Abstract
Background
There is currently no single treatment that mitigates all harms caused by severe acute respiratory syndrome coronavirus 2 infection. Tocilizumab, an interleukin-6 antagonist, may have a role as an adjunctive immune-modulating therapy.
Methods
This was an observational retrospective study of hospitalized adult patients with confirmed coronavirus disease 2019 (COVID-19). The intervention group comprised patients who received tocilizumab; the comparator arm was drawn from patients who did not receive tocilizumab. The primary outcome was all-cause mortality censored at 28 days; secondary outcomes were all-cause mortality at discharge, time to clinical improvement, and rates of secondary infections. Marginal structural Cox models via inverse probability treatment weights were applied to estimate the effect of tocilizumab. A time-dependent propensity score–matching method was used to generate a 1:1 match for tocilizumab recipients; infectious diseases experts then manually reviewed these matched charts to identify secondary infections.
Results
This analysis included 90 tocilizumab recipients and 1669 controls. Under the marginal structural Cox model, tocilizumab was associated with a 62% reduced hazard of death (adjusted hazard ratio [aHR], 0.38; 95% CI, 0.21 to 0.70) and no change in time to clinical improvement (aHR, 1.13; 95% CI, 0.68 to 1.87). The 1:1 matched data set also showed a lower mortality rate (27.8% vs 34.4%) and reduced hazards of death (aHR, 0.47; 95% CI, 0.25 to 0.88). Elevated inflammatory markers were associated with reduced hazards of death among tocilizumab recipients compared with controls. Secondary infection rates were similar between the 2 groups.
Conclusions
Tocilizumab may provide benefit in a subgroup of patients hospitalized with COVID-19 who have elevated biomarkers of hyperinflammation, without increasing the risk of secondary infection.
Keywords: COVID-19, IL-6, SARS-CoV-2, retrospective, tocilizumab
In this retrospective study of adult patients hospitalized with COVID-19, tocilizumab was associated with a 62% reduction in hazard of death. Tocilizumab may have a role as adjunctive therapy for COVID-19 among patients with hyperinflammation including elevated IL-6.
In December 2019, reports of what would soon be labeled coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 began to emerge. As of November 19, 2020, worldwide infections are estimated to be 56.4 million and deaths to be 1.4 million. The United States is the current epicenter of the global pandemic, with over 11.5 million cases and 250 000 deaths [1].
Elevations in interleukin-6 (IL-6) and C-reactive protein (CRP) have been associated with poor outcomes from COVID-19 [2–4]. This has led to the hypothesis that a second illness phase seen in a minority of patients is a hyperinflammatory state [5], analogous to cytokine release syndrome (CRS) with chimeric antigen receptor (CAR) T-cell therapy [6], which is also seen in both SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV) infections [7]. In the hyperinflammatory states associated with CAR-T therapy and certain rheumatologic diseases, anti-IL-6 therapy has proven beneficial [8–11]. Though some studies show that IL-6 levels in severe COVID-19 are lower than in other causes of acute respiratory distress syndrome (ARDS) [12], there remains interest in using immunomodulatory therapies to dampen the inflammatory cascade in affected patients.
Treatment for COVID-19 is evolving as therapeutic trials progress. Remdesivir, an antiviral, may shorten time to recovery, particularly when used early in the disease course [13, 14]. Dexamethasone has been shown to reduce mortality in COVID-19 among those requiring supplemental oxygen [15], and a meta-analysis of corticosteroid trials found reduced mortality, regardless of oxygen support or symptom duration [16]. Numerous other immunomodulatory drugs are currently under investigation [17–19]. Tocilizumab, a humanized monoclonal antibody against the IL-6 receptor (IL-6R), has been used to treat rheumatologic diseases [20] and CRS due to CAR-T therapy [10]. Given its relative global availability, tocilizumab was used off-label at many sites in the first few months of the COVID-19 pandemic. Several recent studies have yielded conflicting results about the effects of tocilizumab in COVID-19, including secondary infection risk [21–29]. Careful retrospective analyses of the off-label use of tocilizumab can assist in understanding whether the drug has utility. This study aims to describe outcomes including secondary infection risk and identify clinical and laboratory features that predict benefit among hospitalized patients with COVID-19 and evidence of a hyperinflammatory state who received off-label tocilizumab within the Johns Hopkins Health System (JHHS).
METHODS
Study Population
This is an observational, retrospective, nonrandomized study of adult patients (>18 years) with confirmed COVID-19, hospitalized within JHHS between March 15 and June 27, 2020. JHHS is a 5-hospital system in the Baltimore/Washington DC area with ~2300 beds. COVID-19 was diagnosed via SARS-CoV-2 RNA detection on polymerase chain reaction (PCR) using nasal swab, nasopharyngeal swab, or bronchoalveolar lavage (BAL). The intervention group was patients who received tocilizumab for off-label treatment of COVID-19, and the comparator arm was drawn from patients with COVID-19 who did not receive tocilizumab. Patients were excluded if they were younger than 18 years old or if they died or were discharged within 24 hours after hospitalization. Some of the included patients have been part of prior analyses [4].
Data Source
The COVID-19 Precision Medicine Analytics Platform (PMAP) Registry (JH-CROWN) includes clinical and laboratory data from all patients admitted to JHHS with suspected or confirmed SARS-CoV-2 infection. Data were extracted from the electronic medical record and also included Research Electronic Data Capture (REDCap) data from manual chart abstraction. A group of infectious diseases specialists adjudicated incident infections and preexisting immunosuppression (use of corticosteroid or chemotherapy, history of solid organ or bone marrow transplant). Cases in which there was uncertainty were discussed jointly by all adjudicating infectious diseases specialists before reaching a final decision.
Treatment
Off-label tocilizumab administration at JHHS requires approval of the 5-member JHHS Formulary COVID-19 Drug Approval Committee. Patients are potentially eligible to receive tocilizumab if they meet prespecified criteria, including progressive hypoxemia, vital sign instability, and elevated inflammatory markers (Supplementary Table 1). When a clinician wants to prescribe tocilizumab, a request is submitted for consideration to the designated member at each hospital on the JHHS Formulary COVID-19 Drug Approval Committee, who then presents the case request by conference call to the other available committee members. If approval is obtained, patients receive a single intravenous dose of tocilizumab, usually 8 mg/kg (range, 6–8 mg/kg), not to exceed 800 mg [10, 20, 30].
Outcomes
The primary outcomes were all-cause in-hospital mortality, right-censored at 28 days, and all-cause mortality at time of discharge. The secondary outcome was clinical improvement, defined as a decrease of 2 points on the World Health Organization (WHO) Ordinal Scale for Clinical Improvement or hospital discharge [31]. Secondary infection rate was also analyzed as a safety outcome [32–37].
Statistical Analysis
Given the nonrandomized nature of tocilizumab receipt, marginal structural Cox models [38] via inverse probability treatment weights (IPTWs) were applied to estimate the treatment effect of tocilizumab on the primary outcomes by estimating the hazards of death after adjusting for the tocilizumab assignment bias and informative censoring bias of discharge. The marginal structural model has the advantages of utilizing the whole data set and capturing the effect of longitudinal changes in laboratory and clinical measurements on survival as a time-to-event outcome. The outcome of this model is the hazard of mortality, and it is particularly beneficial when the values of previous time-dependent variables may predict future values as well as the likelihood of nonrandom treatment assignment (in this case, receipt of tocilizumab). This model is particularly adept at mitigating bias of nonrandom treatment assignment in analyzing the causal effect of treatment on the outcome of mortality. To calculate the weighting for each record (per patient, per day) in the marginal structural model, numerous covariates were included in computing the probability of tocilizumab exposure. Time-invariant (fixed) covariates included race, age, sex, body mass index (BMI), and Charlson Comorbidity Index (CCI). Time-dependent (varying) covariates included clinical measures of disease severity, such as SpO2/FiO2 (S:F), respiratory rate, temperature, systolic blood pressure, diastolic blood pressure, pulse, oxygen supplementation device, and code status (whether a patient had a do-not-resuscitate [DNR]/do-not-intubate [DNI] order). S:F was selected, as it can be calculated without obtaining an arterial blood gas (not available on all patients) and has a good correlation with more traditional measures of ARDS severity such as PaO2/FiO2 (P:F) [39]. Time-varying laboratory parameters included C-reactive protein (CRP), white blood cell (WBC) count, absolute lymphocyte count (ALC), hemoglobin (Hgb), albumin, alanine aminotransferase (ALT), glomerular filtration rate (GFR), D-dimer, ferritin, and IL-6. Missing values were imputed using multiple imputation by chained equations (mice) with the predictive mean matching method [40]. To assess the adjusted hazard ratio of tocilizumab treatment on death, a set of demographics and clinical variables were included in Cox regression models.
Clinical improvement was defined as live discharge from the hospital without worsening of WHO score, or at least a 2-point decrease in WHO score during hospitalization. A marginal structural Cox model was applied with the same set of covariates adjusted for the bias of both treatment exposure and death as a censoring event.
Due to the large size of the comparator arm, manual chart review for adjudication of all incident infections was not feasible. Therefore, we used a time-dependent propensity score–matching method to balance between treatment and control arms by making them similar (only randomly different) regarding prespecified covariates (see the Supplementary Data for details). After matching, these 180 patients (90 tocilizumab recipients, 90 matched controls) were compared for their survival over time and risk of secondary infection.
Post hoc analyses included duration of ventilation and concomitant medication administration (remdesivir, dexamethasone) and were compared using Welch’s 2-sample t test. Data were analyzed using R, version 3.6.2 [41].
RESULTS
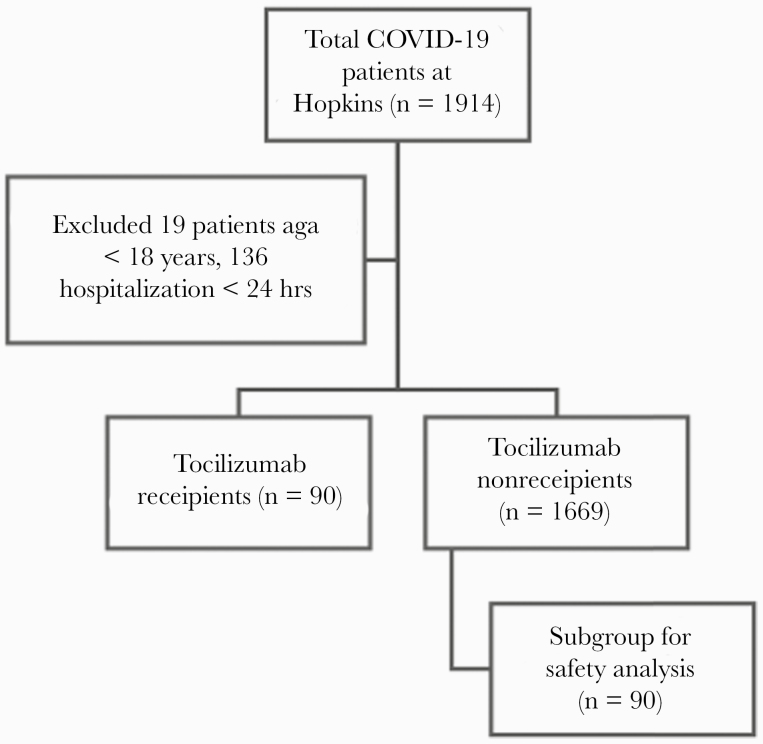
Of 1914 patients admitted to JHHS between March 15 and June 27, 2020, with COVID-19, 1759 were included in the analysis (90 tocilizumab recipients, 1669 controls). A total of 1759 were included in the primary efficacy analyses (mortality and clinical improvement), while 180 (90 tocilizumab recipients, 90 controls) were included in the safety analysis (Figure 1) and survival curve (Figure 2). The majority of patients (90%) received the 8-mg/kg dose. The median time from hospitalization to tocilizumab receipt (range, interquartile range [IQR]) was 2.6 (0.1–11.7, 1.4–4.7) days. The median follow-up (range, IQR) was 6.5 (1.2–87.2, 3.3–12.1) days; the primary outcome was right-censored at 28 days. Almost all patients who received tocilizumab (88/90; 97.8%) required at least 2 liters per minute (L/min) of supplemental oxygen via nasal cannula, and 78/90 (86.7%) achieved an end point (either death or discharge from the hospital) at the date of data censoring (June 27, 2020).
Figure 1.
Study cohort and flowchart. Abbreviation: COVID-19, coronavirus disease 2019.
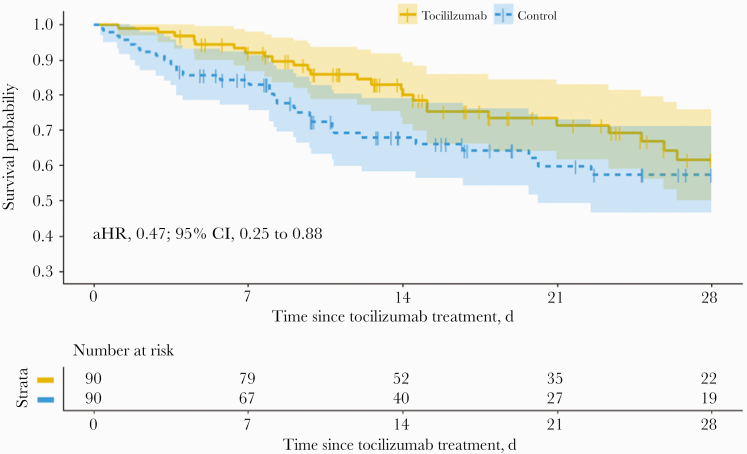
Figure 2.
Survival within a matched data set of tocilizumab receipients vs non-tocilizumab-treated controls (n = 180). Abbreviation: aHR, adjusted hazard ratio.
Baseline characteristics between the tocilizumab recipients and all (unmatched) controls were numerically different (column 2, Table 1). Tocilizumab recipients were more frequently male, with longer hospitalizations, more severe respiratory failure, and more comorbidities (including 5.6% vs 1.8% with history of solid organ transplantation). After matching using both the marginal structural Cox model and propensity score, balance on key covariates was improved (Table 1). There were similar numbers of patients treated with other therapies, but more control patients received dexamethasone. Representation of comorbidities was similar between the arms. Manual chart review of 180 patients identified 15 immunosuppressed patients (10 intervention, 5 control) (Supplementary Table 2).
Table 1.
Patient Characteristics at Baseline, Including Tocilizumab Recipients, All Controls, and Matched Controls
| Tocilizumab (n = 90) | Control Group (n = 1669) | Matched Group (n = 90) | |
|---|---|---|---|
| Demographics | |||
| Median age (IQR), y | 63 (9) | 61 (14) | 63.5 (10) |
| Male | 71 (78.9) | 864 (51.8) | 70 (77.8) |
| Race Black | 28 (31.1) | 585 (35.1) | 31 (34.4) |
| Race Hispanic | 30 (33.3) | 473 (28.3) | 31 (34.4) |
| Race White | 15 (16.7) | 448 (26.8) | 15 (16.7) |
| Race other | 17 (18.9) | 163 (9.8) | 13 (14.4) |
| Median BMI (IQR), kg/m2 | 28.4 (3.4) | 28.6 (4.7) | 27.7 (4.4) |
| Ever DNR/DNI | 38 (42.2) | 388 (23.2) | 41 (45.6) |
| Comorbidities | |||
| Hypertension | 44 (48.9) | 793 (47.5) | 36 (40) |
| Coronary artery disease | 29 (32.2) | 517 (31) | 33 (36.7) |
| Congestive heart failure | 18 (20) | 250 (15) | 20 (22.2) |
| Chronic kidney disease | 14 (15.6) | 199 (11.9) | 13 (14.4) |
| Diabetes | 35 (38.9) | 476 (28.5) | 34 (37.8) |
| Asthma | 4 (4.4) | 138 (8.3) | 3 (3.3) |
| COPD/chronic lung disease | 10 (11.1) | 260 (15.6) | 17 (18.9) |
| Cancer | 6 (6.7) | 169 (10.1) | 9 (10) |
| Liver disease | 4 (4.4) | 86 (5.2) | 4 (4.4) |
| HIV | 1 (1.1) | 21 (1.3) | 1 (1.1) |
| Transplant | 5 (5.6) | 23 (1.4) | 0 (0) |
| CCI 0 | 34 (37.8) | 660 (39.5) | 31 (34.4) |
| CCI 1–4 | 53 (58.9) | 944 (56.6) | 54 (60) |
| CCI ≥5 | 3 (3.3) | 65 (3.9) | 5 (5.6) |
| Concomitant medications | |||
| Hydroxychloroquine | 25 (27.8) | 381 (22.8) | 24 (26.7) |
| Azithromycin | 56 (62.2) | 670 (40.1) | 50 (55.6) |
| Dexamethasone | 6 (6.7) | 75 (4.5) | 13 (14.4) |
| Prednisone | 10 (11.1) | 87 (5.2) | 2 (2.2) |
| Heparin (prophylaxis or treatment dose) | 69 (76.7) | 1217 (72.9) | 71 (78.9) |
| Remdesivir | 17 (18.9) | 157 (9.4) | 17 (18.9) |
| Trial participation | 1 (1.1) | 54 (3.2) | 10 (11.1) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; DNR/DNI, do-not-resuscitate, do-not-intubate; IQR, interquartile range.
In the unadjusted data set, tocilizumab recipients had a higher mortality rate (27.8% vs 11.0%). However, using the marginal structural Cox model, patients who received tocilizumab had a 62% reduced hazard of death at 28 days (adjusted hazard ratio [aHR], 0.38; 95% CI, 0.21 to 0.70; P = .002), compared with controls (Table 2). This was also true for the 1:1 matched data set, which showed a lower mortality rate in tocilizumab recipients (27.8% vs 34.4%) and reduced hazards of death (aHR, 0.47; 95% CI, 0.25 to 0.88; P = .02) (Figure 2). To analyze any differential effects of tocilizumab on mortality within subgroups, patients were separated into strata of various demographics, vital signs, and laboratory results at the time of hospitalization. Grouping thresholds were selected based on a heuristic method as well as clinical knowledge. Certain subgroups appeared to receive greater mortality benefit with tocilizumab than others, including male gender, age >55 years (particularly 55–65), few comorbidities (CCI <5), and more normal vital sign values (pulse <100, MAP ≥60, temperature <38.3°C). Mortality benefit was seen across all strata of S:F groupings. Elevated ferritin (>800 ng/mL), albumin level (≥3 g/dL), ALT level (≥30 U/L), and WBC count (≥12 ×103/mm3), as well as lower CRP (<16 mg/dL) and D-dimer both <1 and ≥4 mg/L, were associated with reduced hazards of death among tocilizumab recipients compared with nonrecipients. Among patients with IL-6 >55 pg/mL, tocilizumab was associated with reduced hazard of death (aHR, 0.34; 95% CI, 0.18 to 0.64; P = .0008). When mortality data were censored only at time of death or discharge (rather than 28 days), the effect was mildly attenuated due to few patients remaining at risk for the outcome (aHR, 0.39; 95% CI, 0.21 to 0.71; P = .002) (Supplementary Table 3).
Table 2.
Hazard Ratio of Death Based on Covariates, Stratified by Treatment Arm (Tocilizumab Recipients vs Controls)
| Tocilizumab (n = 90), No. (%) | Controls (n = 1669), No. (%) | Hazard Ratio (95% CI) | |
|---|---|---|---|
| Demographics | |||
| Gender | |||
| Male | 71 (78.9) | 864 (51.8) | 0.319 (0.161 to 0.629) |
| Female | 19 (21.1) | 805 (48.2) | 0.499 (0.142 to 1.753) |
| Age, y | |||
| <55 | 24 (26.7) | 654 (39.2) | 0.146 (0.009 to 2.294) |
| 55–<65 | 24 (26.7) | 310 (18.6) | 0.012 (0.001 to 0.132) |
| ≥65 | 42 (46.7) | 705 (42.2) | 0.395 (0.22 to 0.709) |
| BMI, kg/m2 | |||
| <30.5 | 60 (66.7) | 1029 (61.7) | 0.302 (0.162 to 0.561) |
| ≥30.5 | 30 (33.3) | 640 (38.3) | 0.349 (0.15 to 0.81) |
| CCI | |||
| <5 | 72 (80) | 1310 (78.5) | 0.272 (0.134 to 0.552) |
| ≥5 | 18 (20) | 359 (21.5) | 0.541 (0.189 to 1.546) |
| Vitals | |||
| SpO2/FiO2 ratio | |||
| <100 (severe) | 8 (8.9) | 9 (0.5) | N/A |
| 100–<200 (moderate) | 24 (26.7) | 90 (5.4) | 0.316 (0.121 to 0.822) |
| 200–< 300 (mild) | 24 (26.7) | 191 (11.4) | 0.225 (0.086 to 0.59) |
| ≥300 (non-ARDS) | 34 (37.8) | 1379 (82.6) | 0.321 (0.124 to 0.831) |
| Pulse, beats/min | |||
| <100 | 40 (44.4) | 977 (58.5) | 0.209 (0.08 to 0.547) |
| ≥100 | 50 (55.6) | 692 (41.5) | 0.705 (0.353 to 1.41) |
| Respiratory rate, breaths/min | |||
| <30 | 32 (35.6) | 1310 (78.5) | 0.204 (0.062 to 0.67) |
| ≥30 | 58 (64.4) | 359 (21.5) | 0.376 (0.171 to 0.827) |
| Mean arterial pressure, mmHg | |||
| <60 | 15 (16.7) | 150 (9) | 1.583 (0.631 to 3.974) |
| ≥60 | 75 (83.3) | 1519 (91) | 0.222 (0.117 to 0.424) |
| Temperature, °C | |||
| <38.3 | 47 (52.2) | 1235 (74) | 0.408 (0.214 to 0.779) |
| ≥38.3 | 43 (47.8) | 434 (26) | 0.336 (0.109 to 1.038) |
| Laboratory results | |||
| Albumin, g/dL | |||
| <2.5 | 8 (8.9) | 101 (6.1) | 4.403 (0.723 to 26.82) |
| 2.5–<3 | 23 (25.6) | 222 (13.3) | 0.644 (0.183 to 2.273) |
| ≥3 | 59 (65.6) | 1346 (80.6) | 0.23 (0.113 to 0.466) |
| ALT, U/L | |||
| <30 | 37 (41.1) | 828 (49.6) | 0.45 (0.192 to 1.056) |
| ≥30 | 53 (58.9) | 841 (50.4) | 0.283 (0.122 to 0.653) |
| GFR, mL/min | |||
| <15 | 7 (7.8) | 94 (5.6) | 0.486 (0.019 to 12.41) |
| 15–<30 | 18 (20) | 116 (7) | 1.005 (0.327 to 3.084) |
| 30–<60 | 15 (16.7) | 322 (19.3) | 0.145 (0.045 to 0.466) |
| 60–<90 | 28 (31.1) | 452 (27.1) | 0.125 (0.027 to 0.575) |
| ≥90 | 22 (24.4) | 685 (41) | 0.279 (0.016 to 4.784) |
| CRP, mg/dL | |||
| <16 | 50 (55.6) | 1359 (81.4) | 0.234 (0.087 to 0.627) |
| 16–<27 | 29 (32.2) | 236 (14.1) | 0.597 (0.21 to 1.698) |
| ≥27 | 11 (12.2) | 74 (4.4) | 0.011 (1e-04 to 1.090) |
| D-dimer, mg/L | |||
| <1 | 25 (27.8) | 860 (51.5) | 0.288 (0.086 to 0.958) |
| 1–<2 | 33 (36.7) | 401 (24.0) | 0.302 (0.087 to 1.057) |
| 2–<4 | 9 (10) | 210 (12.6) | 0.251 (0.062 to 1.01) |
| ≥4 | 23 (25.6) | 198 (11.9) | 0.209 (0.052 to 0.838) |
| Ferritin, ng/mL | |||
| <500 | 11 (12.2) | 698 (41.8) | 1.316 (0.47 to 3.681) |
| 500–<800 | 14 (15.6) | 317 (19) | 0.682 (0.15 to 3.087) |
| 800–<2000 | 45 (50) | 477 (28.6) | 0.256 (0.102 to 0.644) |
| ≥2000 | 20 (22.2) | 177 (10.6) | 0.243 (0.063 to 0.941) |
| WBC, ×103/mm3 | |||
| <12 | 78 (86.7) | 1465 (87.8) | 0.42 (0.219 to 0.805) |
| ≥12 | 12 (13.3) | 204 (12.2) | 0.073 (0.011 to 0.486) |
| IL-6, pg/mL | |||
| <55 | 10 (11.1) | 736 (44.1) | 0.898 (0.094 to 8.583) |
| ≥55 | 80 (88.9) | 933 (55.9) | 0.336 (0.178 to 0.635) |
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; CCI, Charlson Comorbidity Index; IL-6, interleukin-6; SpO2/FiO2, pulse oximetric saturation/fractional inspired oxygen; WBC, white blood cell.
For the secondary outcome of clinical improvement, patients treated with tocilizumab had no benefit in time to clinical improvement under the marginal structural Cox model (aHR, 1.13; 95% CI, 0.68 to 1.87; P = .63). Using the propensity score–matched data set, among patients who survived 28 days or were discharged alive, there was no difference in duration of intubation between tocilizumab recipients (16.2 days) and controls (13.1 days; 95% CI for difference, –7.8 to 1.5; P = .18).
The safety analysis comparing secondary infection rates showed no difference between the arms (Supplementary Table 4), using both liberal infection definitions including “possible” pneumonia (40 infections per arm) and more restrictive “probable” and “proven” infections only (26 in the treatment arm, 25 in the control arm; 95% CI for difference, –0.15 to 0.13; P = 1.00). There were 10 cases of fungal infections (4 tocilizumab recipients, 6 controls) and 2 instances of herpesvirus reactivation (1 per arm). These infections did not appear to confer additional risk of mortality (31.3% and 31.0% mortality with and without secondary infection, respectively).
DISCUSSION
This study suggests that tocilizumab may be beneficial for patients hospitalized with COVID-19 who have elevated biochemical markers of inflammation [22, 42, 43]. Hazard of death was significantly lower among tocilizumab recipients, using both a marginal structural Cox model and traditional propensity score matching. Men >55 years of age with few comorbidities and moderate elevation in inflammatory markers were among those who benefited most. There was no difference between tocilizumab recipients and controls in time to clinical improvement or risk of secondary infection.
This study has several strengths. Tocilizumab approval within JHHS was based on set institutional criteria, which may have mitigated some effect of provider preference. While other retrospective studies matched on baseline characteristics alone [28], our study applied a marginal structural Cox model to account for disease progression before the receipt of tocilizumab and included a large number of significant demographic, clinical, and laboratory covariates. Length of follow-up was longer than some studies and showed that the mortality benefit of tocilizumab extends out to at least 28 days. We used robust statistical methods to address confounding by indication, including 2 different matching methods that yielded similar results. Adjudication of secondary infection was performed by a panel of infectious disease experts using a set of guidelines-based criteria and showed no excess infections with both stringent and liberal definitions [32–36].
Overall, our findings converge with those of several other published retrospective studies. Two studies of tocilizumab with concurrent controls found reduced mechanical ventilation and death [22, 44]. Another study using historical controls found higher survival, recovery, and respiratory function [43]. A single-arm study of mostly intubated patients found that clinical and laboratory parameters improved after tocilizumab administration [45]. A study of mechanically ventilated patients found a 45% reduction in hazards of death, despite more secondary infections in tocilizumab recipients, as well as improvement on the WHO ordinal scale [37]. A smaller study found numerical trends that were similar to ours but, perhaps due to the smaller sample size results, were not statistically significant [23]. Two meta-analyses have yielded conflicting results about the efficacy of tocilizumab in reducing COVID-19 mortality [46, 47].
There are now press release results available for 2 sponsor-initiated tocilizumab randomized controlled trials and published results of 3 investigator-initiated trials. Though the first sponsored trial of 450 participants with SpO2 <94% found no benefit in clinical status or mortality, time to discharge was 8 days shorter for tocilizumab recipients; it remains unclear how ill patients were at inclusion [27]. A subsequent sponsored trial, which intentionally enrolled minority racial and ethnic groups, found that receipt of tocilizumab was associated with 44% reduction in the composite outcome of death or need for mechanical ventilation (12.2% vs 19.3% at day 28) [26]. A recently published open-label randomized trial of patients with P:F 200–300 found no effect of tocilizumab on clinical worsening; however, only intensive care unit (ICU)–eligible patients were included, mortality rates were low (0.8% at day 14, 2.4% at day 30), and 12 of 66 control patients later received tocilizumab. Additionally, patients in the control arm were less sick (based on CRP, IL-6, ferritin, D-dimer) and treated more often with antivirals compared with tocilizumab recipients, despite randomization [25]. A second open-label randomized trial of patients with moderate to severe pneumonia requiring at least 3L oxygen via nasal cannula found no effect of tocilizumab on clinical worsening; benefit was seen at day 14 but not day 28 [24]. Of note, this study recruited less ill patients (non-ICU, on nasal cannula only), 47% of tocilizumab recipients received a second dose on day 3, and control arm patients received more immunomodulatory therapies including anakinra, eculizumab, and dexamethasone. In the only published randomized, double-blind, placebo-controlled trial to date, tocilizumab use was not effective for preventing intubation or death. However, the trial was small (243 patients), and the confidence intervals for efficacy comparisons were wide [29]. All 3 published trials found lower rates of secondary infection in the tocilizumab arms. Studies of similar drugs targeting inflammatory cytokines have yielded mixed results [48–50] and highlight the need for additional well-designed, masked randomized controlled trials.
Similar to other studies, we found that elevated inflammatory markers were associated with worse outcomes overall (Supplementary Table 5) [2, 51], including higher mortality for all patients with IL-6 ≥55 pg/mL [52, 53]. It remains unclear whether IL-6 is a marker of disease progression or directly contributes to pathogenesis in COVID-19 [54]. We found that for patients with IL-6 ≥55 pg/mL there was a 66% reduction in hazard of death among those who received tocilizumab compared with controls. The average IL-6 level of treated patients in the published randomized trials of tocilizumab ranged from 23.6 pg/mL to 50.4 pg/mL [25, 29]. In our study, 89% of treated patients had an IL-6 level >55 pg/mL with a mean level of 865.6 pg/mL and a median level of 239.8 pg/mL. The fact that our study showed a robust mortality benefit in contrast to these recent randomized trials may indicate that elevated IL-6 levels, likely a proxy for a hyperinflammatory state, are necessary in order for patients to benefit from tocilizumab.
Limitations
From a heterogeneous cohort, our matching generated comparable groups in terms of numerous covariates relevant to the end points of death and clinical improvement. However, there could be unmeasured variables that biased our treatment effect estimates. Though our study and some others have not found excess secondary infection risk, this observation may not be generalizable [55], and the risk of certain infectious complications, such as herpesvirus reactivation, may be uniquely elevated after tocilizumab [56]. Additionally, larger tertiary care centers where these observational studies are taking place may have more robust infectious disease expertise to help mitigate these infectious risks and prevent ensuing deaths. Therapeutic guidance at Johns Hopkins evolved over the study period, which could have introduced biases of secular trends. Infection fatality rates due to COVID-19 have overall declined since these data were collected, which may limit direct translation of these observations into clinical practice. To address secular trends in treatments and mortality, controls were selected from the same time period as tocilizumab recipients, and both arms had a similar tempo of recruitment over time (Supplementary Figure 1). Nonetheless, most of the included patients received tocilizumab before data supporting remdesivir and dexamethasone had emerged. The treatment landscape for COVID-19 is dynamic, and the role of tocilizumab in combination with dexamethasone and remdesivir remains to be elucidated. Additionally, tocilizumab was given at a median of 2.6 days after admission in our study, but the optimal timing of administration in the disease course remains unknown.
CONCLUSIONS
We found that among patients hospitalized with COVID-19 and elevated biochemical markers suggestive of a hyperinflammatory state, administration of tocilizumab reduced the hazard of death by 62%. Particular subgroups who appeared to benefit included older males with few comorbidities and elevated inflammatory markers such as IL-6. There was no evidence of an increased risk of hospital-acquired infection among tocilizumab recipients. The optimal dosing strategy, timing of administration, and combined use with other therapeutics remain to be elucidated. While we await the results of additional randomized trials, our study has identified clinical and laboratory features that predict potential benefits from tocilizumab in hospitalized patients with COVID-19 and evidence of hyperinflammation.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We are grateful to the patients whose data are summarized in this study. The data utilized for this publication were part of the JH-CROWN: the COVID PMAP Registry, which is based on the contributions of many patients and clinicians. JH-CROWN received funding from Hopkins inHealth, the Johns Hopkins Precision Medicine Program.
Financial support. This trial did not receive separate funding. E.H.I. is supported by T32 GM066691-17. K.W., Y.X., M.R., and B.G. are partially funded by U3REP150540, a grant from the US Office of the Assistant Secretary for Preparedness and Response (ASPR), administered through the Office of Preparedness and Response at the Maryland State Department of Health. The JH-CROWN registry is funded by Hopkins inHealth.
Potential conflicts of interest. P.G.A. is on the DSMB for Humanigen. R.K.A. has received grant/study support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, and Takeda/Shire. T.J. has a consultancy with Takeda Oncology and is on the advisory board for CareDx and Bristol Myers Squibb. M.T.M. reports research grants to his institution from Gilead Sciences. B.G.P. has received funding from Bristol Myers Squibb and AstraZeneca. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the US Armed Forces. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
IRB approval. The institutional review boards at each of the 5 participating hospitals approved this study as minimal risk and waived consent requirements.
References
- 1.Johns Hopkins Coronavirus Resource Center. COVID-19 map. Available at: https://coronavirus.jhu.edu/map.html. Accessed 19 November 2020.
- 2. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature 2020; 583:437–40. [DOI] [PubMed] [Google Scholar]
- 3. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130:2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient Trajectories Among Persons Hospitalized for COVID-19. Ann Intern Med. Published online September 22, 2020:M20-3905. doi: 10.7326/M20-3905. [DOI] [Google Scholar]
- 5. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 2020; 71:762–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jain T, Bar M, Kansagra AJ, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant 2019; 25:2305–21. [DOI] [PubMed] [Google Scholar]
- 7. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Behrens EM. Macrophage activation syndrome in rheumatic disease: what is the role of the antigen presenting cell? Autoimmun Rev 2008; 7:305–8. [DOI] [PubMed] [Google Scholar]
- 9. Yokota S, Itoh Y, Morio T, et al. Tocilizumab in systemic juvenile idiopathic arthritis in a real-world clinical setting: results from 1 year of postmarketing surveillance follow-up of 417 patients in Japan. Ann Rheum Dis 2016; 75:1654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 2018; 23:943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neelapu SS, Tummala S, Kebriaei P, et al. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘ALL.’ Nat Rev Clin Oncol 2018; 15:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med 2020; 180:1152. [DOI] [PubMed] [Google Scholar]
- 13. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. RECOVERY Collaborative Group. Effect of dexamethasone in hospitalized patients with COVID-19 – preliminary report 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.06.22.20137273v1.full.pdf. Accessed 22 August 2020.
- 16. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Massachusetts General Hospital. COVID-19 clinical trials Available at: https://www.massgeneral.org/news/coronavirus/research/therapies/coronavirus-clinical-trials. Accessed 6 July 2020.
- 18. Johns Hopkins Institute for Clinical and Translational Research. Therapeutic protocols for hospitalized patients Available at: https://ictr.johnshopkins.edu/coronavirus/active-therapeutic-protocols/therapeutichospitalized/. Accessed 6 July 2020.
- 19. Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020; 5:eabd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang X, Peck R. Clinical pharmacology of tocilizumab for the treatment of patients with rheumatoid arthritis. Expert Rev Clin Pharmacol 2011; 4:539–58. [DOI] [PubMed] [Google Scholar]
- 21. Price CC, Altice FL, Shyr Y, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized patients with coronavirus disease 2019: survival and clinical outcomes. Chest 2020; 158:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2020; 2:e474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campochiaro C, Della-Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med 2020; 76:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia. JAMA Intern Med 2021; 181:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2020; 324:1048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roche. Roche’s phase III EMPACTA study showed Actemra/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID-19 associated pneumonia 2020. Available at: https://www.roche.com/dam/jcr:2ca93ba2-739c-4b69-a971-2a94fca2d818/en/18092020-mr-empacta.pdf. Accessed 5 October 2020.
- 27. Roche. Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia 2020. Available at: https://www.roche.com/dam/jcr:6d8de90d-2e31-43c8-b4e1-0a24a2675015/en/29072020-mr-covacta.pdf. Accessed 5 October 2020.
- 28. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med 2020; e206252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020; 383:2333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gardner RA, Ceppi F, Rivers J, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood 2019; 134:2149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization (WHO). Novel coronavirus: COVID-19 therapeutic trial synopsis 2020. Available at: https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf.
- 32. De Pauw B, Walsh TJ, Donnelly JP, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pergam SA, Limaye AP; AST Infectious Diseases Community of Practice Varicella zoster virus in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13622. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention (CDC). Ventilator-associated events 2020. Available at: https://www.cdc.gov/nhsn/PDFs/pscManual/10-VAE_FINAL.pdf. Accessed 26 October 2020.
- 35. Centers for Disease Control and Prevention (CDC). Identifying healthcare-associated infections (HAI) for NHSN surveillance 2020. Available at: http://www.cdc.gov/nhsn/PDFs/pscManual/2PSC_IdentifyingHAIs_NHSNcurrent.pdf. Accessed 26 October 2020.
- 36. Lee DH, Zuckerman RA; AST Infectious Diseases Community of Practice Herpes simplex virus infections in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13526. [DOI] [PubMed] [Google Scholar]
- 37. Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis 2020: 1–10. doi: 10.1093/cid/ciaa954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hernán MA, Brumback BA, Robins JM. Estimating the causal effect of zidovudine on CD4 count with a marginal structural model for repeated measures. Stat Med 2002; 21:1689–709. [DOI] [PubMed] [Google Scholar]
- 39. Rice TW, Wheeler AP, Bernard GR, et al. ; National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007; 132:410–7. [DOI] [PubMed] [Google Scholar]
- 40. van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw 2011; 45:1–67. [Google Scholar]
- 41. R: A Language and Environment for Statistical Computing. R Core Team; 2019. [Google Scholar]
- 42. Kewan T, Covut F, Al–Jaghbeer MJ, Rose L, Gopalakrishna KV, Akbik B. Tocilizumab for treatment of patients with severe COVID–19: A retrospective cohort study. EClinicalMedicine 2020; 24:100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Capra R, De Rossi N, Mattioli F, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med 2020; 76:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Menzella F, Fontana M, Salvarani C, et al. Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation. Crit Care 2020; 24:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jordan SC, Zakowski P, Tran HP, et al. Compassionate Use of Tocilizumab for Treatment of SARS-CoV-2 Pneumonia [published online ahead of print June 23, 2020]. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lan SH, Lai CC, Huang HT, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents 2020; 56:106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao J, Cui W, Tian BP. Efficacy of tocilizumab treatment in severely ill COVID-19 patients. Crit Care 2020; 24:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sanofi and Regeneron provide update on Kevzara® (sarilumab) phase 3 U.S. trial in COVID-19 patients 2020. Available at: https://www.sanofi.com/en/media-room/press-releases/2020/2020-07-02-22-30-00. Accessed 7 July 2020.
- 49. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol 2020; 2:e393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim GW, Lee NR, Pi RH, et al. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res 2015; 38:575–84. [DOI] [PubMed] [Google Scholar]
- 51. Knight SR, Ho A, Pius R, et al. ; ISARIC4C investigators Risk stratification of patients admitted to hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ 2020; 370:m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J Med Virol 2020; 92:2283–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Galván-Román JM, Rodríguez-García SC, Roy-Vallejo E, et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study [published online ahead of print September 30, 2020]. J Allergy Clin Immunol 2020; doi: 10.1016/j.jaci.2020.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 2020; 19:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frigault MJ, Nikiforow S, Mansour MK, et al. Tocilizumab not associated with increased infection risk after CAR T-cell therapy: implications for COVID-19? Blood 2020; 136:137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Busani S, Bedini A, et al. Two fatal cases of acute liver failure due to HSV-1 infection in COVID-19 patients following immunomodulatory therapies [published online ahead of print August 25, 2020]. Clin Infect Dis 2020: 1–14. doi: 10.1093/cid/ciaa1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.