study we focus on the latter problem, which is commonly referred to as the biofilm diffusion barrier. On the one hand it is caused by physicochemical interactions that can occur between antibiotics and biofilm constituents such as polysaccharides, extracellular DNA or enzymes, all of which can inactivate or slow down the diffusion of antimicrobial agents. On the other hand, hindered penetration also stems from the fact that sessile cells are packed tightly together in dense microcolonies of tens to hundreds of micrometers in size [[9], [10], [11], [12], [13]]. Even though most drug molecules are small enough to diffuse through the spaces between cells, the net flux towards deeper cell layers is reduced since they all have to pass through those narrow openings. Therefore, innovative strategies that can somehow interfere with the dense structure of biofilms to enhance drug penetration of current interest, including for wound care [14].
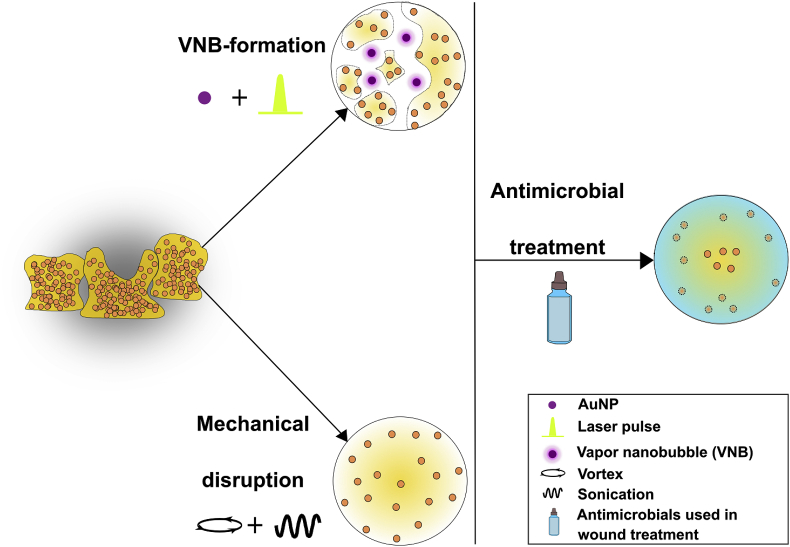
One approach is to make use of anti-biofilm agents, for instance enzymatic approaches like the use of dispersin B that has been shown to degrade critical components of the extracellular polymeric substances (EPS) matrix of methicillin resistant S. aureus in a chronic wound mouse model [15,16]. Quorum sensing inhibitors are another example of agents that can interfere with bacterial communication that regulates biofilm formation in chronic wounds [17,18]. However, broad applicability of such pharmacological approaches is limited as they target very specific components of the biofilm. Physical methods on the other hand may be more flexible as their mechanism typically does not depend on the specific composition of a biofilm. Along these lines we have recently reported on a new approach that is based on the combination of nanotechnology and laser treatment to locally disturb biofilm integrity, improving the penetration and effectiveness of antibiotics [19]. In this approach biofilms are first incubated with small gold nanoparticles that have the ability to penetrate deep into biofilms (Fig. 1). Such gold nanoparticles can absorb the energy of high-intensity, short laser pulses, causing their temperature to rise to several hundreds of degrees within nanoseconds. The water surrounding the particles then quickly evaporates, resulting in expanding and imploding water vapor nanobubbles (VNB) [20,21]. The generated pressure waves can locally disturb the organization of tightly packed sessile cells, allowing better penetration of drug molecules deep into the biofilm. It was found that pretreatment of biofilms with VNB substantially improved the efficacy (up to 3000-fold) of the antibiotic tobramycin in biofilms formed by Pseudomonas aeruginosa and Staphylococcus aureus [19].
Fig. 1.
Schematic overview of the experimental protocol. Biofilms are loaded with small cationic AuNP and irradiated with pulsed laser light in order to create vapor nanobubbles (VNB) inside the bacterial cell clusters. Besides VNB-formation, biofilms were also disrupted mechanically by means of vortex/sonication as a positive control. Then, a broad panel of antimicrobials frequently used in wound treatment were added to the treated biofilms, and it was evaluated to which extent their efficacy was improved.
Excellent spatial control is another important advantage of using VNB to disrupt wound biofilms. Indeed, as laser light can be perfectly controlled in time and space, unwanted side effects like tissue damage are likely to be reduced to a minimum. Furthermore, VNB-formation only causes biofilm deformation at the location of the exploding nanobubbles without causing the bacteria to be released into the surrounding medium. This is an important consideration when translating this concept to the clinic with minimal risk for systemic spread and sepsis [22]. Another important feature of laser-induced VNB is that, contrary to ultrasound treatment, net transfer of heat to the surrounding healthy tissue is avoided, as all the heat within the AuNP is efficiently converted into mechanical energy [23].
In the present study, we investigated the use of laser-induced VNB for the treatment of P. aeruginosa and S. aureus biofilms, which are often found in infected wounds. Its potential to enhance the efficacy of a broad range of commercially available wound products was tested, including povidone-iodine (Pvp-I), chlorhexidine (Chx), benzalkonium chloride (BzCl), cetrimonium bromide (Cetr) and mupirocin (Mupi). We compared the antimicrobial activity after VNB treatment with the activity against undisturbed biofilms and biofilms that were completely disrupted by vortexing and ultrasound treatment. Our results showed that VNB treatment enhanced the antimicrobial effect of the tested compounds to the same level as forced disruption by sonication and vortexing, clearly demonstrating that laser-induced VNB are an effective way to overcome the diffusion barrier. A potentiating effect was found for BzCl (~21x) in P. aeruginosa biofilms, and Cetr (~24x) and Mupi (~53x) in S. aureus biofilms, which could even be increased to a complete loss of survival after repeated VNB-formation in case of Mupi. Instead, for the other tested combinations, such as Pvp-I, Chx and Cetr in P. aeruginosa biofilms and BzCl in S. aureus biofilms, it turned out that there was no diffusion barrier. Together our results highlight the importance of the diffusion barrier in biofilms, and the potential of laser-induced VNB to enhance the efficacy of those antimicrobials which suffer from a diffusion barrier in biofilms.
Results
Combining VNB treatment and disinfectants in P. aeruginosa biofilms
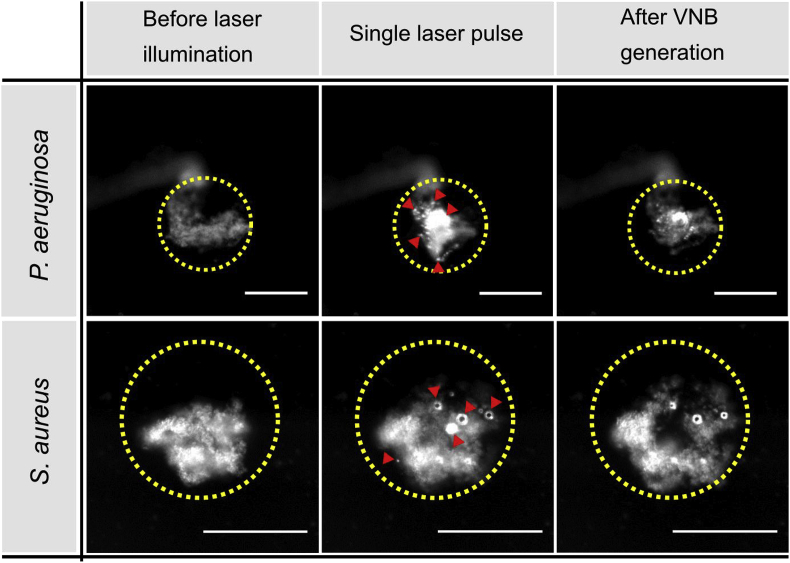
The effect of VNB treatment on the efficacy of various wound-related disinfectants was first evaluated on P. aeruginosa biofilms, as this is an important pathogen often found in biofilm-infected wounds [24]. Cationic 70 nm AuNP (which were previously shown to be able to penetrate P. aeruginosa biofilms [19]) were added to the biofilms, and biofilms were subsequently illuminated with pulsed laser light (7 ns, 561 nm). The laser beam had a diameter of approximately 150 μm and was scanned across the biofilm so that each location received 1 or maximum 2 laser pulses (there is a certain overlap between consecutive pulses to ensure that each location of the biofilm receives at least one pulse). Laser pulses were applied with a fluence of 1.7 J cm−2, which is well above the VNB fluence threshold of 70 nm AuNP in water [19], so that VNB were effectively formed. As shown in Fig. 2, dark field microscopy confirmed that VNB were formed, which generally resulted in a visible alteration of the morphology of the cell clusters.
Fig. 2.
Vapor nanobubble mediated biofilm disruption in P. aeruginosa (upper panel) and S. aureus (lower panel) biofilms. Dark field images were taken before, during and immediately after VNB generation by a single nanosecond laser pulse of 561 nm at a laser fluence of 1.7 J cm−2. The location of the laser pulse is indicated by a yellow circle. The red arrow heads point towards the location of vapor nanobubbles which are visible as small bright spots due to increased light scattering during their lifetime. Scale bar = 100 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
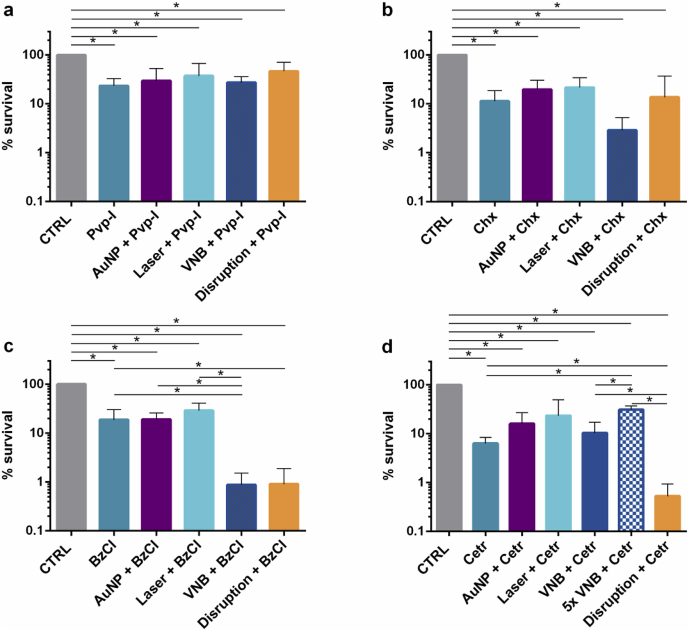
After laser irradiation, the biofilms were treated for 5 min with either Pvp-I, Chx, BzCl or Cetr at concentrations of 0.01%, 0.04%, 0.06% or 0.15% (w/v) respectively. Cell survival was quantified by plate counting after addition of neutralizing broth. In case of Pvp-I, treating P. aeruginosa biofilms by VNB did not result in an enhanced efficacy of the disinfectant (Fig. 3 a). A similar effect was seen for Chx, where no significant differences were found between the survival of Chx treated or VNB + Chx treated P. aeruginosa sessile cells (Fig. 3 b). When P. aeruginosa biofilms were treated with BzCl, however, a ~21 x increased effect of BzCl was observed after VNB formation compared to BzCl treatment alone (p = 0.03) (Fig. 3 c). This enhanced activity was only observed after formation of VNB, as the controls of BzCl with AuNP (no laser) or BzCl with laser (no AuNP) had no additional effect compared to BzCl treatment. Finally, when P. aeruginosa biofilms were treated with Cetr, which, like BzCl, is a quaternary ammonium compound, no significant additional effect of VNB pre-treatment was found in P. aeruginosa biofilms (p = 1) (Fig. 3 d). It is important to note that we showed previously that the generation of VNB by itself does not significantly affect the bacterial viability of both P. aeruginosa as S. aureus biofilms under exactly the same conditions [19]. The observed effects on the efficacies of the antimicrobial agents are thus solely attributed to the secondary effects of VNB generation on the biofilm structure and not a direct effect of VNB mediated killing of sessile cells.
Fig. 3.
Anti-biofilm effect of pre-treatment with VNB and subsequent addition of various wound-related disinfectants on P. aeruginosa biofilms: (a) povidone-iodine (Pvp-I), (b) chlorhexidine (Chx), (c) benzalkonium chloride (BzCl), (d) cetrimonium bromide (Cetr). CTRL: 0.9% NaCl solution, AuNP + disinfectant: addition of AuNP with subsequent disinfectant treatment, Laser + disinfectant: laser control (without AuNP) and subsequent disinfectant treatment, VNB + disinfectant: laser-induced VNB treatment followed by the addition of disinfectant, Disruption + disinfectant: complete biofilm disruption by vortexing/sonication and subsequent disinfectant treatment. Each anti-biofilm effect was tested in at least 3 biological repeats and each biological repeat consisted of 3 technical repeats (n ≥ 3 × 3) (p < 0.05).
In view of the positive results we obtained previously with P. aeruginosa biofilms and the antibiotic tobramycin [19], it was at first surprising that only 1 out of 4 tested disinfectants gave an additional effect after VNB treatment. This led us to believe that very likely there is only a diffusion barrier for BzCl, but not for the other three disinfectants in P. aeruginosa biofilms. In order to verify this hypothesis, we performed a positive control where biofilms were completely disrupted by 2 rounds of vortexing and sonication before treatment with the same disinfectants. As can be seen in Fig. 3, apart from Cetr these forced disruption experiments correlated well with the VNB results. Fig. 3 a and 3 b confirmed that the efficacy of Pvp-I and Chx could not be enhanced by disrupting the biofilm, indicating that there is no substantial diffusion barrier for those compounds in P. aeruginosa biofilms. For BzCl on the other hand, a ~21 x reduction was observed in the amount of surviving cells, corresponding remarkably well with the VNB results. Only for Cetr there was a mismatch with the VNB results. As shown in Fig. 3 d, a ~12 fold enhanced activity of Cetr was seen in the positive control, while this was not the case after VNB treatment. This could be due to the fact that forming VNB 1x only was insufficient to improve Cetr diffusion. Indeed, in previous research [19] we already showed that repeated VNB formation could in some cases further enhance drug penetration. Therefore, we repeated the laser treatment 5 times before adding Cetr, but as shown in Fig. 3 d, no further enhancement of Cetr was obtained by repeated VNB formation. Somewhat surprisingly, there was a significant 5-fold increase in the amount of surviving cells after 5x VNB + Cetr treatment compared to only adding Cetr to P. aeruginosa biofilms. However, since we did not observe such trends for the other antimicrobial agents and bacterial biofilms, we believe that, in spite of being statistically different, its biological relevance is disputable.
Combining VNB treatment with disinfectants and mupirocin in S. aureus biofilms
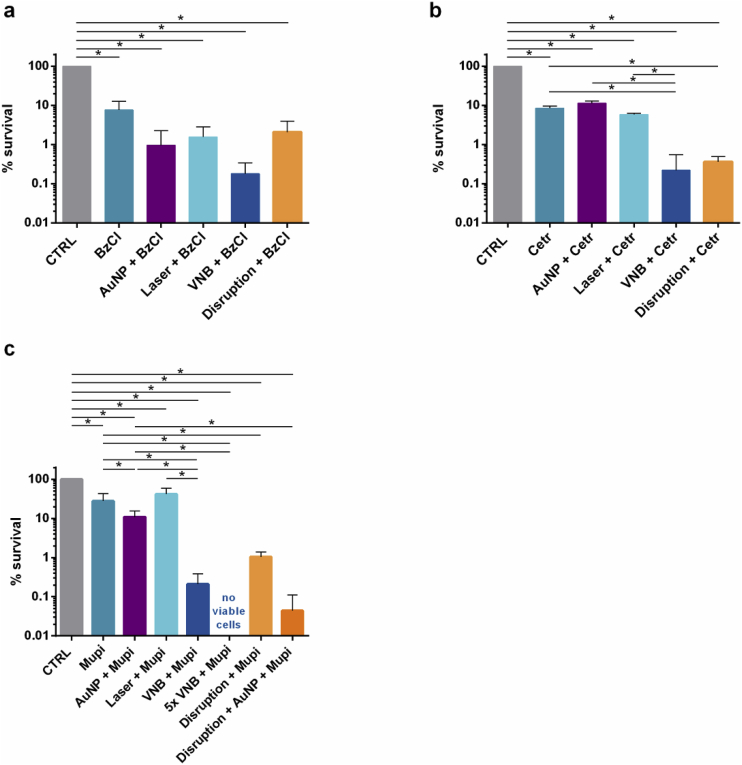
We continued our studies on gram-positive S. aureus as another pathogen that is often dominating wound-associated biofilms [24]. Because the forced disruption of P. aeruginosa biofilms suggested that there is no diffusion barrier for Pvp-I and Chx, we excluded these disinfectants from the testing panel, and continued our study with BzCl and Cetr – for which we did see an enhancement of their efficacy after disrupting the biofilms – and evaluated their potential in combination with VNB in S. aureus biofilms. In addition, the use of quaternary ammonium derivates in the treatment of S. aureus infections is of interest since Gram-positive bacteria tend to be more susceptible towards such membrane-targeting agents due to their more vulnerable cell wall compared to Gram-negative bacteria [25]. Next to BzCl and Cetr, we also investigated the effect of VNB treatment in combination with mupirocin (Mupi), an antibiotic often used to treat skin and soft tissue MRSA infections [26,27]. The formation of VNB again resulted in a visible deformation of S. aureus cell clusters, as shown in Fig. 2. In this case the activity of BzCl was not enhanced after VNB treatment (Fig. 4 a), while a ~24 fold increased killing was seen with Cetr (Fig. 4 b). Importantly, this enhanced effect of Cetr was only observed in combination with VNB, but not with AuNP (p = 0.61) or laser treatment only (p = 0.51). For mupirocin, it was noted that the combination with AuNP alone (AuNP + Mupi) already resulted in a ~3 times lower cell survival (Fig. 4 c), a somewhat surprising finding as it was not observed for any of the other tested compounds. Importantly, however, an extra ~53 times enhancement of mupirocin was seen after VNB-formation.
Fig. 4.
Anti-biofilm effect of pre-treatment by laser-induced VNB followed by the addition of (a) benzalkonium chloride (BzCl), (b) cetrimonium bromide (Cetr) or (c) the antibiotic mupirocin (Mupi) in S. aureus biofilms. CTRL: 0.9% NaCl solution, AuNP + antimicrobial agent: addition of AuNP with subsequent antimicrobial agent treatment, Laser + antimicrobial agent: laser and subsequent antimicrobial agent treatment, VNB + antimicrobial agent: laser-induced VNB treatment followed by the addition of the antimicrobial agent, Disruption + antimicrobial agent: complete biofilm disruption by vortexing/sonication and antimicrobial agent treatment, Disruption + AuNP + Mupi: complete biofilm disruption in presence of AuNP and subsequent mupirocin treatment. Each antibiofilm effect was tested in at least 3 biological repeats and each biological repeat consisted of 3 technical repeats (n ≥ 3 × 3) (p < 0.05).
Again we found a good correspondence between these VNB results and positive controls where the biofilm was completely disrupted by vortexing and sonication. For BzCl indeed no significantly increased effect was found on disrupted biofilms, indicating that this compound does not experience a diffusion barrier in S. aureus biofilms. The activity of Cetr on the other hand increased ~39 times on disrupted biofilms, similar to what we found for VNB. In case of mupirocin, the disrupted biofilms were treated with a combination of AuNP and mupirocin due to the above mentioned effect of AuNP on this compound. In this case a ~250 fold enhanced activity was found of mupirocin on disrupted S. aureus biofilms. This is even more than what was obtained with VNB, indicating that treatment of the S. aureus biofilms with one laser pulse as we did so far may not have completely resolved the diffusion barrier for mupirocin. Indeed, when the laser treatment was repeated 5x on S. aureus biofilms, none of the cells survived following the addition of mupirocin (Fig. 4 c).
Discussion
In the past decade, the clinical impact of biofilms in chronic wounds has been increasingly recognized. One of the reasons why biofilm formation decreases the effectiveness of antimicrobial treatment is because of hindered diffusion of antimicrobials through biofilms. Indeed, several studies reported on reduced diffusion of disinfectants through wound biofilms, likely due to interaction with the extracellular polymeric substance (EPS) matrix [28,29]. For instance, De Beer et al. followed chlorine penetration in mixed biofilms of P. aeruginosa and Klebsiella pneumoniae by measuring chlorine concentrations with microelectrodes, finding that the chlorine concentration inside the clusters was only 20% of that of the incubating solution [30]. Reaction-diffusion interaction with biofilm constituents was found to be responsible for the hindered diffusion [31,32]. As another example, Ganeshnarayan et al. showed that biofilm diffusion of the quaternary ammonium derivative cetylpyridinium chloride was slowed down by electrostatic interactions with poly-N-acetylglucosamine matrix polysaccharides [33].
In order to improve drug diffusion, a plethora of anti-biofilm agents have been investigated for this purpose, such as enzymes and amino acids, targeting specific components of the extracellular polymeric substance (EPS) matrix, or molecules that target pathways involved in biofilm disruption such as the cyclic-di-GMP pathway, quorum sensing and others [34,35]. Fleming et al. showed that the addition of glycoside hydrolase to mature P. aeruginosa and S. aureus wound biofilms degraded EPS polysaccharides which increased the efficiency of subsequent antibiotic treatment [36]. In another study, Sanchez et al. reported on the potential of d-amino acids to disrupt mature biofilms of P. aeruginosa and S. aureus clinical wound isolates, thereby increasing the effectiveness of several antimicrobial agents [37]. However, despite promising results in vitro, clinical translation of these molecular disruption agents may be hampered because of several reasons. First, off-target side effects can occur since many of those targets are not only found in bacteria but in human tissues as well [34]. Second, the highly variable nature of the EPS composition that depends on the type of organism, substrate and host interactions [28], combined with the fact that biofilm signaling molecules are species and even strain dependent, add another level of complexity that restricts the broad applicability of such approaches [38]. Third, as some agents rely on cellular processes such as interference with cyclic-di-GMP and iron metabolism, cells have to be metabolically active in order to be affected which is not always the case [28]. Lastly, although it was previously accepted that these anti-biofilm agents targeted against the biofilm should be less prone to drive resistance in contrast to classic antimicrobial agents [34], a recent study indicated that this is not always the case, as shown for the quorum sensing inhibitor baicalin hydrate in Burkholderia cenocepacia biofilms [39].
Due to these shortcomings, rather than a pharmacological approach, it is attractive to explore physical methods to interfere with the biofilm integrity. Physical forces are largely independent of the bacterial microflora and matrix composition, making them likely more universally applicable. Currently, the gold standard for physically disrupting and removing biofilms in wound beds is still repeated sharp debridement [34,40]. However, as debridement is often painful and imprecise in removing biofilms, there is an interest in newer and more refined methods [35,40]. While electric currents have been proven successful as anti-biofilm strategy and reportedly may also promote wound healing, clinical translation of this method is hampered because of heating of the surrounding tissue and cytotoxicity [28]. Similarly, several studies reported on ultrasound-mediated wound biofilm disruption in vitro, and its potential to decrease exudate, slough and patient pain [41]. Another experimental approach is the use of laser-generated shockwaves which have been shown to disrupt S. epidermidis biofilms grown on pig skin [42]. In this technique, metal coated surfaces were placed in close proximity of biofilms and irradiated with laser light, which consequently created shockwaves by means of laser-assisted ablation of metal films. For both ultrasound as laser-generated shock wave therapy, further studies are needed to assess their efficacy and rule out possible detrimental damage such as heating and off-target side effects in a clinical setting [28,43]. Recently we presented the use of laser-induced VNB as a new and refined approach to interfere with the biofilm structure to enhance drug diffusion and efficacy [19]. A particular advantage of this technique is that it can be applied locally with a high spatial control [44,45]. Combined with the fact that it is not associated with heating effects [23], it is expected to minimize off-target effects to the surrounding healthy tissue. Furthermore, since the VNB shockwaves are produced locally on a nano- and microscale from AuNP within the biofilm, it does not cause bacteria to become spread in the environment, as previously demonstrated [19]. Hence it minimizes the risk of recolonization of disrupted bacteria, which could lead to biofilm formation in other locations of the human body and even life-threatening sepsis [34,46]. Lastly, it is of note that the application of lasers in dermatology has increased dramatically in recent years, and they have been used safely and effectively in a wide variety of treatments, including vascular and pigmented lesions, tattoos, scars, and psoriasis [47]. In the present study, we specifically investigated the potential of treating biofilms with laser-induced VNB in the context of wound infections. In particular, we investigated the potentiating effect of VNB treatment on antimicrobial agents that are commonly used to treat infected wounds. Since VNB treatment enhances drug diffusion, we also included positive controls where the biofilms were completely disrupted by 2 rounds of vortexing and sonication as a benchmark for the maximum achievable effect. This is a similar approach as reported by Leung et al. who mechanically disrupted biofilms by means of sonication and tested the susceptibility of these disorganized biofilms to antimicrobial agents as compared to intact biofilms [48]. Treatment of P. aeruginosa biofilms with VNB did not increase the efficacy of Pvp-I, Chx and Cetr, while the activity of BzCl was enhanced 21-fold. Similar results were found on disrupted biofilms, the only exception being Cetr for which a small but significant effect was observed. These results show that Pvp-I and Chx do not experience a diffusion barrier in P. aeruginosa biofilms. For Pvp-I this was not that surprising as it is known to be able to penetrate biofilms, which contributes to its frequent use as antiseptic for wound treatment [49,50]. We did, however, expect to find hindered diffusion for Chx as it is a highly positively charged molecule which might get sequestered by negatively charged matrix components (similar to e.g. tobramycin [51]). Looking into literature, contradicting findings have been reported regarding Chx’s penetration through biofilms. Some studies report on EPS-mediated retardation of Chx diffusion [[52], [53], [54], [55]], while others state that Chx penetration is not an issue [56,57], which is more in line with our findings. For BzCl on the other hand, we did find hindered diffusion in P. aeruginosa biofilms, which could be due to its positively charged quaternary ammonium head group and long alkyl tail. Indeed, it has been proposed that for quaternary ammonium compounds, charged interactions between negatively charged matrix components and cationic ammonium groups on the one hand and hydrophobic interactions with alkyl chains on the other hand play an important role in their affinity towards biofilm matrices [58,59]. Hindered BzCl penetration through P. aeruginosa biofilms was also observed in the study of Bridier et al., who visualized the spatiotemporal pattern of BzCl-penetration and inactivation of P. aeruginosa biofilms by confocal laser scanning microscopy. They found that, initially, BzCl only affected cells in peripheral layers of the biofilm clusters, whereas cells located in the center of the clusters became gradually inactivated during the course of the disinfectant treatment [60]. Surprisingly, however, when the structurally similar quaternary ammonium compound Cetr was tested, no significantly enhanced activity was seen after VNB pre-treatment of P. aeruginosa biofilms, while its activity increased by 12 times in disrupted biofilms. While the exact reason for this difference is not clear, it suggests that when P. aeruginosa biofilms are subjected to vortexing/sonication, some pathways become affected that play a role in the protection against Cetr, which are not affected by VNB-mediated disruption. Indeed, it has been reported before that the way in which disruption is induced can affect the susceptibility of disrupted cells against antimicrobials. For instance, in the study of Chambers et al. it was shown that the susceptibility phenotype of disrupted P. aeruginosa cells to antimicrobials differed between glutamate and nitric oxide-mediated disruption, showing that different types of disruption agents can result in disrupted cells with different antimicrobial sensitivity phenotypes [61].
When the activity of BzCl and Cetr was tested in S. aureus, it became clear that biofilm penetration is not only molecule- but also biofilm-dependent. Contrary to P. aeruginosa biofilms, BzCl had no additional effect on VNB pre-treated S. aureus biofilms. The same was found on disrupted S. aureus biofilms, showing that for BzCl there is no diffusion barrier in these biofilms. Our results are in line with the study of Campanac et al. [62] where disruption of S. aureus biofilms also did not restore their sensitivity towards BzCl. Also for Cetr the opposite trend was found in S. aureus biofilms, with a 24x enhanced activity in VNB pre-treated biofilms, which could be confirmed in disrupted biofilms. This emphasizes that biofilm diffusion studies cannot be generalized but depend on the specific combination of microbial species and antimicrobial agent. When testing mupirocin on S. aureus biofilms, finally, pre-treatment with VNB enhanced its killing efficiency by 53 times, with even complete eradication when laser irradiation was 5x repeated. This corresponded well to the 250 times enhancement that we found on completely disrupted S. aureus biofilms, confirming the presence of a strong diffusion barrier for mupirocin in S. aureus biofilms.
Although our study clearly demonstrates that VNB treatment of biofilms can enhance the efficacy of several relevant antimicrobials that suffer from a diffusion barrier in a particular type of biofilm, we believe that the importance of this type of novel treatment will become even more convincing in more complex in vivo situations. The reasoning for this is that biofilms residing in clinical wounds have a more complex structure and EPS matrix compared to in vitro grown biofilms in 96-well plates. Thus, the contribution of impaired mass transport, and hence the potential of VNB to resolve this problem, is expected to be even more important in mature wound biofilms. Therefore, it will be of interest to evaluate this concept in more complex biofilm wound models integrating different aspects of the wound environment, such as the presence of plasma proteins, keratinocytes, and host immune responses [63,64]. As dynamic interactions between multiple species play an important role in the persistence of chronic wounds, it is also of interest to evaluate whether VNB treatment has the same positive effect on multispecies biofilms rather than simple monospecies communities.
Conclusion
Disrupting P. aeruginosa or S. aureus wound biofilms by laser-induced VNB is a promising route to enhance the efficiency of antimicrobial agents that experience hindered diffusion in biofilm-related wound infections. Forced disruption experiments generally offered a good prediction of the extent of improvement that is attainable with VNB pre-treatment. Even though further work is needed to evaluate this novel concept on more complex biofilm wound models and in vivo, these promising results show the potential of laser-induced VNB to help combating tenacious biofilm implicated wound infections.
Materials and methods
Materials and strains
P. aeruginosa LESB58 (LMG 27622) and S. aureus Mu50 were used. Lysogeny Agar/Broth and Dey-Engley Neutralizing Broth (DENB) were purchased from Lab M Limited (Lancashire, UK). Simulated wound fluid (1:1 fetal bovine serum: 0.9% NaCl (w/v) in 0.1% peptone) was prepared in-house. Fetal bovine serum was purchased from HyClone (Pierce, Rockford, IL, USA), NaCl was obtained from Applichem (Darmstadt, Germany) and peptone from BD Diagnostics (New Jersey, USA). AuNP were purchased from Nanopartz (Loveland, USA) and were diluted in ultrapure water prior to use. Poly(vinylpyrrolidone)-Iodine (Pvp-I), chlorhexidine digluconate (20% in water) (Chx), benzalkonium chloride (BzCl) and hexadecyltrimethylammonium bromide (Cetr) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Mupirocin was obtained from Toronto Research Chemicals (Toronto, Canada).
Biofilm formation
Biofilms were grown aerobically in 96-well SensoPlates (Greiner Bio-One, USA) with microscopy grade borosilicate glass bottom for 24 h at 37 °C. Biofilms of P. aeruginosa were grown in Lysogeny Broth, while S. aureus biofilms were grown in simulated wound fluid, which was found to be necessary in order for thick biofilms to be formed. The wells of the 96-well SensoPlate were filled with 100 μL of the bacterial suspension, and incubated at 37 °C. After 4 h, the adhered cells were washed with physiological saline (0.9% NaCl (w/v)), covered with medium and incubated for another 20 h at 37 °C.
Formation and visualization of VNB inside biofilms
After 24 h of growth, the supernatant was removed and 100 μL of an aqueous dispersion of AuNP (1.4E+10 AuNP mL−1) was added to the biofilm. To allow complete penetration, the biofilms were incubated for 15 min at room temperature. Next, the supernatant was removed and 100 μL physiological saline was added to each well. A home-made optical set-up was used to generate VNBs inside the biofilms, as previously described [19]. The set-up is built around an inverted TE2000 epi-fluorescence microscope (Nikon, Nikon BeLux, Brussels, Belgium) equipped with a Plan Fluor 10 × 0.3 NA objective lens (Nikon). An Optical Parametric Oscillator (OPO) laser (OpoletteTM HE 355 LD, OPOTEK Inc., Faraday Ave, CA, USA) produces laser pulses of 7 ns tuned to 561 nm in order to excite the localized surface plasmon resonance of the AuNP while at the same time being compatible with optical filters in the set-up. The energy of each laser pulse is monitored with an energy meter (J-25MB-HE&LE, Energy Max-USB/RS sensors, Coherent) synchronized with the pulsed laser. In this study, a laser pulse fluence of 1.7 J cm−2 was used (calculated as the pulse energy of a single laser pulse divided by the laser beam area (150 μm diameter)).
VNB formation was visualized by dark-field images before, during and immediately after irradiation with a laser pulse, based on the light scattering properties of VNB. The camera (EMCCD camera, Cascade II:512, Photometrics, Tucson, USA) was synchronized with the pulsed laser by an electronic pulse generator (BNC575, Berkeley Nucleonics Corporation, CA, USA) to compensate for the short nature of VNB generation (lifetime < 1 μs). For dark field imaging, the biofilms were cultured 24 h at 37 °C in 50 mm glass bottom dishes (No. 1.5 coverslip) (MatTek Corporation, Ashland, USA).
Disinfection procedures
After laser treatment, 100 μL supernatant was removed and the biofilm was covered with 120 μL of disinfectant, antibiotic or control solution (0.9% NaCl (w/v)), as displayed in Table 1. Concentrations and contact times were chosen to achieve a suboptimal killing of the sessile cells. After the prescribed contact time, the disinfectants were neutralized by adding 80 μL of 2.5x concentrated DENB. After 5 min neutralization time, 200 μL supernatant was removed and the sessile cells were washed with physiological saline. In case of mupirocin, biofilms were treated for 24 h at 37 °C, followed by a washing step with physiological saline. The sessile cells were harvested by 2 rounds of 5 min vortexing (900 rpm, Titramax 1000, Heidolph Instruments, Schwabach, Germany) and 5 min sonication (Branson 3510, Branson Ultrasonics Corp., Danbury, CT, USA). Next, the effect of the treatments was evaluated by plate counting.
Table 1.
Concentrations and contact times of the antimicrobial agents used in this study.
|
P. aeruginosa |
S. aureus |
|||||
|---|---|---|---|---|---|---|
| Concentration (w/v) | Contact time | CFU/biofilm CTRL | Concentration (w/v) | Contact time | CFU/biofilm CTRL | |
| Pvp-I | 0.01% | 5 min | 1.6 × 10ˆ7 ± 8.3 × 10ˆ6 |
x | x | x |
| Chx | 0.04% | 5 min | 1.8 × 10ˆ7 ± 1.2 × 10ˆ7 |
x | x | x |
| BzCl | 0.06% | 5 min | 1.6 × 10ˆ7 ± 8.2 × 10ˆ6 |
0.06% | 5 min | 5.8 × 10ˆ6 ± 3.9 × 10ˆ6 |
| Cetr | 0.15% | 5 min | 1.8 × 10ˆ7 ± 8.6 × 10ˆ6 |
0.15% | 5 min | 1.8 × 10ˆ6 ± 9.1 × 10ˆ5 |
| Mupi | x | x | x | 0.01% | 24 h | 4.1 × 10ˆ6 ± 3.7 × 10ˆ6 |
Biofilm disruption procedure
Disruption of 24 h-old biofilms was done by washing the sessile cells with physiological saline followed by 2 rounds of 5 min vortexing and 5 min sonication. Next, the disrupted cells were treated with 2.67x concentrated solutions of the antimicrobial agents (taking into account the dilution factor introduced by the disruption procedure) as described in Table 1. After neutralization with DENB, the number of CFU/biofilm per condition was determined by plating.
Statistical analysis
SPSS Statistics 24 (SPSS, Chicago, IL, USA) was used to analyze the data. To assess the normality of the data sets, the Shapiro–Wilk test was used. The one-way analysis of variance test and independent samples T-test were used for normally distributed data. The Kruskal–Wallis test and Mann–Whitney U test were used for non-normally distributed data. Differences with a p-value < 0.05 were considered significant.
Acknowledgments
This research was funded by Agency for Innovation by Science and Technology, University Ghent Special Research Fund/Concerted Research Actions (01G02215), and European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant Agreement [648124]). R. Xiong is a postdoctoral fellow of the Research Foundation-Flanders (FWO-Vlaanderen) (1500418N).
Contributor Information
E. Teirlinck, Email: Eline.Teirlinck@ugent.be.
K. Braeckmans, Email: Kevin.Braeckmans@UGent.be.
References
- 1.Sen C.K. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han G., Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34:599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robson M.C. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin N Am. 1997;77:637–650. doi: 10.1016/s0039-6109(05)70572-7. [DOI] [PubMed] [Google Scholar]
- 4.Percival S.L., Thomas J.G., Williams D.W. Biofilms and bacterial imbalances in chronic wounds: anti-Koch. Int Wound J. 2010;7:169–175. doi: 10.1111/j.1742-481X.2010.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjarnsholt T. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 2008;16:2–10. doi: 10.1111/j.1524-475X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 6.Schultz G. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25:744–757. doi: 10.1111/wrr.12590. [DOI] [PubMed] [Google Scholar]
- 7.Percival S.L., Hill K.E., Malic S., Thomas D.W., Williams D.W. Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms. Wound Repair Regen. 2011;19:1–9. doi: 10.1111/j.1524-475X.2010.00651.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Acker H., Van Dijck P., Coenye T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014;22:326–333. doi: 10.1016/j.tim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Stewart P.S. Diffusion in biofilms. J Bacteriol. 2003;185:1485–1491. doi: 10.1128/JB.185.5.1485-1491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart P.S. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol Bioeng. 1998;59:261–272. doi: 10.1002/(sici)1097-0290(19980805)59:3<261::aid-bit1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Forier K. Probing the size limit for nanomedicine penetration into Burkholderia multivorans and Pseudomonas aeruginosa biofilms. J Control Release. 2014;195:21–28. doi: 10.1016/j.jconrel.2014.07.061. [DOI] [PubMed] [Google Scholar]
- 12.Messiaen A.-S., Forier K., Nelis H., Braeckmans K., Coenye T. Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messiaen A.-S., Nelis H., Coenye T. Investigating the role of matrix components in protection of Burkholderia cepacia complex biofilms against tobramycin. J Cyst Fibros. 2014;13:56–62. doi: 10.1016/j.jcf.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Bowler P.G. Antibiotic resistance and biofilm tolerance: a combined threat in the treatment of chronic infections. J Wound Care. 2018;27:273–277. doi: 10.12968/jowc.2018.27.5.273. [DOI] [PubMed] [Google Scholar]
- 15.Clinton A., Carter T. Chronic wound biofilms: pathogenesis and potential therapies. Lab Med. 2015;46:277–284. doi: 10.1309/LMBNSWKUI4JPN7SO. [DOI] [PubMed] [Google Scholar]
- 16.Gawande P.V. Antibiofilm efficacy of DispersinB(®) wound spray used in combination with a silver wound dressing. Microbiol insights. 2014;7:9–13. doi: 10.4137/MBI.S13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das L., Singh Y. Biotechnological Applications of quorum sensing inhibitors 111–126. Springer Singapore; 2018. Quorum sensing inhibition: a target for treating chronic wounds. [DOI] [Google Scholar]
- 18.Brackman G., Coenye T. Quorum sensing inhibitors as anti-biofilm agents. Curr Pharmaceut Des. 2015;21:5–11. doi: 10.2174/1381612820666140905114627. [DOI] [PubMed] [Google Scholar]
- 19.Teirlinck E. Laser-induced vapour nanobubbles improve drug diffusion and efficiency in bacterial biofilms. Nat Commun. 2018;9:4518. doi: 10.1038/s41467-018-06884-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapotko D. Plasmonic nanoparticle-generated photothermal bubbles and their biomedical applications. Nanomedicine. 2009;4:813–845. doi: 10.2217/nnm.09.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong R. Cytosolic delivery of nanolabels prevents their asymmetric inheritance and enables extended quantitative in vivo cell imaging. Nano Lett. 2016;16:5975–5986. doi: 10.1021/acs.nanolett.6b01411. [DOI] [PubMed] [Google Scholar]
- 22.Fleming D., Rumbaugh K. The consequences of biofilm dispersal on the host. Sci Rep. 2018;8:10738. doi: 10.1038/s41598-018-29121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong R. Comparison of gold nanoparticle mediated photoporation: vapor nanobubbles outperform direct heating for delivering macromolecules in live cells. ACS Nano. 2014;8:6288–6296. doi: 10.1021/nn5017742. [DOI] [PubMed] [Google Scholar]
- 24.Serra R. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infect Ther. 2015;13:605–613. doi: 10.1586/14787210.2015.1023291. [DOI] [PubMed] [Google Scholar]
- 25.Jennings M.C., Minbiole K.P.C., Wuest W.M. Quaternary ammonium compounds: an antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect Dis. 2015;1:288–303. doi: 10.1021/acsinfecdis.5b00047. [DOI] [PubMed] [Google Scholar]
- 26.Sritharadol R., Nakpheng T., Wan Sia Heng P., Srichana T. Development of a topical mupirocin spray for antibacterial and wound-healing applications. Drug Dev Ind Pharm. 2017;43:1715–1728. doi: 10.1080/03639045.2017.1339077. [DOI] [PubMed] [Google Scholar]
- 27.Antonov N.K. High prevalence of mupirocin resistance in Staphylococcus aureus isolates from a pediatric population. Antimicrob Agents Chemother. 2015;59:3350–3356. doi: 10.1128/AAC.00079-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koo H., Allan R.N., Howlin R.P., Stoodley P., Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 2017;15:740–755. doi: 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bridier A., Briandet R., Thomas V., Dubois-Brissonnet F. Resistance of bacterial biofilms to disinfectants: a review. Biofouling. 2011;27:1017–1032. doi: 10.1080/08927014.2011.626899. [DOI] [PubMed] [Google Scholar]
- 30.De Beer D., Srinivasan R., Stewart P.S. Direct measurement of chlorine penetration into biofilms during disinfection. Appl Environ Microbiol. 1994;60:4339–4344. doi: 10.1128/aem.60.12.4339-4344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X., Stewart P.S. Chlorine penetration into artificial biofilm is limited by a reaction-diffusion interaction. Environ Sci Technol. 1996;30:2078–2083. [Google Scholar]
- 32.Xue Z., Hessler C.M., Panmanee W., Hassett D.J., Seo Y. Pseudomonas aeruginosa inactivation mechanism is affected by capsular extracellular polymeric substances reactivity with chlorine and monochloramine. FEMS Microbiol Ecol. 2013;83:101–111. doi: 10.1111/j.1574-6941.2012.01453.x. [DOI] [PubMed] [Google Scholar]
- 33.Ganeshnarayan K., Shah S.M., Libera M.R., Santostefano A., Kaplan J.B. Poly-N-Acetylglucosamine matrix polysaccharide impedes fluid convection and transport of the cationic surfactant cetylpyridinium chloride through bacterial biofilms. Appl Environ Microbiol. 2009;75:1308–1314. doi: 10.1128/AEM.01900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming D., Rumbaugh K. Approaches to dispersing medical biofilms. Microorganisms. 2017;5:15. doi: 10.3390/microorganisms5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ammons M.C.B. Anti-biofilm strategies and the need for innovations in wound care. Recent Pat Antiinfect Drug Discov. 2010;5:10–17. doi: 10.2174/157489110790112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming D., Chahin L., Rumbaugh K. Glycoside hydrolases degrade polymicrobial bacterial biofilms in wounds. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01998-16. e01998-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez C.J. D-amino acids enhance the activity of antimicrobials against biofilms of clinical wound isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2014;58:4353–4361. doi: 10.1128/AAC.02468-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omar A., Wright J., Schultz G., Burrell R., Nadworny P. Microbial biofilms and chronic wounds. Microorganisms. 2017;5:9. doi: 10.3390/microorganisms5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sass A. Various evolutionary trajectories lead to loss of the tobramycin-potentiating activity of the quorum sensing inhibitor baicalin hydrate in Burkholderia cenocepacia biofilms. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.02092-18. e02092-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolcott R.D., Kennedy J.P., Dowd S.E. Regular debridement is the main tool for maintaining a healthy wound bed in most chronic wounds. J Wound Care. 2009;18:54–56. doi: 10.12968/jowc.2009.18.2.38743. [DOI] [PubMed] [Google Scholar]
- 41.Chang Y.-J.R., Perry J., Cross K. Low-frequency ultrasound debridement in chronic wound healing: a systematic review of current evidence. Plast Surg. 2017;25:21–26. doi: 10.1177/2292550317693813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francis N.C., Yao W., Grundfest W.S., Taylor Z.D. Laser-generated shockwaves as a treatment to reduce bacterial load and disrupt biofilm. IEEE Trans Biomed Eng. 2017;64:882–889. doi: 10.1109/TBME.2016.2581778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gnanadhas D.P. Successful treatment of biofilm infections using shock waves combined with antibiotic therapy. Sci. Rep. 2015;5:17440. doi: 10.1038/srep17440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong R. Fast spatial-selective delivery into live cells. J Control Release. 2017;266:198–204. doi: 10.1016/j.jconrel.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 45.Xiong R. Selective labeling of individual neurons in dense cultured networks with nanoparticle-enhanced photoporation. Front Cell Neurosci. 2018;12:80. doi: 10.3389/fncel.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplan J.B. Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. J Dent Res. 2010;89:205–218. doi: 10.1177/0022034509359403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Husain Z., Alster T.S. The role of lasers and intense pulsed light technology in dermatology. Clin. Cosmet Investig Dermatol. 2016;9:29–40. doi: 10.2147/CCID.S69106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung K.-P. Control of oral biofilm formation by an antimicrobial decapeptide. J Dent Res. 2005;84:1172–1177. doi: 10.1177/154405910508401215. [DOI] [PubMed] [Google Scholar]
- 49.Bigliardi P.L. Povidone iodine in wound healing: a review of current concepts and practices. Int. J Surg. 2017;44:260–268. doi: 10.1016/j.ijsu.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 50.Bigliardi P. An asian perspective on povidone iodine in wound healing. Dermatology. 2017;233:223–233. doi: 10.1159/000479150. [DOI] [PubMed] [Google Scholar]
- 51.Tseng B.S. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ Microbiol. 2013;15:2865–2878. doi: 10.1111/1462-2920.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takenaka S., Trivedi H.M., Corbin A., Pitts B., Stewart P.S. Direct visualization of spatial and temporal patterns of antimicrobial action within model oral biofilms. Appl Environ Microbiol. 2008;74:1869–1875. doi: 10.1128/AEM.02218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen Y. Experimental and theoretical investigation of multispecies oral biofilm resistance to chlorhexidine treatment. Sci Rep. 2016;6:27537. doi: 10.1038/srep27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen Y., Stojicic S., Haapasalo M. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J Endod. 2011;37:657–661. doi: 10.1016/j.joen.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 55.He Y. Stress relaxation analysis facilitates a quantitative approach towards antimicrobial penetration into biofilms. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corbin A., Pitts B., Parker A., Stewart P.S. Antimicrobial penetration and efficacy in an in vitro oral biofilm model. Antimicrob Agents Chemother. 2011;55:3338–3344. doi: 10.1128/AAC.00206-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hope C.K., Wilson M. Analysis of the effects of chlorhexidine on oral biofilm vitality and structure based on viability profiling and an indicator of membrane integrity. Antimicrob Agents Chemother. 2004;48:1461–1468. doi: 10.1128/AAC.48.5.1461-1468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandt C., Barbeau J., Gagnon M.-A., Lafleur M. Role of the ammonium group in the diffusion of quaternary ammonium compounds in Streptococcus mutans biofilms. J Antimicrob Chemother. 2007;60:1281–1287. doi: 10.1093/jac/dkm382. [DOI] [PubMed] [Google Scholar]
- 59.Stewart P.S. Antimicrobial tolerance in biofilms. Microbiol Spectr. 2015;3:10–1128. doi: 10.1128/microbiolspec.MB-0010-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bridier A., Dubois-Brissonnet F., Greub G., Thomas V., Briandet R. Dynamics of the action of biocides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2011;55:2648–2654. doi: 10.1128/AAC.01760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chambers J.R., Cherny K.E., Sauer K. Susceptibility of Pseudomonas aeruginosa dispersed cells to antimicrobial agents is dependent on the dispersion cue and class of the antimicrobial agent used. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00846-17. e00846-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campanac C., Pineau L., Payard A., Baziard-Mouysset G., Roques C. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob Agents Chemother. 2002;46:1469–1474. doi: 10.1128/AAC.46.5.1469-1474.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalton T. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brackman G. Dressings loaded with cyclodextrin-hamamelitannin complexes increase Staphylococcus aureus susceptibility toward antibiotics both in single as well as in mixed biofilm communities. Macromol Biosci. 2016;16:859–869. doi: 10.1002/mabi.201500437. [DOI] [PubMed] [Google Scholar]