Abstract
We studied the pattern and duration of viral ribonucleic acid (RNA) shedding in 32 asymptomatic and 11 paucisymptomatic coronavirus disease 2019 cases. Viral RNA shedding in exhaled breath progressively diminished and became negative after 6 days of a positive reverse-transcription polymerase chain reaction test. Therefore, the duration of isolation can be minimized to 6 days.
Keywords: asymptomatic, COVID-19, cycle threshold paucisymptomatic, SARS-CoV-2, viral shedding
The transmission dynamics of coronavirus disease 2019 (COVID-19) has been extensively studied in symptomatic individuals. However, as the outbreak progressed, it became evident that many cases are asymptomatic but have the same infectivity rate as that of symptomatic infections [1, 2]. Several studies have shown that the chances of asymptomatic infections are increased in crowded and family-clustered settings [3, 4], which is a matter of concern for densely populated countries where isolation is difficult to achieve. Viral load is another contributory factor determining infectivity [5, 6]. Studies have shown that viral load in asymptomatic individuals can be the same as symptomatic patients, indicating that people without obvious symptoms can potentially transmit the infection [7]. The transmission dynamics of COVID-19 in asymptomatic and paucisymptomatic individuals is poorly understood. The present study aimed to analyze the pattern, duration, and trends of viral ribonucleic acid (RNA) shedding in nasopharyngeal (NP) secretions and exhaled breath of asymptomatic and paucisymptomatic secondary cases of COVID-19. These findings might help in strengthening the effectiveness of COVID-19 control measures and thus limit the spread of the disease.
METHODS
This was a prospective observational time-to-event study in which 44 participants, above 18 years of age, who had known history of contact with symptomatic COVID-19 cases and tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were enrolled. They were traced within 24 hours of contact and tested by reverse-transcription polymerase chain reaction (RT-PCR). Accordingly, the day of contact was considered as Day 0 and the day of positive RT-PCR test result was considered as Day 1. All positive contacts were isolated in a designated community quarantine facility and carefully monitored until 2 consecutive negative RT-PCR results were obtained at least 24 hours apart. Any participant who developed symptomatic illness during the period was shifted to the COVID-19 ward of All India Institute of Medical Sciences, Jodhpur, and excluded from the study. Written informed consent was obtained from each participant after approval by the institute’s Ethics Committee (Ref. no. AIIMS/IEC/2020-21/3034), and it conforms to standards currently applied in India. The cases were classified as asymptomatic and paucisymptomatic based on the definitions set by the World Health Organization [8] and Rivett et al [9] (see Supplementary Table 1 for more details on case definitions). The participants were instructed to wear a single mask for the whole day. Nasopharyngeal swab and corresponding day’s mask were collected from each participant and tested for SARS-CoV-2 RNA by RT-PCR, using primer and probe sequences described by Corman et al [10]. Collection and transport of specimens and PCR protocol are described in detail in the Supplementary Data.
The cycle threshold (Ct) values for NP swabs and masks of respective days were calculated and analyzed using test of normality by Kolmogorov-Smirnov and Shapiro-Wilk tests. Because all data were not found to follow normal distribution, non-parametric tests were used. For comparison of repeated measures, Friedman χ 2 test was used to test the significance between the Ct values over the days. Furthermore, to observe the significance between 2 paired data, Wilcoxon-Mann-Whitney rank test was used and P < .01 was used as level of significance. The association between the median Ct values was calculated using Spearman rank correlation coefficient. Statistical analysis was performed using IBM SPSS version 20.0 (IBM Corp., Armonk, NY).
RESULTS
Of 44 participants, 32 (73%) were asymptomatic and 11 (25%) were paucisymptomatic. One participant turned symptomatic during the course of study and was excluded from evaluation. Thirty-six (82%) cases were males and 8 (18%) were females. Asymptomatic and paucisymptomatic cases were more common in <50-year-old age group comprising 72% of the total cases (42% in 30- to 50-year-old age group followed by 30% in 18- to 30-year-old age group), whereas 16% and 12% of the cases belonged to 50- to 60-year-old and >60-year-old age groups, respectively. In paucisymptomatic cases, nonpersistent cough was the most common symptom (8 of 11, 73%). Other symptoms included sore throat, myalgia, headache, respiratory distress, and fatigue. Thirteen (30%) patients had underlying comorbidities, such as hypertension (9 of 43; 21%), type 2 diabetes mellitus (5 of 43; 12%) and asthma, ischemic heart disease, and cholelithiasis (1 of 43; 2% each). The comorbid conditions were more commonly observed in males (12 of 13; 92%) than in females (1 of 13; 8%). Asymptomatic infection was documented in 3 pregnant women.
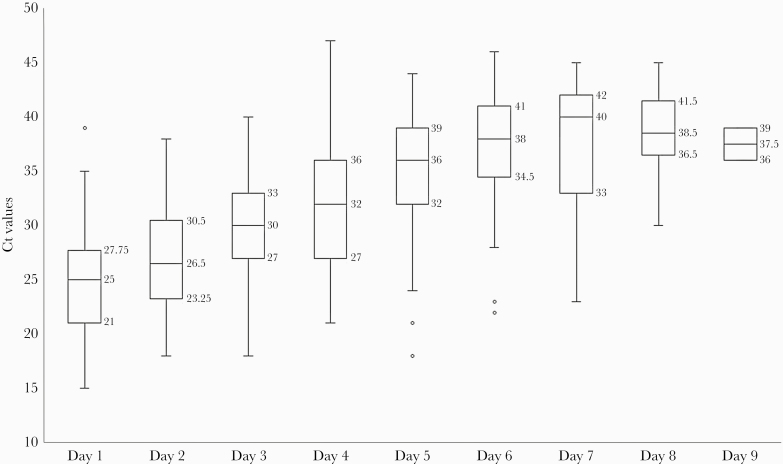
Viral RNA shedding in NP swabs progressively diminished from Day 1 through Day 9, as evidenced by an increase in the median Ct values in RT-PCR. The overall Ct values over the days were compared and found to be statistically significant (Friedman χ 2 = 133.42, P < .001). The Ct values for different days were compared using Wilcoxon signed-rank test. The median Ct value was 25 (Q1 = 21, Q3 = 27.75) on Day 1, which increased to 30 (Q1 = 27, Q3 = 33) on Day 3 (P < .001), 32 (Q1 = 27, Q3 = 36) on Day 4 (P < .002), 36 (Q1 = 32, Q3 = 39) on Day 5 (P < .001), and 38 (Q1 = 34.5, Q3 = 41) on Day 6 (P < .004). Thereafter, it increased to 40 (Q1 = 33, Q3 = 42) on Day 7 but was not significant (P = .170). The NP swabs of none of the participants tested positive after Day 9 of recruitment (Figure 1).
Figure 1.
Day-wise distribution of viral ribonucleic acid (RNA) shedding in nasopharyngeal (NP) swabs in asymptomatic and paucisymptomatic coronavirus disease 2019 cases. Viral RNA shedding progressively diminished from Day 1 through Day 9, as evidenced by an increase in median cycle threshold (Ct) values in reverse-transcription polymerase chain reaction. The NP swabs of none of the participants tested positive after Day 9 of recruitment. Comparison between the median Ct values over the days was statistically significant (χ 2 = 133.42, P < .001).
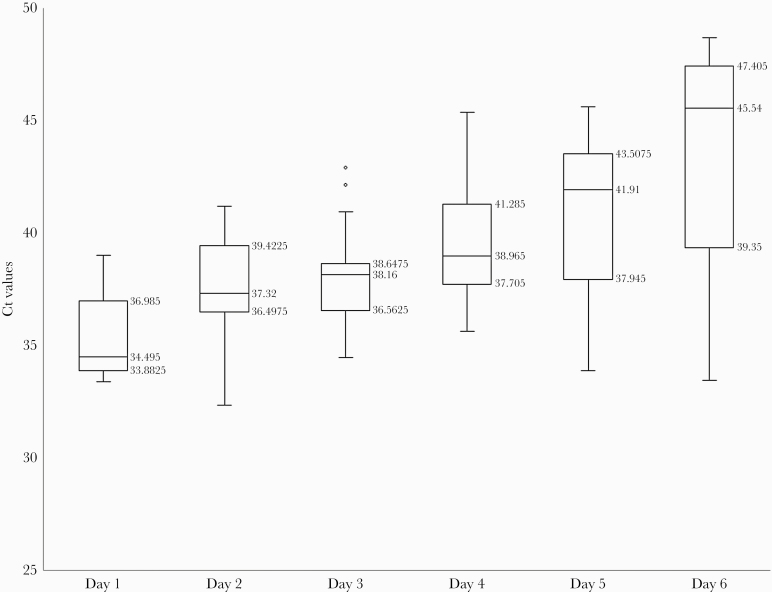
The number of participants shedding the virus in exhaled air (masks) showed a rising trend from Day 1 (10 of 43; 23%) to Day 4 (36 of 43; 84%), followed by a decline with only 5 (12%) cases shedding the virus on Day 6. In addition, there was a gradual decline in the viral RNA shedding, as evidenced by an increase in the median Ct values from Day 1 to Day 6 with a significant difference as per Friedman χ 2 test for repeated measures (χ 2 = 55.78, P < .001). The viral RNA shedding in exhaled air (masks) for 6 consecutive days based on median Ct values was compared using Wilcoxon-Mann-Whitney rank test. The median Ct value was 34.49 (Q1 = 33.4, Q3 = 36.98) on Day 1, which increased to 38.16 (Q1 = 36.56, Q3 = 38.65) on Day 3 (P = .03), and 38.96 (Q1 = 37.7, Q3 = 41.28) on Day 4 (P = .012). It further increased to 41.91 (Q1 = 37.95, Q3 = 43.50) on Day 5 (P = .112), and 45.54 (Q1 = 39.35, Q3 = 47.40) (P = .463) on Day 6, but these increases were not significant. Viral RNA shedding in exhaled air (masks) was not detected after Day 6 of recruitment (Figure 2).
Figure 2.
Day-wise distribution of viral ribonucleic acid (RNA) shedding in exhaled air (on masks). Comparative analysis of the average viral RNA shedding on masks for 6 consecutive days showed a gradual decline in the viral shedding, as evidenced by an increase in the median cycle threshold values from Day 1 to Day 6 with a significant difference (χ 2 = 55.78, P < .001).
The median Ct values of NP swabs and masks were assessed for correlation by using scatter plot and a linear association was obtained. The Spearman rank correlation coefficient was calculated and found to have significant positive correlation (r = 1.0) between the viral RNA shedding in NP swabs and masks for 6 days.
DISCUSSION
Our results suggest a likelihood of transmission of SARS-CoV-2 through exhaled breath of asymptomatic and paucisymptomatic cases during the first 6 days after a positive RT-PCR test, with the day of positive test being Day 1. So far, very few studies have demonstrated the degree and duration of SARS-CoV-2 shedding in asymptomatic and paucisymptomatic cases [11, 12]. The present study is the first to have documented detection of SARS-CoV-2 RNA in exhaled air (masks) and correlated the degree and duration of viral RNA shedding between NP swabs and masks worn on respective days. Previous studies have shown that asymptomatic and paucisymptomatic cases occur in all age groups from infants [13, 14] to middle-aged adults [15] and even the elderly [16]. According to Hu et al [15], asymptomatic cases were relatively young, with a median age of 14 years. In the present study, we observed that adults between 18 to 50 years of age accounted for the majority (72%) of the asymptomatic cases, which is a matter of concern because this age group predominantly comprises the working population who are more likely to travel frequently and thus have more chances of transmitting the infection to healthy contacts.
Efficient viral transmission is determined by several factors such as replication competency of the virus, viral load in the specimen, and the behavior and environmental factors associated with the infected individual [8]. The viral load and duration of viral shedding can be variable. Zhou et al [6] observed that asymptomatic patients have a lower viral load than symptomatic ones, and the mean duration of viral shedding in asymptomatic cases was 7 days. However, another study showed no difference in viral load between asymptomatic and symptomatic individuals with viral shedding detected until Day 11 after contact [15]. Sia et al [17] reported that hamsters infected with SARS-CoV-2 could transmit the infection to healthy hamsters on Day 1 after infection, but not on Day 6. In the present study, we observed that viral RNA shedding in both NP swabs and exhaled breath progressively diminished over time, becoming undetectable in the latter after Day 6. In addition, a significant positive correlation was observed between the viral RNA shedding in NP swabs and masks for 6 days. These findings suggest that although NP swabs of asymptomatic and paucisymptomatic cases test positive until Day 9, they shed the virus in exhaled breath and are likely to transmit the infection to contacts during the first 6 days after a positive test.
According to the current estimates, asymptomatic cases comprise 4%−41% of the total cases [18]. These cases most often go unnoticed until some of their family members or close contacts develop symptomatic illness. Hence, to avoid missing infected individuals, it is essential to screen all close contacts of confirmed or suspected cases. Reverse-transcription PCR of NP swabs remains the mainstay of diagnosis of COVID-19. However, mere detection of SARS-CoV-2 RNA in clinical specimens is not a measure of infectivity and does not necessarily mean that the person is infectious [8, 19]. For the virus to be infective, it should be capable of replicating efficiently in cultured cells. However, virus culture requires specialized laboratory and may be less sensitive than RT-PCR. In the present study, we observed a constant decrease in viral shedding over time with no detectable viral RNA in exhaled breath after 6 days of a positive RT-PCR test. Based on these findings, it would be prudent to curtail the quarantine duration of asymptomatic and paucisymptomatic individuals to 6 days, which would remove the unnecessary long periods of isolation, affecting individual well being, society, and access to healthcare.
Limitations
Our study is a small cohort that is not representative of the whole population of the country. Multicentric studies involving larger cohorts are required for better understanding of the transmission dynamics and the potential role of asymptomatic and paucisymptomic secondary cases in the spread of COVID-19. We used Ct values as an indirect measure of viral load. However, quantification of viral load could be best achieved by viral culture.
CONCLUSIONS
Asymptomatic and paucisymptomatic cases of COVID-19 remain potentially infectious during the first 6 days after a positive RT-PCR test. Considering that viral RNA shedding in these individuals decrease over time and becomes undetectable in exhaled breath after Day 6, it would be prudent to consider release of such individuals from isolation after 6 days of a positive RT-PCR test. This would remove the unnecessary long periods of isolation, affecting individual well being, society, and access to healthcare. Further research is needed to understand the transmission dynamics of COVID-19 in asymptomatic and paucisymptomatic cases, which will help in developing effective preventive strategies and Thus limit the spread of the disease.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This study was funded by an intramural project (Grant Number AIIMS/2020–21/3034).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Hassan SA, Sheikh FN, Jamal S, et al. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus 2020; 12:e7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao Z, Xu Y, Sun C, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect 2020. doi: 10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020; 323:1406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis 2020; 20:410− 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral RNA shedding and transmissibility of COVID-19. Nat Med 2020; 26:672− 5. [DOI] [PubMed] [Google Scholar]
- 6. Zhou R, Li F, Chen F, et al. Viral dynamics in asymptomatic patients with COVID-19. Int J Infect Dis 2020; 96:288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 Viral load in upper respiratory specimens of infected patients. N Engl J Med 2020; 382:1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. Criteria for releasing COVID-19 patients from isolation. Scientific Brief Geneva: World Health Organization; 2020. [Google Scholar]
- 9. Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife 2020; 9:e58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020; 25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang XL, Zhang XL, Zhao XN, et al. Transmission potential of asymptomatic and paucisymptomatic severe acute respiratory syndrome coronavirus 2 infections: a 3-family cluster study in China. J Infect Dis 2020; 221:1948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng HY, Jian S, Liu D, et al. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med 2020; 180:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kam KQ, Yung CF, Cui L, et al. A well infant with coronavirus disease 2019 with high viral load. Clin Infect Dis 2020; 71:847− 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin C, Ding Y, Xie B, et al. Asymptomatic novel coronavirus pneumonia patient outside Wuhan: the value of CT images in the course of the disease. Clin Imaging 2020; 63:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci 2020; 63:706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoehl S, Rabenau H, Berger A, et al. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med 2020; 382:1278–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sia SF, Yan LM, Chin AWH, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020; 583:834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Byambasuren O, Cardona M, Bell K, Clark J, McLaws M-L, Glasziou P. Estimating the extent of true asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. JAMMI 2020. doi: 10.3138/jammi-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Atkinson B, Petersen E. SARS-CoV-2 shedding and infectivity. Lancet 2020; 395:1339–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.