Abstract
Background
During the coronavirus disease 2019 (COVID-19) outbreaks, health care workers (HCWs) are at a high risk of infection. Strategies to reduce in-hospital transmission between HCWs and to safely manage infected HCWs are lacking. Our aim was to describe an active strategy for the management of COVID-19 in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–infected HCWs and investigate its outcomes.
Methods
A prospective cohort study of SARS-CoV-2-infected health care workers in a tertiary teaching hospital in Barcelona, Spain, was performed. An active strategy of weekly polymerase chain reaction screening of HCWs for SARS-CoV-2 was established by the Occupational Health department. Every positive HCW was admitted to the Hospital at Home Unit with daily assessment online and in-person discretionary visits. Clinical and epidemiological data were recorded.
Results
Of the 590 HCWs included in the cohort, 134 (22%) were asymptomatic at diagnosis, and 15% (89 patients) remained asymptomatic during follow-up. A third of positive cases were detected during routine screening. The most frequent symptoms were cough (68%), hyposmia/anosmia (49%), and fever (41%). Ten percent of the patients required specific treatment at home, while only 4% of the patients developed pneumonia. Seventeen patients required a visit to the outpatient clinic for further evaluation, and 6 of these (1%) required hospital admission. None of the HCWs included in this cohort required intensive care unit admission or died.
Conclusions
Active screening for SARS-CoV-2 among HCWs for early diagnosis and stopping in-hospital transmission chains proved efficacious in our institution, particularly due to the high percentage of asymptomatic HCWs. Follow-up of HCWs in Hospital at Home units is safe and effective, with low rates of severe infection and readmission.
Keywords: coronavirus, COVID-19, health care workers, Hospital at Home, SARS-CoV-2
Since its outbreak in Wuhan, China, in December 2019, the novel coronavirus disease 2019 (COVID-19) pandemic has reached more than 47 million infected individuals and caused 1.2 million deaths worldwide [1]. The rapid spread of the virus and the severity of the disease have been challenging to health systems globally. Alternatives to conventional hospitalization, such as home care in Hospital at Home (HaH) units or other outpatient resources have previously been proposed to manage the risk of health system collapse in other high-demand situations [2, 3].
HCWs, defined as any paid professional involved in the care of patients, are at high health risk during pandemics [4]. Risks observed by the Prevention Service, and also acknowledged by the World Health Organization, include pathogen exposure, long working hours, psychological distress, fatigue, occupational burnout, stigma, and physical and psychological violence [5]. HCW nosocomial infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been widely reported to be highly prevalent in countries such as the United States, China, or Italy [6–9]. In Spain, nearly 41 000 HCWs were infected during the first wave, with 53 fatalities [10]. In many cases, personal protection equipment shortage was an issue, greatly endangering professionals on the front line of care provision. Different strategies for control and surveillance of health workers at risk have been proposed by international organisations; however, no global strategy has been defined for caring for infected health care workers.
Our aim in this study is to describe the characteristics and outcomes of an active strategy of COVID-19 management of infected HCWs in a HaH Unit in coordination with the Human Resources and Occupational Health departments in the Hospital Clinic Barcelona, Spain.
METHODS
Design
This was a prospective cohort study including HCWs with a confirmed SARS-CoV-2 infection by real-time polymerase chain reaction (rt-PCR) on nasopharyngeal swabs between March 9 and May 4, 2020.
Setting
The Hospital Clinic is a public tertiary teaching hospital with 6403 health care workers, serving 560 000 people in the metropolitan area of Barcelona, Spain. The Hospital Clinic’s Hospital at Home Unit (HaH) started providing hospital-level specialized health care to patients at home in 1996 [11]. Care of infected HCWs during HaH admission included daily virtual medical and nurse visits (phone and video calls), a round-the-clock call center, medical and nurse visits at home on demand, blood tests, and transfer to an adapted outpatient hospital facility for either further tests (eg, chest x-ray) or planned conventional hospitalization if required. Patients were discharged from HaH a week after symptom remission, with guidelines for re-consulting in case of clinical deterioration. Working reinstatement was decided by the Human Resources and Work Health department according to government protocols.
Participants
Per hospital policy, weekly control rt-PCR was performed by the Prevention Service on every HCW working in COVID-19 areas in our hospital. Additional rt-PCRs were performed on all HCWs self-reporting symptoms compatible with COVID-19. All HCWs from the Hospital Clinic Barcelona with a positive rt-PCR were admitted to the HaH unit. Only those HCWs with baseline comorbidities or showing moderate symptoms underwent chest x-ray and blood tests at the time of admission. Active protocols at the time were followed to provide specific COVID-19 treatment. All patients initiating treatment underwent an electrocardiogram to rule out QT segment abnormalities to prevent potential pro-arrhythmic effects associated with antiviral treatment.
Patients were followed up in the HaH unit until symptom remission. Those who remained asymptomatic had a minimum follow-up of 1 week.
Patient Consent Statement
The study protocol was evaluated and approved by the Ethical Board of the Hospital Clinic (HCB/2020/0444). A waiver for informed consent was provided due to the state of infectious disease emergency. Admission to the Hospital at Home program was voluntary, as was every medical procedure performed.
Statistical Analysis
Categorical variables collected included sex, type of HCW occupation (administration, maintenance, nurses, physicians, other), active smoking habit, comorbidities (dyslipidemia, hypertension, chronic lung disease, ischemic heart disease, chronic kidney disease, neoplasm), reason for performing nasopharyngeal swab for SARS-CoV-2 infection diagnosis through rt-PCR (routine, high-risk contact, symptoms, unknown), presence of symptoms at diagnosis, presence of symptoms during follow-up, type of symptoms (cough, fever, dyspnea, hyposmia/anosmia, dysgeusia, gastrointestinal symptoms including diarrhea, and abdominal pain), performance of blood analysis, performance of chest x-ray, radiological findings (normal, unilateral interstitial infiltrates, bilateral infiltrates), administration of antiviral treatment (lopinavir/ritonavir, hydroxychloroquine, azithromycin, remdesivir, tocilizumab), treatment received at home, treatment withdrawal, oxygen requirements at home, outpatient clinic visit, readmission to hospital, admission to intermediate care/intensive care unit (ICU), mechanical ventilation (invasive or noninvasive), and death. Continuous variables included number of HCW admissions per day in the HaH, age, time from symptom initiation to diagnosis, time from diagnosis to symptom initiation in asymptomatic patients at diagnosis, serum C-reactive protein concentration, procalcitonin, lymphocyte count, lactate dehydrogenase, d-dimer, ferritin, aspartate aminotransferase, alanine aminotransferase, gammaglutamyl transferase, alkaline phosphatase, bilirubin, and length of stay at HaH. Categorical variables are reported as percentages and continuous variables as medians and interquartile ranges (IQRs). Data were analyzed with SPSS, version 21.0.
RESULTS
A total of 590 HCWs (9.21% of the institution’s total staff) tested positive for COVID-19 during the study period and were therefore admitted to the HaH (Figure 1). Patient characteristics are summarized in Table 1. Patients were predominantly female (76%) with a median age of 40 years. A small proportion of patients in our cohort presented comorbidities, including hypertension in 4.6% and dyslipidemia in 3.7%. Notably, 6% had a history of chronic lung disease (mainly asthma). The largest professional group was composed of nursing staff, who accounted for 34% of HCWs, followed by nurse aide assistants (25%) and physicians (18%). Almost a third (32%) of the positive cases were detected by routine screening, while nearly half of the positive tests (49%) were performed in HCWs actively seeking attention due to self-reported symptoms.
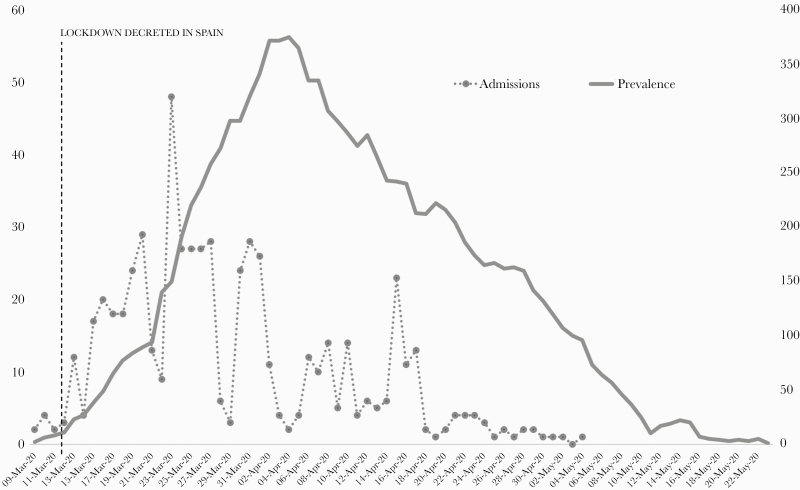
Figure 1.
Timeline of admissions (total numbers, right vertical axis) and prevalence (percentage over total number of admissions per day, left axis) of positive health care workers in the Hospital at Home unit.
Table 1.
Baseline Characteristics of 590 Health Care Workers With COVID-19 Admitted to the Hospital at Home During the Study Period
| Baseline Characteristics | |
|---|---|
| Age, median (IQR), y | 40 (30–50) |
| Male sex, No. (%) | 141 (24) |
| Occupation, No. (%) | |
| - Administration | 34 (5.8) |
| - Maintenance | 13 (2.2) |
| - Certified Nurse Assistants | 150 (25.4) |
| - Nurses | 199 (33.7) |
| - Physicians | 107 (18.1) |
| - Other | 33 (5.6) |
| Current smoking habit, No. (%) | 26 (4.4) |
| Dyslipidemia, No. (%) | 22 (3.7) |
| Hypertension, No. (%) | 27 (4.6) |
| Chronic lung disease, No. (%) | 34 (5.8) |
| Ischemic heart disease, No. (%) | 4 (0.7) |
| Chronic kidney disease, No. (%) | 3 (0.5) |
| Neoplasm, No. (%) | 16 (2.7) |
| Characteristics of the infection | |
| Reason for performing nasopharyngeal swab, No. (%) | |
| - Routine | 189 (32) |
| - High-risk contact | 10 (1.7) |
| - Symptoms | 293 (49.5) |
| - Unknown | 98 (16.6) |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range.
Clinical Features
Globally, 77% COVID-19-positive patients reported some symptom when specifically asked in the first assessment. The mean time from showing symptoms to diagnosis was 4.16 days. The most frequent symptom reported in this group of patients was cough (222 patients, 48.8%), followed by fever (156 patients, 34%), hyposmia/anosmia (10.5%), dyspnea (17 patients, 4%), gastrointestinal symptoms (diarrhea; 13 patients, 3%), and dysgeusia (5 patients, 1.1%). The most frequent combination of symptoms was fever and cough, present in 16.5% of the patients at the time of diagnosis (Table 2).
Table 2.
Clinical Features of 590 Health Care Workers With COVID-19 Admitted to the Hospital at Home During the Study Period
| Clinical Features | |
|---|---|
| Symptomatic at diagnosis, No. (%) | 455 (77.1) |
| Time from symptom initiation to diagnosis, mean (IQR), d | 4.16 (1–5) |
| - Cough | 222 (48.8) |
| - Fever | 156 (34.3) |
| - Dyspnea | 17 (3.7) |
| - Hyposmia/anosmia | 48 (10.5) |
| - Dysgeusia | 5 (1.1) |
| - Gastrointestinal symptoms | 13 (2.9) |
| - Fever + cough | 75 (16.5) |
| - Fever + dyspnea | 7 (1.5) |
| - Fever + hyposmia | 6 (1.3) |
| - Fever + gastrointestinal symptoms | 4 (0.9) |
| Asymptomatic at diagnosis, No. (%) | 135 (22.9) |
| Never developed symptoms during follow-up | 89 (15.1) |
| Time from diagnosis to symptom initiation, mean (IQR), d | 2.59 (1.25–3) |
| Symptoms at any point of follow-up, No. (%) | 501 (84.9) |
| - Cough | 342 (68.3) |
| - Fever | 207 (41.3) |
| - Dyspnea | 108 (21.6) |
| - Hyposmia/anosmia | 246 (49.1) |
| - Dysgeusia | 222 (44.3) |
| - Gastrointestinal symptoms | 155 (30.9) |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range.
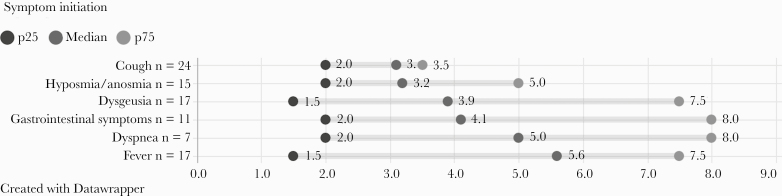
One hundred thirty-four patients (22%) in the cohort were asymptomatic at the time of diagnosis. Of these, 89 (66.4%, 15% of the total cohort) did not develop symptoms at any time during follow-up. The remaining 45 patients (33.6%; 7.6% overall) had at least 1 symptom during follow-up. The median time from positive PCR to having symptoms (IQR) was 2.6 (1.25–3) days in these patients. The median time to each of the symptoms is presented in Figure 2. When comparing baseline characteristics (age, comorbidities, occupation) between symptomatic and asymptomatic patients, we did not find any differences (data not shown).
Figure 2.
Symptom onset diagram in patients who were asymptomatic at diagnosis who presented symptoms during follow-up (days from positive polymerase chain reaction to symptom manifestation; n = 46).
Outcomes
The median length of stay in HaH (IQR) was 15 (12–19) days. Only 47 (8%) patients underwent blood analysis (C Reactive Protein, basic metabolic panel, complete blood count, ferritin, D-dimer, procalcitonin). The main blood analysis findings are depicted in Table 3. Regarding blood analysis, patients had low median levels of C-reactive protein (3.07 mg/dL), ferritin (330 ng/mL), D-dimer level (550 ng/mL), and procalcitonin (0.14 ng/mL). Notably, no concomitant bacterial infections or superinfections were detected. Nine percent (n = 55) of the patients required a chest x-ray; abnormalities were found in 63% of these, with bilateral infiltrates in 20 patients (36% of the x-ray performed). Ten percent of the HCWs started treatment at home. All of these received a combination of hydroxychloroquine and azithromycin, whereas just a third (31%) started lopinavir/ritonavir treatment. Seven patients (1.2%) required oxygen treatment at home.
Table 3.
Clinical Assessment and Outcomes of 590 Health Care Workers With COVID-19 Admitted to the Hospital at Home During the Study Period
| Blood Analysis Results | |
|---|---|
| Blood analysis performed, No. (%) | 47 (8) |
| C-reactive protein, median (IQR), mg/dL | 3.07 (0.4–4.82) |
| Procalcitonin, median (IQR), ng/mL | 0.14 (0.03–0.78) |
| Lymphocytes, median (IQR), per 106/L | 810 (220–1325) |
| Lactate dehydrogenase, median (IQR), U/L | 218 (168–262) |
| D-dimer, median (IQR), U/L | 550 (200–550) |
| Ferritin, median (IQR), ng/mL | 330 (132–405) |
| Aspartate aminotransferase, median (IQR), U/L | 29 (20–37) |
| Alanine aminotransferase, median (IQR), U/L | 27 (16–34) |
| Gammaglutamyl transferase, median (IQR), U/L | 32 (15–41) |
| Alkaline fosfatase, median (IQR), U/L | 63 (49–72) |
| Total bilirubin, median (IQR), mg/dL | 0.49 (0.4–0.6) |
| Radiological findings, No. (%) | |
| Chest x-ray performed | 55 (9.3) |
| Normal chest x-ray | 26 (47.3) |
| Unilateral interstitial infiltrates | 4 (7.3) |
| Bilateral infiltrates | 20 (36.4) |
| Treatment characteristics, No. (%) | |
| Received treatment at home | 61 (10.3) |
| Lopinavir/ritonavir | 19 (31.1) |
| Hydroxicloroquine | 59 (96.7) |
| Azithromycin | 60 (98.4) |
| Remdesivir | 3 (4.9) |
| Tocilizumab | 4 (6.5) |
| Treatment withdrawal | 4 (6.5) |
| Oxygen requirements at home | 7 (1.2) |
| Outcomes | |
| Total HaH length of stay, median (IQR), d | 15 (12–19) |
| Outpatient clinic visit | 17 (2.9) |
| Readmission to hospital | 6 (1) |
| Admission to intermediate care/ICU | 0 |
| Invasive or noninvasive mechanical ventilation | 0 |
| Death | 0 |
Abbreviations: COVID-19, coronavirus disease 2019; HaH, Hospital at Home; ICU, intensive care unit; IQR, interquartile range.
Seventeen patients (2.9%) required hospital assessment during HaH stay, and only 6 patients (1%) required hospital admission. Of these 6 patients, 4 received anti-IL6 treatment with tocilizumab, and 3 (4.9%) received antiviral treatment with remdesivir. None of the patients required ICU admission or mechanical ventilation. None of the patients died.
DISCUSSION
During the first wave of COVID-19 in Spain, 590 HCWs were infected by SARS-CoV-2 in our institution, representing 9.21% of the overall Hospital Clinic Barcelona staff and 6.7% of the total of COVID-19 patients diagnosed in the hospital in the same period (n = 8768). In Italy, 26 675 HCWs were reported positive, accounting for 11.7% of the total confirmed cases in Italy [6]. In the United States, among 315 531 COVID-19 cases reported to the Centers for Disease Control and Prevention (CDC) from February 12 to April 9, 2020, 9282 (19%) were identified as HCWs [8]. In Spain, 41 000 HCWs were reported to be infected on April 16, 24% of total cases [10]. However, variations in population-testing strategies within health systems and pandemic timings must be considered when interpreting these data. In Spain, this percentage is probably overestimated, as testing was prioritized in HCWs. It was carried out irregularly in the general population, depending on setting and test availability. It is interesting to point out that 76% of the infected HCWs were female workers, reflecting the higher proportion of women in our center (72% of the workers).
In our institution, both technical actions (assessment of protection equipment, training in occupational risk prevention, action protocols) and health actions (active and passive surveillance of the population) were carried out in order to guarantee the safety and health of HCWs. Weekly screening of SARS-CoV-2 in every HCW was established in order to minimize transmission between HCWs or from HCWs to patients. Also, PCR screening was performed in those HCWs self-reporting symptoms. This strategy has been perceived as positive among HCWs, as it brings confidence and ease regarding reduction in the risk of transmission to relatives outside the hospital. From a public health point of view, this strategy allowed our center to detect and separate 590 HCWs from the system into sick leave, preventing potential horizontal transmission. It is interesting to note that 32% of these PCRs were performed as screening on asymptomatic workers who had symptoms only 2.59 days after a positive PCR. Moreover, 22% never had any symptoms, suggesting that active screening for SARS-CoV-2 between HCWs may be fundamental in the early detection of asymptomatic but potentially contagious HCWs and therefore stopping in-hospital transmission chains. When analyzing the temporal trends in the number of infected HCWs, it is noticeable that the peak of incidence occurs within the first 14 days of confinement (Figure 1), suggesting that a great burden of infection has an extrahospital origin, mitigated afterwards by mobility and social restrictions, as well as in-hospital preventive measures.
From a clinical standpoint, our data are comparable to previous studies describing the clinical features of COVID-19 [9]. The most frequent clinical presentation of COVID-19 in those symptomatic at diagnosis was cough (49%), followed by fever (34%) and hyposmia (10.5%). Other symptoms were less frequently reported, such as dyspnea in <4%, gastrointestinal symptoms (mainly diarrhea) in 3%, and dysgeusia in only 1%. It is interesting to point out that a vast majority (84.9%) of the HCWs in our cohort developed symptoms at some point during follow-up, with cough being the most frequent (68%), followed by fever (41%). Symptoms less frequently reported at diagnosis appeared later in the course of the disease, such as hypo/anosmia in half of the patients, dysgeusia in 44%, and gastrointestinal symptoms in 30%.
It is of great interest to point out that 15% of our cohort never developed symptoms of COVID-19. To date, only small studies have reported data regarding the frequency of asymptomatic COVID-19 infection, with results ranging between 18% and 88% [12, 13].
Overall, the clinical course was benign in our cohort. Only 10% of our patients required antiviral treatment following local protocols. Less than 3% of the patients required in-hospital admission, and a marginal 1% required readmission to the hospital. None of the patients required ICU admission or died, in contrast to other cohorts of HCWs such as those reported by the CDC in the United States, where 2% required ICU admission and 0.3% died [8]. This can be explained by an evident selection bias, but also attributed to a relatively healthy and young cohort and to the active surveillance provided by close monitorization in the Hospital at Home unit.
Our study has 2 main limitations. First, data regarding the origin of infection were scarce, and in most cases, it was impossible to determine. Second, our cohort included only patients with a mild to moderate clinical course who may have not provided epidemiological data in a different setting, but these data cannot be extrapolated to severe COVID-19 patients. These data could serve to better depict the epidemiology and clinical evolution in nonsevere patients and provide a greater understanding of the disease.
In conclusion, during global challenges to health systems such as the actual COVID-19 pandemic, HCWs are at great risk of biohazard exposure due to increased demand for health assistance, compounded by the difficulties in guaranteeing adequate provision of protective equipment. According to our data, asymptomatic infection is as frequent as 15%; therefore an active strategy of SARS-CoV-2 screening is critical to stopping in-hospital transmission. Close monitoring of clinical status and early detection of complications are critical in the follow-up of HCWs with COVID-19. In the context of the current COVID-19 pandemic, an active strategy of monitoring and treatment at home by Hospital at Home units seems to be safe and efficacious alternatives to self-monitoring or conventional hospital admission.
Acknowledgments
Members of the Hospital Clinic Hospital at Home team. Gemma Martinez, Antoni Castells (Nursing and Medical Direction), Faust Feu, Roser Cadenas (Quality and Clinical Safety Direction), Pilar Varela, Sonia Barroso, Marta Tortajada, Victoria Olive (Direcció per a les Persones), David Nicolás, Emmanuel Coloma, Marta Bodro, Celia Cardozo, Juan M Pericás, Júlia Calvo, Alfons López-Soto, Álex Soriano, Josep M Nicolás (Institut Clinic de Medicina i Dermatologia), Sara Llufriu, Gerard Anmella (Institut Clinic de Neurociències), Anna Camos, Felipe Spencer, Lucía Escudero, Marina Dotti, M Teresa Carrión, Valeria Opazo, Alba Parrado, Joan Giralt, Carolina Bernal, Barbara Romero, Clàudia Boquera, Miriam Sánchez, Silvia Feu, Anna Casablanca, Ana Cayado, Xavier Carreras, J Pablo Figueroa, Sara Marín, Rafa Castro, Cristian Oliva (Institut Clinic d’Oftalmologia), Andrea Arenas, Eugenia Butori, Orla Torrallardona, Pol Maymo, Eduarda Alves, Laura García, Irene Pereta, Eva Castells, Nuria Seijas, Begoña Ibáñez, Carme Grané, Carme Hernández, Jùlia Serralabós, Elisenda Alvès, Neus Rabaneda, Judit Hidalgo, Maribel Avalos, Anna Carbonell, Núria Subirana, Regina Navas, Carmen Aranda, Magali Rodríguez, Marta Salas, Adolfo Suárez, Ana Fernández, Alba Martínez, Ariadna Barta, Cristina Escobar, Laura Moreno, Mohammed Jawara, Susana Cano, Mariana Román, Maria Martinez, David Jiménez, Elisabeth Rosero, Mariana Román, Lourdes Llop, Maria Asenjo (Hospital at Home Unit); all from Hospital Clínic de Barcelona, Barcelona, Spain.
Financial support. This work did not receive any specific funding.
Potential conflicts of interest. The authors declare that they have no relevant or material financial interests that relate to the research described in this paper. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. D.N. and J.M.P. designed the study; F.S., A.A., A.C., E.B., G.A., and P.M. systematically collected the data; J.M.P. and D.N. obtained permissions; J.M.P. and D.C. performed the statistical analyses and drafted the tables; D.N.O. and O.T.-M. wrote the first draft of the manuscript; all other authors contributed by gathering data, critically revising the manuscript, and providing final approval.
Contributor Information
Hospital Clinic Hospital at Home Team:
Gemma Martinez, Antoni Castells, Faust Feu, Roser Cadenas, Pilar Varela, Sonia Barroso, Marta Tortajada, Victoria Olive, David Nicolás, Emmanuel Coloma, Marta Bodro, Celia Cardozo, Juan M Pericás, Júlia Calvo, Alfons López-Soto, Álex Soriano, Josep M Nicolás, Sara Llufriu, Gerard Anmella, Anna Camos, Felipe Spencer, Lucía Escudero, Marina Dotti, M Teresa Carrión, Valeria Opazo, Alba Parrado, Joan Giralt, Carolina Bernal, Barbara Romero, Clàudia Boquera, Miriam Sánchez, Silvia Feu, Anna Casablanca, Ana Cayado, Xavier Carreras, J Pablo Figueroa, Sara Marín, Rafa Castro, Cristian Oliva, Andrea Arenas, Eugenia Butori, Orla Torrallardona, Pol Maymo, Eduarda Alves, Laura García, Irene Pereta, Eva Castells, Nuria Seijas, Begoña Ibáñez, Carme Grané, Carme Hernández, Jùlia Serralabós, Elisenda Alvès, Neus Rabaneda, Judit Hidalgo, Maribel Avalos, Anna Carbonell, Núria Subirana, Regina Navas, Carmen Aranda, Magali Rodríguez, Marta Salas, Adolfo Suárez, Ana Fernández, Alba Martínez, Ariadna Barta, Cristina Escobar, Laura Moreno, Mohammed Jawara, Susana Cano, Mariana Román, Maria Martinez, David Jiménez, Elisabeth Rosero, Mariana Román, Lourdes Llop, and Maria Asenjo
References
- 1. Johns Hopkins University. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html. Accessed 4 November 2020.
- 2. Pericás JM, Aibar J, Soler N, et al. Should alternatives to conventional hospitalisation be promoted in an era of financial constraint? Eur J Clin Invest 2013; 43:602–15. [DOI] [PubMed] [Google Scholar]
- 3. Cardozo C, Nicolás D, Bodro M, et al. Influenza at hospital at home: a safe option during epidemic season. Paper presented at: World Hospital at Home Congress; 5-6 April, 2019; Madrid, Spain. [Google Scholar]
- 4. Centers for Disease Control and Prevention (CDC). Interim U.S. guidance for risk assessment and work restrictions for health care personnel with potential exposure to COVID-19. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.Accessed 5 August 2020.
- 5. World Health Organization. Coronavirus disease (COVID-19) outbreak: rights, roles and responsibilities of health workers, including key considerations for occupational safety and health. 2020. Available at: https://apps.who.int/iris/rest/bitstreams/1272583/retrieve.
- 6. Epicentro. Istituto Superiore de Sanità. Integrated Surveillance of COVID-19 in Italy. Available at: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-dashboard. Accessed 5 August 2020. [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention (CDC). Characteristics of health care personnel with COVID-19—United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Red Nacional de Vigilancia Epidemiológica. Informe sobre la situación de COVID-19 en España. Informe COVID-19 no 322020. Available at: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/InformesCOVID-19/Informenº32.SituacióndeCOVID-19enEspañaa21demayode2020.pdf. Accessed 7 August 2020.
- 11. Hernández C, Aibar J, Seijas N, et al. Implementation of home hospitalization and early discharge as an integrated care service: a ten years pragmatic assessment. Int J Integr Care 2018; 18:12. doi: 10.5334/ijic.3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020; 382:2163–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. EuroSurveill 2020; 25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180.. [DOI] [PMC free article] [PubMed] [Google Scholar]