Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections on mink farms are increasingly observed in several countries, leading to the massive culling of animals on affected farms. Recent studies showed multiple (anthropo)zoonotic transmission events between humans and mink on these farms. Mink-derived SARS-CoV-2 sequences from The Netherlands and Denmark contain multiple substitutions in the S protein receptor binding domain (RBD). Molecular modeling showed that these substitutions increase the mean binding energy, suggestive of potential adaptation of the SARS-CoV-2 S protein to the mink angiotensin-converting enzyme 2 (ACE2) receptor. These substitutions could possibly also impact human ACE2 binding affinity as well as humoral immune responses directed to the RBD region of the SARS-CoV-2 S protein in humans. We wish to highlight these observations to raise awareness and urge for the continued surveillance of mink (and other animal)-related infections.
Keywords: SARS-CoV-2, host adaptation, mink, protein structure, humoral immunity, public health
Recently, the first reports on the infection and spread of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on mink farms in The Netherlands were published (Oreshkova et al. 2020; Oude Munnink et al. 2020). On several mink farms workers experienced coronavirus-related symptoms prior to the outbreaks, leading to the assumption that the workers introduced the virus within these farms. Interestingly, these studies identified multiple substitutions in the receptor binding domain (RBD) of the SARS-CoV-2 spike protein: Y453F, F486L, and N501T (Oreshkova et al. 2020; Oude Munnink et al. 2020) (Genbank accession numbers MT396266, MT457390-MT457401). In addition, the Y453F substitution was also present in mink-derived sequences from Denmark (Genbank accession numbers MT919525-MT919536). Additionally, experimental infection of ferrets showed the emergence of the N501T substitution within days post-infection (Richard et al. 2020). These three substitutions involve key positions in the RBD that interact with the angiotensin-converting enzyme 2 (ACE2) receptor (Lan et al. 2020). Similarly, several of these substitutions were also observed in strains obtained from human cases, related to infections on mink farms (Oude Munnink et al. 2020).
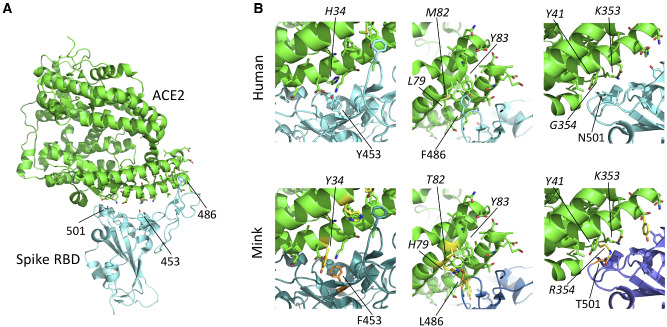
Multiple ACE2 residues that differ between human and mink ACE2, interact with the Y453F, F486L or N501T residues (Table 1, Fig. 1) (Damas et al. 2020) of SARS-CoV-2 spike RBD. These differences may underlie the selective pressure for the virus to adapt to the new host, optimizing its spike protein binding affinity to the mink ACE2-receptor. On visual inspection, substitutions Y453F, F486L and N501T appear to provide a possible better fit to the mink ACE2 receptor residues (Fig. 1) (Lan et al. 2020). To investigate this hypothesis, we calculated binding energy changes resulting from the RBD substitutions Y453F, F486L and N501T, which are in contact with the residues that differ between the human and mink ACE2 receptor (Table 1). This was done by simulating annealing energy minimizations through short molecular dynamics (MD) simulations in YASARA (Krieger and Vriend 2014). To validate our approach, we also estimated binding energy changes of RBD substitutions that were found to alter receptor-binding affinity with human ACE2 in a recent deep mutational scanning study (Supplementary Fig. S1) (Starr et al. 2020). Although we only found a weak correlation between our binding energy estimates and the experimentally measured effects of these RBD substitutions on human ACE2 receptor binding for this limited data set, we could still make qualitative inferences from the energy estimates on the potential binding effects of the RBD substitutions found in minks and their corresponding ACE2 receptor.
Table 1.
Overview SARS-CoV-2 ACE2 receptor binding domain substitutions in mink-related sequences and their interacting residues within ACE2.
| Substitution | Interacting residues in human ACE2 | Interacting residues in mink ACE2 |
|---|---|---|
| Y453F | H34 | Y34 |
| F486L | L79, M82, Y83 | H79, T82, Y83 |
| N501T | Y41, K353, G354, | Y41, K353, R354 |
Figure 1.
Structure of the SARS-CoV-2 RBD bound to ACE2 and location of substitutions within the mink-derived SARS-CoV-2 RBD. (A) Overview of SARS CoV-2 spike RBD interactions with ACE2. ACE2 is shown in green and RBD in light blue. Positions of residues 453, 486, and 501 are indicated. (B) Comparisons of interactions of human derived SARS-CoV-2 RBD–ACE2 (upper panels) and mink-derived SARS-CoV RBD–ACE2 interfaces (lower panels). ACE2 is shown in green and RBD in different shades of blue. Residues 453, 486, and 501 are indicated. Substitutions within mink RBD and ACE2 are indicated in orange and yellow, respectively. Modeling of substitutions and pictures produced by PyMol (The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC) on structure PDB 6MOJ (Lan et al. 2020).
Our analyses showed an overall increase in mean binding energies between substitutions Y453F, F486L and N501T and the mink-like ACE2 receptor in the same range as those substitutions that improved binding with human ACE2 receptor (Supplementary Fig. S1 and Table S1), suggesting that these RBD substitutions found in minks may indeed improve binding of the spike protein to mink ACE2 compared to human ACE2 (see Supplementary Material). These findings indicate that adaptation toward improved mink ACE2 usage could be an explanation for part of the current SARS-CoV-2 evolutionary pattern in mink-farm related infections in The Netherlands.
Such naturally occurring RBD substitutions might be expected after a (anthropo)zoonotic event. For instance, lion-derived SARS-CoV-2 sequences contain a Y505H RBD substitution, tiger-derived sequences contain a F456Y RBD substitution and in a mouse-adapted SARS-CoV-2 strain RBD substitutions Q493K, Q498Y and P499T are selected after serial passaging (Leist et al. 2020; McAloose et al. 2020). All of these positions in the RBD have been reported to interact with the ACE2 receptor (Lan et al. 2020). Such substitutions may also have an impact on SARS-CoV-2 affinity to human ACE2. While the aforementioned DMS study showed a lower affinity for human ACE2 for the tiger and lion-derived RBD substitutions, both our computational estimates and the deep mutational scanning study showed that the Y453F and N501T substitutions also increase binding affinity to human ACE2 (Starr et al. 2020). As such, it is possible that these viruses could successfully infect humans and possibly even transmit among humans (Starr et al. 2020). Nonetheless, there is currently no evidence of ongoing transmission in humans of SARS-CoV-2 with these substitutions in The Netherlands, based on the ongoing genomic surveillance efforts.
However, recent outbreaks have been reported at more than 200 mink farms in Denmark. Additionally, over 200 human SARS-CoV-2 sequences collected in Denmark, contain the Y453F RBD substitution as detailed in the rapid risk assessment by the European Centre for Disease Prevention & Control (ECDC 2020). The F486L and N501T substitutions were not detected in the human-derived Danish sequences. Although the introduction of the Y453F SARS-CoV-2 variant in the human population was likely due to the outbreaks at mink farms, many of the infected humans in Denmark had no direct link to the affected mink farms, indicating the possibility of sustained transmission of the Y453F variant among humans (Serum Statens Institute 2020). Whether or not this is related to additional spike substitutions identified in Denmark should be investigated further.
These identified RBD substitutions could also affect the level of protective immunity against SARS-CoV-2 in humans. Antibodies that are elicited after a SARS-CoV-2 infection in humans mainly target the spike protein (Brouwer et al. 2020). Antibodies targeting the RBD of the spike protein offer protection by inhibiting the attachment of SARS-CoV-2 to target cells (Tai et al. 2020). Therefore, it is not unreasonable to assume that the substitutions occurring in the RBD might alter the potential level of protection provided by antibodies directed at this region, either post-infection or vaccination. For instance, the Y453F mutation was recently shown to be an escape mutation for the monoclonal antibody RGN10933 (Baum et al. 2020). In addition, in a recent case report involving a patient receiving Regeneron treatment, escape mutations were identified on several positions the S protein, including positions 486 and 501 (Choi et al. 2020). It is therefore of the utmost importance to determine whether or not the neutralizing capacity of human sero-convalescent sera remains effective against viral variants with the identified substitutions.
In summary, mink-derived SARS-CoV-2 strains encode substitutions in areas of the genome crucial to ACE2 receptor binding and could potentially affect neutralizing antibody responses in humans as well. With this reflection we would like to highlight these observations, raise awareness and urge for the continued surveillance of mink (and other animal)-related infections. Furthermore, we wish to stress the importance of genomic surveillance in a OneHealth setting including animal-derived SARS-CoV-2 viruses.
Supplementary data
Supplementary data are available at Virus Evolution online.
Conflict of interest: None declared.
Supplementary Material
Acknowledgements
We would like to gratefully acknowledge the originating and submitting laboratories and authorities for the timely sharing of their data, allowing for the early risk assessment by public health institutes.
Contributor Information
Matthijs R A Welkers, Department of Medical Microbiology & Infection Prevention, Amsterdam University Medical Centers, location AMC, Meibergdreef 9, 1105AZ, Amsterdam, The Netherlands; Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Antonie van Leeuwenhoeklaan 9, 3721 MA Bilthoven, The Netherlands.
Alvin X Han, Department of Medical Microbiology & Infection Prevention, Amsterdam University Medical Centers, location AMC, Meibergdreef 9, 1105AZ, Amsterdam, The Netherlands.
Chantal B E M Reusken, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Antonie van Leeuwenhoeklaan 9, 3721 MA Bilthoven, The Netherlands.
Dirk Eggink, Department of Medical Microbiology & Infection Prevention, Amsterdam University Medical Centers, location AMC, Meibergdreef 9, 1105AZ, Amsterdam, The Netherlands; Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Antonie van Leeuwenhoeklaan 9, 3721 MA Bilthoven, The Netherlands.
References
- Baum A. et al. (2020) ‘ Antibody Cocktail to SARS-CoV-2 Spike Protein Prevents Rapid Mutational Escape seen with Individual Antibodies’, Science, 369: 1014–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer P. J. M. et al. (2020) ‘ Potent Neutralizing Antibodies from COVID-19 Patients Define Multiple Targets of Vulnerability’, Science, 369: 643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B. et al. (2020) ‘ Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host’, The New England Journal of Medicine, 383: 2291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas J. et al. (2020) ‘ Broad Host Range of SARS-CoV-2 Predicted by Comparative and Structural Analysis of ACE2 in Vertebrates’, Proceedings of the National Academy of Sciences of the United States of America, 117: 202010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC. Rapid Risk Assessment: Detection of New SARS-CoV-2 Variants Related to Mink. 2020. <https://www.ecdc.europa.eu/sites/default/files/documents/RRA-SARS-CoV-2-in-mink-12-nov-2020.pdf>.
- Krieger E., Vriend G. (2014) ‘ YASARA View—Molecular Graphics for All Devices—from Smartphones to Workstations’, Bioinformatics, 30: 2981–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J. et al. (2020) ‘ Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor’, Nature, 581: 215–20. [DOI] [PubMed] [Google Scholar]
- Leist S. R. et al. (2020) ‘ A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice’, Cell, 183: 1070–85.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAloose D. et al. (2020) ‘ From People to Panthera: Natural SARS-CoV-2 Infection in Tigers and Lions at the Bronx Zoo. Meng X-J (ed.)’, MBio, 11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova N. et al. (2020) ‘ SARS-CoV-2 Infection in Farmed Minks, The Netherlands, April and May 2020’, Eurosurveillance, 25: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink B. B. et al. (2020) ‘ Transmission of SARS-CoV-2 on Mink Farms between Humans and Mink and Back to Humans’, Science, 5901: eabe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M. et al. (2020) ‘ SARS-CoV-2 is Transmitted via Contact and via the Air between Ferrets’, Nature Communications, 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serum Statens Institute. Risikovurdering-human-sundhed-ved-fortsat-minkavl-03112020. 2020. <https://files.ssi.dk/Risikovurdering-human-sundhed-ved-fortsat-minkavl-03112020> accessed 4 Nov 2020.
- Starr T. N. et al. (2020) ‘ Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding’, Cell, 182: 1295–310.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W. et al. (2020) ‘ Characterization of the Receptor-Binding Domain (RBD) of 2019 Novel Coronavirus: Implication for Development of RBD Protein as a Viral Attachment Inhibitor and Vaccine’, Cellular & Molecular Immunology, 17: 613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.