Abstract
Objectives
Concern has been raised in the rheumatology community regarding recent regulatory warnings that HCQ used in the coronavirus disease 2019 pandemic could cause acute psychiatric events. We aimed to study whether there is risk of incident depression, suicidal ideation or psychosis associated with HCQ as used for RA.
Methods
We performed a new-user cohort study using claims and electronic medical records from 10 sources and 3 countries (Germany, UK and USA). RA patients ≥18 years of age and initiating HCQ were compared with those initiating SSZ (active comparator) and followed up in the short (30 days) and long term (on treatment). Study outcomes included depression, suicide/suicidal ideation and hospitalization for psychosis. Propensity score stratification and calibration using negative control outcomes were used to address confounding. Cox models were fitted to estimate database-specific calibrated hazard ratios (HRs), with estimates pooled where I2 <40%.
Results
A total of 918 144 and 290 383 users of HCQ and SSZ, respectively, were included. No consistent risk of psychiatric events was observed with short-term HCQ (compared with SSZ) use, with meta-analytic HRs of 0.96 (95% CI 0.79, 1.16) for depression, 0.94 (95% CI 0.49, 1.77) for suicide/suicidal ideation and 1.03 (95% CI 0.66, 1.60) for psychosis. No consistent long-term risk was seen, with meta-analytic HRs of 0.94 (95% CI 0.71, 1.26) for depression, 0.77 (95% CI 0.56, 1.07) for suicide/suicidal ideation and 0.99 (95% CI 0.72, 1.35) for psychosis.
Conclusion
HCQ as used to treat RA does not appear to increase the risk of depression, suicide/suicidal ideation or psychosis compared with SSZ. No effects were seen in the short or long term. Use at a higher dose or for different indications needs further investigation.
Trial registration
Registered with EU PAS (reference no. EUPAS34497; http://www.encepp.eu/encepp/viewResource.htm? id=34498). The full study protocol and analysis source code can be found at https://github.com/ohdsi-studies/Covid19EstimationHydroxychloroquine2.
Keywords: HCQ; safety; epidemiology, RA; psychosis; depression
Rheumatology key messages
This is the largest study on the neuropsychiatric safety of hydroxychloroquine, including >900 000 users internationally.
We found no association between hydroxychloroquine treatment for RA and depression, suicide or psychosis compared with sulfasalazine.
These findings do not support stopping hydroxychloroquine for RA based on concerns raised in COVID-19 patients.
Introduction
Hydroxychloroquine (HCQ) has received much scientific and public attention during the coronavirus disease 2019 (COVID-19) pandemic as a leading therapeutic and prophylactic target [1, 2]. Commonly used for autoimmune disorders (e.g. SLE) and inflammatory arthritis, HCQ was released for emergency use for COVID-19 due to its postulated antiviral efficacy in cellular studies [3–9]. HCQ is currently being used in >217 registered ongoing clinical trials for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as of 12 June 2020 [10, 11]. Results to date have been conflicting, with emerging data suggesting a lack of clinical efficacy against COVID-19 [12–18]. Case report literature suggests that chloroquine, the compound from which HCQ was derived, is associated with neurological and psychiatric side effects when used as an antimalarial treatment or prophylaxis [19]. Similar potential side effects that have been described in the use of HCQ include neuropsychiatric side effects such as psychosis, depression and suicidal behaviour [20–22]. Regulatory authorities have received reports of new-onset psychiatric symptoms associated with the increased use of high-dose HCQ during the pandemic [23]. While chloroquine and HCQ have multiple mechanisms of action, a major action is the disruption of lysosomal functioning and autophagy [24]. These actions to some degree mimic lysosomal storage diseases, disorders that are characterized by neurodevelopmental delay and neurodegeneration when manifested in the more common form in childhood, but also associated with neuropsychiatric manifestations in adulthood [25, 26].
New reports of serious side effects associated with HCQ used in COVID-19 are concerning to the rheumatology community, leading to confusion and anxiety for patients who are taking HCQ for autoimmune conditions. Given the previous reports of neuropsychiatric symptoms with HCQ, together with a plausible mechanism for such phenomena, we performed a review of the literature to determine what was already known about the potential risks of psychosis, depression and suicide associated with HCQ use from literature database inception until 14 May 2020 (Supplementary Appendix Section 1, available at Rheumatology online). Interrogation of adverse event registers have identified potential associations between HCQ and psychiatric disorders [11]. Case reports and case series describing new-onset psychosis, bipolar disorder, seizures and depression associated with HCQ and chloroquine use for rheumatologic disorders and malaria prophylaxis can be found as early as 1964 [20, 27–35]. No clinical trial or observational study was found that had investigated the incidence of new-onset neuropsychiatric symptoms associated with HCQ use.
Considering the widespread use of HCQ in rheumatology, we therefore aimed to determine whether there is an association between incident HCQ use for RA (the most common indication for the drug) and the onset of acute psychiatric events, including depression, suicide and psychosis, compared with SSZ.
Methods
Study design
A new-user cohort, active-comparator design was used, as recommended by methodological guidelines for observational drug safety research [36]. The study protocol is registered in the European Union Post-Authorisation Studies Register as EUPAS34497 [37].
SSZ was used as the active comparator for HCQ, as both SSZ and HCQ are second-line conventional synthetic DMARDs (csDMARDs) used in addition to or instead of MTX. While it is acknowledged that the drugs are not exactly equivalent, SSZ was felt to be the closest possible drug to HCQ in an RA cohort. Aware that there are other rheumatologic indications for using HCQ, such as SLE, we designed the study to include propensity score (PS) stratification and matching to prevent confounding. We used a set of diagnostic tools to check the PS adjustments in each dataset for any imbalances that may have remained despite stratification and also used negative control outcomes to identify if unobserved confounding had occurred. Analyses were not completed and are not reported if imbalance remained despite PS stratification or there appeared to be a large proportion of negative control outcomes outside our level of tolerance. All of these diagnostic tools were assessed while results were blinded and can be freely reviewed online. Further details are given in the statistical analysis section.
Data sources
Electronic health records and administrative claims data from the UK and USA were used, previously mapped to the Observational Medical Outcomes Partnership (OMOP), common data model (CDM). The study period covered from September 2000 until the latest data available at the time of extraction in each database. Data from 10 data sources were analysed in a federated manner using a distributed network strategy in collaboration with the Observational Health Data Science and Informatics (OHDSI) and European Health Data and Evidence Network communities (EHDEN). The data used included primary care electronic medical records from the UK [Clinical Practice Research Datalink (CPRD) and IQVIA Medical Research Data (IMRD)]; specialist ambulatory care electronic health records from Germany [IQVIA Database Analyzer Germany (DAGermany)]; electronic health records in a sample of US inpatient and outpatient facilities in the Optum® de-identified Electronic Health Record dataset (Optum EHR) and IQVIA US Ambulatory Electronic Medical Records (AmbEMR); and US claims data from the IBM MarketScan® Commercial Claims Database (CCAE), Optum® de-identified Clinformatics® Data Mart Database–Date of Death (Clinformatics), IBM MarketScan® Medicare Supplemental Database (MDCR), IBM MarketScan® Multi-State Medicaid Database (MDCD) and IQVIA OpenClaims (OpenClaims). In addition, data were obtained and analysed from electronic primary care data from the Netherlands (IPCI database) and Spain (SIDIAP) and from Japanese claims (JMDC), but none of these analyses were deemed appropriate due to low/no event counts in at least one of the cohorts. A more detailed description of all these data sources is available in Supplementary Appendix Section 2, available at Rheumatology online.
Follow-up
Participants were followed up from the date of initiation (first dispensing or prescription) of HCQ or SSZ (index date) as described in detail in Supplementary Appendix Section 3.1, available at Rheumatology online. SSZ was proposed as an active comparator, as it shares a similar indication as a second-line csDMARD for RA. Two different follow-up periods were prespecified to look at short- and long-term effects, respectively. First, a fixed 30 day time window from the index date was used to study short-term effects, where follow-up included from day 1 post-index until the earliest of loss to follow-up/death, outcome of interest or 30 days from therapy initiation, regardless of compliance/persistence with the study drug. Second, in a long-term (on treatment) analysis, follow-up went from day 1 post-index until the earliest of therapy discontinuation (with a 14 day additional washout), outcome of interest or loss to follow-up/death. Continued treatment episodes were constructed based on dispensing/prescription records, with a 90 day refill gap allowed to account for stockpiling.
Participants
All subjects registered in any of the contributing data sources for at least 365 days prior to the index date, ≥18 years of age, with a history of RA (as defined by a recorded diagnosis any time before or on the same day as therapy initiation) and starting either HCQ or SSZ during the study period were included.
Potential participant counts and age-, sex- and calendar year-specific incidence per database were produced for transparency and reviewed to check for data inconsistencies and face validity and are available for inspection at https://data.ohdsi.org/Covid19CohortEvaluationExposures/, labelled as ‘New users of hydroxychloroquine with previous rheumatoid arthritis’ and ‘New users of sulfasalazine with previous rheumatoid arthritis’.
Outcomes and confounders
Code lists for the identification of the study population, for the study exposures and for the relevant outcomes were created by clinicians with experience in the management of RA and by clinical epidemiologists using ATLAS, a science analytics platform that provides a unified interface for researchers [38]. Exposures and outcomes were reviewed by experts in Observational Medical Outcomes Partnership vocabulary and in the use of the proposed data sources. A total of three outcomes were analysed: depression, suicide or suicidal ideation and hospital admission for psychosis. Detailed outcome definitions with links to code lists are fully detailed in Supplementary Appendix Section 3.2, available at Rheumatology online [39, 40]. Cohort counts for each of the outcomes in the entire source database and age-, sex- and calendar year–specific incidence rates were explored for each of the contributing databases and reviewed to check for data inconsistencies and face validity. These are available for inspection at https://data.ohdsi.org/Covid19CohortEvaluationSafetyOutcomes/.
A list of negative control outcomes was generated for which there is no biologically plausible or known causal relationship with the use of HCQ or SSZ. These outcomes were identified based on previous literature, clinical knowledge (reviewed by two clinicians), product labels and spontaneous reports and confirmed by manual review by two clinicians [41]. The full list of codes used to identify negative control outcomes can be found in Supplementary Appendix Section 4, available at Rheumatology online.
Statistical methods
All analytical source code is available for inspection and reproducibility at https://github.com/ohdsi-studies/Covid19EstimationHydroxychloroquine2. All study diagnostics and the steps described below are available for review at https://data.ohdsi.org/Covid19EstimationHydroxychloroquine2/.
The following steps were followed for each analysis:
1. PS estimation
PS stratification was used to minimize confounding. All baseline characteristics recorded in the participants’ records/health claims were constructed for inclusion as potential confounders (including demographics, past medical history, procedures and medication prescription within 30 and within 365 days prior to drug initiation). Covariate construction details are available in Supplementary Appendix Section 5, available at Rheumatology online. Lasso regression models were fitted to estimate PS as the probability of HCQ vs SSZ use based on patient demographics and medical history, including previous conditions, procedures, healthcare resource use and treatments. The balance of known characteristics that could cause potential confounding were then reviewed while the results were blinded in order to determine whether a dataset was able to contribute to the meta-analysis. This was undertaken in two ways. First, we used the PS scores themselves and the standardized difference between the scores prior to and after PS stratification to determine whether the cohorts of SSZ and HCQ users were imbalanced. Second, we looked at the PS model pictorially in a graph to see if the populations appeared to ‘overlap’ in their characteristics. The full resulting PS models are available for inspection by clicking on ‘Propensity model’ and ‘Propensity scores’ after selecting a database in the results app (https://data.ohdsi.org/Covid19EstimationHydroxychloroquine2/).
2. Study diagnostics
Study diagnostics were explored for each database-specific analysis before progressing to outcome modelling, and included checks for power, observed confounding and potential residual (unobserved) confounding. Only database-outcome analyses that passed all diagnostics below were then conducted and reported, with all others marked as ‘NA’ in the accompanying results app.
Positivity and power were assessed by looking at the number of participants in each treatment arm and the number with the outcome (see the ‘Power’ tab after clicking on a database in the results app). Small cell counts less than five (and resulting estimates) are reported as ‘<5’ to minimize the risk of secondary disclosure of data with patient identification. PS overlap was also plotted to visualize positivity issues and can be seen by clicking on ‘Propensity scores’.
Observed confounding was explored by plotting standardized differences before (x-axis) vs after (y-axis) PS stratification, with standardized differences >0.1 in the y-axis indicating the presence of unresolved confounding, which can be seen by clicking on ‘Covariate balance’ in the results app [36].
Finally, negative control outcome analyses were assessed to identify systematic error due to residual (unobserved) confounding. The results for these are available in the ‘Systematic error’ tab of the results app. The resulting information was used to calibrate the outcome models using empirical calibration [37, 38].
3. Outcome modelling
Cox proportional hazards models conditioned on the PS strata were fitted to estimate hazard ratios (HRs) for each psychological outcome in new users of HCQ (vs SSZ). Empirical calibration based on the previously described negative control outcomes was used to minimize any potential residual confounding with calibrated HRs and 95% CIs estimated [42, 43]. All analyses were conducted for each database separately, with estimates combined in random-effects meta-analysis methods where I2 was ≤40% [44]. The standard errors of the database-specific estimates were adjusted to incorporate estimate variation across databases, where the across-database variance was estimated by comparing each database-specific result to that of an inverse-variance, fixed-effects meta-analysis. No meta-analysis was conducted where I2 for a given drug–outcome pair was >40%.
All analyses were conducted using the CohortMethod package, available at https://ohdsi.github.io/CohortMethod/ and the Cyclops package for PS estimation (https://ohdsi.github.io/Cyclops) [45].
Data sharing
Open science is a guiding principle within the OHDSI. As such, we provide unfettered access to all open-source analysis tools employed in this study via https://github.com/OHDSI/, as well as all data and results artefacts that do not include patient-level health information via http://data.ohdsi.org/Covid19EstimationHydroxychloroquine2. Data partners contributing to this study remain custodians of their individual patient-level health information and hold either institutional review board exemption or approval for participation.
Results
A total of 918 144 HCQ and 290 383 SSZ users were identified. Participant counts in each data source are provided in Supplementary Appendix Section 6, available at Rheumatology online. Before PS stratification, users of HCQ were (compared with SSZ users) more likely female (e.g. 82.0% vs 74.3% in the CCAE database) and less likely to have certain comorbidities such as Crohn’s disease (0.6% vs 1.8% in the CCAE) or psoriasis (3.0% vs 8.9% in the CCAE). The prevalence of a past medical history of SLE was higher in HCQ users as expected (1.5% vs 0.5% in the CCAE), while the use of systemic glucocorticoids was similar (46.1% vs 47.2% in the previous month in the CCAE). The prevalence of depressive disorder was similar in both groups (13.4% vs 13.5% in the CCAE) and so was the history of use of antidepressants in the previous year (36.4% vs 36.4% in the CCAE), which appears in keeping with the prevalence discussed in previous literature [46]. After PS stratification, the prevalence of a past medical history of SLE, depressive disorder and the use of systemic glucocorticoids and antidepressants were balanced with a standard difference of <0.1 between HCQ and SSZ users. As these were balanced, these patients were not excluded from the analyses.
Detailed baseline characteristics for the two pairs of treatment groups after PS stratification in the CCAE are shown in Table 1 as an example, with the balance of SLE, depression and anti-depressant medication use included. Similar tables and a more extensive list of features are provided in Supplementary Appendix Section 7, available at Rheumatology online, and can also be searched for in the results app (click on a given dataset, then click on the population characteristics tab, raw and search for the condition or drug of interest). Study diagnostics including plots of propensity score distribution, covariate balance and negative control estimate distributions are provided in Supplementary Appendix Section 8.
Table 1.
Baseline characteristics of patients with RA who are new users of HCQ vs SSZ, before and after PS stratification, in the CCAE database
| Characteristics | Before PS stratification |
After PS stratification |
||||
|---|---|---|---|---|---|---|
| HCQ, % | SSZ, % | Std diff. | HCQ, % | SSZ, % | Std diff. | |
| Sociodemographics | ||||||
| Age group (years) | ||||||
| 15–19 | 0.6 | 0.6 | 0.00 | 0.6 | 0.6 | 0.00 |
| 20–24 | 1.9 | 1.9 | 0.00 | 1.8 | 2.0 | −0.01 |
| 25–29 | 2.6 | 2.6 | 0.00 | 2.5 | 2.8 | −0.01 |
| 30–34 | 4.5 | 4.6 | 0.00 | 4.5 | 4.3 | 0.01 |
| 35–39 | 7.2 | 7.3 | 0.00 | 7.1 | 7.1 | 0.00 |
| 40–44 | 9.8 | 9.5 | 0.01 | 9.7 | 9.5 | 0.00 |
| 45–49 | 13.7 | 12.9 | 0.02 | 13.6 | 13.5 | 0.00 |
| 50–54 | 18.2 | 18.2 | 0.00 | 18.2 | 18.1 | 0.00 |
| 55–59 | 20.6 | 21.0 | −0.01 | 20.8 | 20.8 | 0.00 |
| 60–64 | 19.0 | 19.7 | −0.02 | 19.4 | 19.8 | −0.01 |
| 65–69 | 1.8 | 1.7 | 0.01 | 1.8 | 1.6 | 0.01 |
| Gender, female | 82.0 | 74.3 | 0.19 | 80.1 | 79.7 | 0.01 |
| Medical history | ||||||
| Acute respiratory disease | 35.5 | 34.3 | 0.03 | 35.1 | 34.7 | 0.01 |
| Chronic liver disease | 3.2 | 3.2 | 0.00 | 3.2 | 3.4 | −0.01 |
| Chronic obstructive lung disease | 4.2 | 4.5 | −0.01 | 4.3 | 4.5 | −0.01 |
| Crohn’s disease | 0.6 | 1.8 | −0.12 | 0.7 | 1.1 | −0.04 |
| Depressive disorder | 13.4 | 13.5 | 0.00 | 13.2 | 13.4 | −0.01 |
| Diabetes mellitus | 13.5 | 13.4 | 0.00 | 13.6 | 13.7 | 0.00 |
| Hypertensive disorder | 34.7 | 34.9 | 0.00 | 34.7 | 35.0 | −0.01 |
| Obesity | 9.3 | 9.1 | 0.00 | 9.2 | 9.4 | −0.01 |
| Psoriasis | 3.0 | 8.9 | −0.25 | 3.8 | 5.2 | −0.07 |
| Renal impairment | 3.1 | 2.8 | 0.02 | 3.0 | 2.8 | 0.01 |
| SLE | 1.5 | 0.5 | 0.10 | 1.3 | 0.9 | 0.03 |
| Schizophrenia | 0.1 | 0.1 | −0.01 | 0.1 | 0.1 | −0.01 |
| Ulcerative colitis | 0.6 | 1.9 | −0.12 | 0.7 | 1.0 | −0.04 |
| Medication use | ||||||
| Agents acting on the renin–angiotensin system | 24.3 | 24.9 | −0.01 | 24.5 | 24.7 | 0.00 |
| Antidepressants | 36.4 | 36.4 | 0.00 | 36.3 | 36.5 | 0.00 |
| Anti-epileptics | 20.3 | 21.0 | −0.02 | 20.4 | 20.2 | 0.00 |
| Anti-inflammatory and anti-rheumatic products | 55.3 | 57.3 | −0.04 | 55.8 | 56.7 | −0.02 |
| Anti-psoriatics | 0.7 | 1.3 | −0.06 | 0.7 | 1.0 | −0.03 |
| Anti-thrombotic agents | 7.4 | 7.3 | 0.01 | 7.4 | 7.3 | 0.00 |
| Immunosuppressants | 39.6 | 53.1 | −0.27 | 43.4 | 43.6 | 0.00 |
| Opioids | 38.5 | 40.8 | −0.05 | 39.0 | 39.3 | −0.01 |
| Psycholeptics | 33.6 | 33.7 | 0.00 | 33.4 | 33.3 | 0.00 |
Std diff.: standardised difference.
The average baseline dose of HCQ was homogeneous, with >97% in the CCAE using an average dose of 420 mg daily and <3% taking an estimated dose >500 mg. All the observed differences between groups were minimized to an acceptable degree (<0.1 standardized mean differences) after PS stratification: in the CCAE, the most imbalanced variable was the use of glucocorticoids on index date, with a prevalence of 36.1% vs 35.8%.
Database-specific and overall counts and rates of the three study outcomes in the short- (30 day) and long-term (‘on treatment’) analyses are reported in detail in Table 2. Depression was the most common of the three study outcomes, with rates in the ‘on treatment’ analysis ranging from 1.99/1000 person-years among HCQ users in the CPRD to 17.74/1000 among HCQ users in the AmbEMR. Suicide/suicidal ideation was the least common outcome, with rates ranging from 0.32/1000 (HCQ users in the AmbEMR and SSZ users in the IMRD) to 14.08/1000 in SSZ users in the MDCD. Database-specific counts and incidence rates (IRs) for all three outcomes stratified by drug use are detailed in full in Supplementary Appendix Section 9, available at Rheumatology online.
Table 2.
Patient counts, event counts and incidence rates (IRs; per 1000 person-years) of key events according to drug use.
| 30-day follow-up |
On-treatment follow up |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients |
Events |
IR (/1000 py) |
Patients |
Events |
IR (/1000 py) |
||||||||
| Outcome | Database | T | C | T | C | T | C | T | C | T | C | T | C |
| Depression | AmbEMR | 55 793 | 15 092 | 155 | 29 | 33.91 | 23.44 | 55 793 | 15 092 | 320 | 80 | 17.74 | 14.34 |
| CCAE | 66 440 | 22 449 | 79 | 28 | 14.64 | 15.36 | 66 440 | 22 449 | 557 | 137 | 8.54 | 9.40 | |
| Clinformatics | 51 676 | 16 812 | 84 | 41 | 20.05 | 30.09 | 51 676 | 16 812 | 657 | 178 | 12.43 | 15.00 | |
| CPRD | 9160 | 11 348 | <5 | 8 | <6.67 | 8.60 | 9160 | 11 348 | 36 | 94 | 1.99 | 3.60 | |
| DAGermany | 3937 | 5109 | <5 | 12 | <15.48 | 28.63 | 3937 | 5109 | 40 | 70 | 15.47 | 19.66 | |
| IMRD | 8844 | 8456 | <5 | 6 | <6.91 | 8.67 | 8844 | 8456 | 38 | 51 | 2.20 | 2.72 | |
| MDCD | 7950 | 2286 | 14 | 6 | 21.61 | 32.29 | 7950 | 2286 | 90 | 13 | 15.81 | 10.12 | |
| MDCR | 15 735 | 5275 | 13 | 6 | 10.14 | 13.98 | 15 735 | 5275 | 97 | 38 | 5.37 | 9.27 | |
| OpenClaims | 620 081 | 183 312 | 654 | 161 | 12.85 | 10.70 | 620 081 | 183 312 | 4810 | 957 | 5.59 | 5.58 | |
| OptumEHR | 78 528 | 20 244 | 321 | 66 | 50.56 | 40.30 | NA | NA | NA | NA | NA | NA | |
| Meta-analysis | 918 144 | 290 383 | <1335 | 363 | <17.77 | 15.28 | 839 616 | 270 139 | 6645 | 1618 | 6.28 | 6.29 | |
| Suicide and suicidal ideation | AmbEMR | NA | NA | NA | NA | NA | NA | 57 660 | 15 357 | 6 | <5 | 0.32 | <0.88 |
| CCAE | 66 533 | 22 471 | 12 | <5 | 2.22 | <2.74 | 66 533 | 22 471 | 81 | 28 | 1.23 | 1.91 | |
| Clinformatics | 51 807 | 16 843 | 12 | <5 | 2.85 | <3.66 | 51 807 | 16 843 | 97 | 30 | 1.80 | 2.50 | |
| CPRD | 9167 | 11 358 | <5 | <5 | <6.66 | <5.37 | 9167 | 11 358 | 7 | 9 | 0.39 | 0.34 | |
| IMRD | 8852 | 8460 | <5 | <5 | <6.91 | <7.22 | 8852 | 8460 | 8 | 6 | 0.46 | 0.32 | |
| MDCD | 7980 | 2296 | <5 | <5 | <7.68 | <26.78 | 7980 | 2296 | 56 | 18 | 9.71 | 14.08 | |
| MDCR | NA | NA | NA | NA | NA | NA | 15 752 | 5278 | 15 | 6 | 0.83 | 1.45 | |
| OpenClaims | 621 067 | 183 550 | 34 | 8 | 0.67 | 0.53 | 621 067 | 183 550 | 321 | 89 | 0.37 | 0.52 | |
| OptumEHR | 79 903 | 20 480 | 18 | 8 | 2.78 | 4.82 | NA | NA | NA | NA | NA | NA | |
| Meta-analysis | 845 309 | 265 458 | <91 | <41 | <1.31 | <1.89 | 838 818 | 265 613 | 591 | <191 | 0.55 | <0.75 | |
| Hospitalization for psychosis | OpenClaims | 620 964 | 183 527 | 95 | 27 | 1.86 | 1.79 | 620 964 | 183 527 | 1108 | 221 | 1.28 | 1.28 |
| OptumEHR | 79 994 | 20 508 | <5 | <5 | <0.77 | <3.01 | NA | NA | NA | NA | NA | NA | |
| Meta-analysis | 700 958 | 204 035 | <100 | <32 | <1.74 | <1.91 | NA | NA | NA | NA | NA | NA | |
T, target therapy; C, comparator therapy; IR, incidence rate; py, person-years at risk; NA, not applicable (not reported because of failed diagnostics or on-treatment follow-up unavailable); AmbEMR, IQVIA Ambulatory EMR; CCAE, IBM Commercial Database; Clinformatics, Optum de-identified Clinformatics Data Mart Database; CPRD, Clinical Practice Research Datalink; DAGermany, IQVIA Disease Analyzer Germany; IMRD, IQVIA UK Integrated Medical Record Data; MDCD, IBM Multistate Medicaid; MDCR, IBM Medicare Supplemental Database; OpenClaims, IQVIA Open Claims; OptumEHR, Optum de-identified Electronic Health Record dataset.
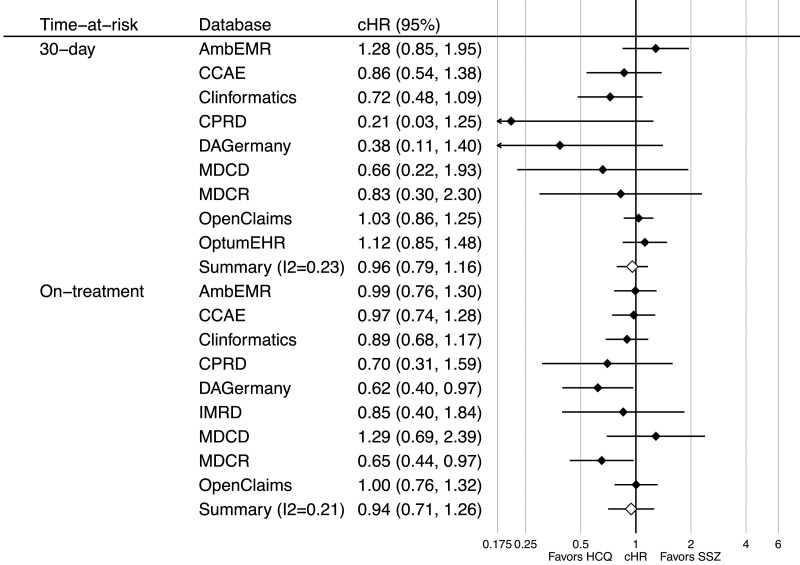
Nine datasets passed cohort diagnostics and contained sufficiently robust data for inclusion into the short-term analyses for depression; six passed for suicide and two passed for psychosis. A small imbalance with the incidence of a past medical history of SLE was seen in the MDCD and with cutaneous lupus in DAGermany. As a result, we excluded both from the psychosis outcome but not for depression, as we did not consider this was a confounder. Short-term (30-day) analyses showed no consistent association between HCQ use and the risk of depression, with database-specific HRs ranging from 0.21 (95% CI 0.03, 1.25) in the CPRD to 1.28 (95% CI 0.85, 1.95) in the AmbEMR, with a meta-analytic HR of 0.96 (95% CI 0.79, 1.16) (See Fig. 1, top). On-treatment analyses showed similar findings, with database-specific HRs from 0.62 (95% CI 0.40, 0.97) in DAGermany to 1.29 (95% CI 0.69, 2.39) in the MDCD, with a meta-analytic HR of 0.94 (95% CI 0.71, 1.26) (Fig. 1, bottom plot). Note only databases passing diagnostics are included within the plot and meta-analysis.
Fig. 1.
Forest plot of the association between short- (top) and long-term (bottom) use of HCQ (vs SSZ) and risk of depression, by database and in the meta-analysis
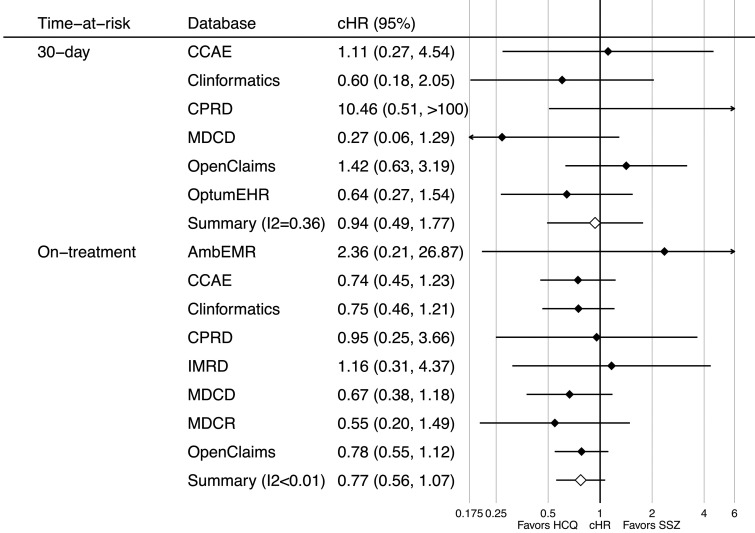
Similarly, no association was seen between the use of HCQ and the risk of suicidal ideation or suicide. In the short-term, HRs ranged from 0.27 (95% CI 0.06, 1.29) in the MDCD to 10.46 (95% CI 0.51, 216.29) in the CPRD, with a meta-analytic HR of 0.94 (95% CI 0.49, 1.77) (Fig. 2, top). Long-term effects were similar, with HRs ranging from 0.55 (95% CI 0.20, 1.49) in the MDCR to 2.36 (95% CI 0.21, 26.87) in the AmbEMR, with a meta-analytic HR of 0.77 (95% CI 0.56, 1.07) (Fig. 2, bottom).
Fig. 2.
Forest plot of the association between short- (top) and long-term (bottom) use HCQ (vs SSZ) and risk of suicidal ideation or suicide, by database and in the meta-analysis
Finally, no association was seen between the use of HCQ (compared with SSZ) and the risk of acute psychosis. Short-term analyses showed database-specific HRs of 0.44 (95% CI 0.05, 3.49) in the OptumEHR and 1.01 (95% CI 0.65, 1.58) in OpenClaims, with a meta-analytic estimated HR of 1.03 (95% CI 0.66, 1.60). Only OpenClaims contributed to the ‘on treatment’ analysis of this event, with an estimated HR of 0.98 (95% CI 0.73, 1.33).
Discussion
Principal findings
This large observational study shows that in routine healthcare treatment of RA, there is no association with the use of HCQ with acute psychosis, depression or suicide as compared with SSZ. These results are seen both in the short-term and long-term risk analyses. While an excess of psychiatric events have been reported during the COVID pandemic in those prescribed HCQ, this risk does not appear to be associated with HCQ prescribed in RA compared with those prescribed SSZ. This study uses data from three countries, with a variety of healthcare systems and modes of routine healthcare data included, enabling the study to produce more generalizable results.
Comparison with other studies
The bulk of the evidence prior to this study consisted of isolated case reports and case series, making it difficult to draw demographic comparisons with previous work. Sato et al. [21] reported that neuropsychiatric adverse events found in the us Food and Drug Administration adverse event reporting system associated with chloroquine use were predominantly in females in the sixth decade of life. The increase in reporting of acute psychiatric disease during the COVID-19 pandemic may be multifactorial, with an increase in external stressors such as social isolation, financial uncertainty and increased misuse of drugs and alcohol [47–49]. Considering that we find no association for HCQ use compared with SSZ with acute psychiatric outcomes in the RA population, evidence points towards external stressors being more likely involved in the aetiology of psychiatric events seen during this pandemic.
Strengths and weaknesses of the study
This study is based on new users of HCQ for RA and therefore the results of this study are most directly relevant to the risk of neuropsychiatric side effects seen in the rheumatologic population. The regulatory warnings of possibly increased acute psychiatric events associated with HCQ warrant investigation in all available datasets to prevent harm in both rheumatology patients and those taking it for emergency use, especially as very few clinical trials include acute psychiatric outcomes. While the general population presenting with COVID-19 may differ from those with RA, within the context of emergency authorization or off-label use of HCQ, all available evidence must be taken into account when considering the risks associated.
Several considerations must be taken into account when interpreting these results. First, the doses used to treat RA are lower than those suggested in current clinical trials for the treatment of SARS-CoV-2 and therefore adverse events seen in the treatment and prophylaxis of COVID-19 may be greater if dose dependent, as is the case with cardiac adverse effects [50, 51].
Second, this study could be affected by outcome misclassification. Only acute psychiatric events presenting to medical services will be captured, and this is especially important for the outcome of suicide. Suicide may not be fully recorded if patients do not reach medical care or cause-of-death information is not linked to the data source, and therefore the true incidence of suicide may be underrecorded [52]. Similarly, this study only focussed on acute psychosis and depression severe enough to be identified in medical consultation in patients with no history of either condition. While we generated phenotypes that underwent full cohort diagnostics, and phenotypes were constructed using a multidisciplinary team of clinicians and bioinformaticians to ensure face validity, it should be noted that no formal validation was undertaken. We took all reasonable steps to ensure the validity of the phenotypes, while considering the risk–benefit trade-off of what could be undertaken within the time frame used to respond to the serious questions raised by regulatory bodies following HCQ use in COVID-19. This study can highlight the association for patients without a prior history of psychosis or depression, but it cannot inform of the risk of acute deterioration after beginning HCQ treatment for those already known to psychiatric services.
Third, depression and hallucinations are listed as potential undesirable effects of SSZ treatment, which may underestimate the true risk, if any, from HCQ [53]. However, the frequency of depression (described as changes in affect in the summary of product characteristics for HCQ) is reported to be common (≥1/100–<1/10) while for SSZ, depression is listed as being uncommon (≥1/1000–<1/100). Therefore it is potentially reassuring for patients that we observed no difference compared with SSZ for which there is a paucity of published evidence suggesting causality [54].
PS stratification and matching as well as a comprehensive examination of potential sources of systematic error were undertaken prior to blinding of the results to identify and reduce the risk of confounding. Baseline characteristics after PS stratification were adequately balanced; of note, the incidence of SLE and a past medical history of depression and antidepressant medication use was balanced between treatment groups. Identifying the balance of these conditions between treatment groups was undertaken prior to unblinding due to the potential neuropsychiatric sequelae of the SLE aside from the potential side effects of pharmacological treatment and the increased likelihood of depression in those with a prior history. This study could also be limited by the fact that patients may overlap and exist in more than one dataset within the USA. The meta-analysis assumes populations to be independent and therefore the obtained estimates may slightly underestimate variance.
Future research
For rheumatologic disorders, future work could expand into investigating the occurrence of acute psychiatric events in SLE patients. This would enable greater understanding of whether neuropsychiatric conditions are related to disease activity or due to pharmacological treatment. Similarly, with the emergency use of HCQ in COVID-19, there is already concern about the potential heightened risk of acute psychiatric disorders due to the increased number of psychosocial stressors present during a pandemic and high-dose use [55]. Future work should consider including acute psychiatric outcomes in order to differentiate between psychiatric conditions generated by the impact of a global pandemic compared with iatrogenic events due to the pharmaceutical therapies used.
Meaning of the study
Exponential growth in research into the best treatment of SARS-CoV-2 infection is generating rapidly evolving evidence for the relative efficacy of pharmaceutical agents. For the rheumatologic community, media attention previously surrounded HCQ as a strong frontrunner in COVID-19 prophylaxis and treatment. The results of the RECOVERY trial, showing that dexamethasone reduced mortality in intensive care patients, have now overtaken HCQ as the leading rheumatologic drug for the pandemic, but the concerns regarding HCQ safety remain for those who take the drug for conventional indications [17, 56]. Cardiovascular safety and reports that it might lack efficacy for both treatment and prophylaxis have halted major HCQ clinical trials [50, 57–60]. The identification of acute psychiatric events associated with HCQ use has raised the need to clarify the risk within general rheumatologic use. Our study identifies no increased risk in RA patients when compared with SSZ and provides evidence to users and clinicians alike that the reports presented during the pandemic are likely to be related to further causes aside from HCQ.
Supplementary Material
Acknowledgements
We thank Catherine Hartley and Eli Harriss of Bodleian Health Care Libraries, University of Oxford, and Runsheng Wang, Joel Swerdel, Zeshan Ghory, Liliana Ciobanu, Michael Kallfelz, Nigel Hughes, Martijn Schuemie, Mitchell M. Conover, Aedin C. Culhane Scott L. DuVall, Dmitry Dymshyts, Seamus Kent, Christophe G. Lambert, Johan van der Lei, Andrea V. Margulis, Michael E. Matheny, Lisa Schilling, Sarah Seager and Oleg Zhuk. Finally, we acknowledge the tremendous work and dedication of the 350 participants from 30 nations in the March 2020 OHDSI COVID-19 Virtual Study-a-thon (https://www.ohdsi.org/covid-19-updates/), without whom this study could not have been realized.
Funding: No funder was involved in data collection, analysis, interpretation, writing or the decision to submit. This work was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC); US National Institutes of Health; US Department of Veterans Affairs; Janssen Research & Development; IQVIA and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant HI16C0992). Personal funding included Versus Arthritis (21605); MRC-DTP (MR/K501256/1) (to J.L.); MRC and FAME (to A.P.U.); Innovation Fund Denmark (5153-00002B) and the Novo Nordisk Foundation (NNF14CC0001) (to B.S.K.H.); VINCI (VA HSR RES 13-457) (to S.L.D., M.E.M. and K.E.L.) and an NIHR Senior Research Fellowship (to D.P.A.). The European Health Data & Evidence Network has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement 806968. The JU receives support from the European Union’s Horizon 2020 Research and Innovation Programme and the European Federation of Pharmaceutical Industries Associations. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Clinician Scientist Award programme, NIHR, Department of Veterans Affairs or the US government, National Health Service or the Department of Health, England.Disclosure statement: All authors have completed the International Committee of Medical Journal Editors’ uniform disclosure form from http://www.icjme.org/conflicts-of-interest/. J.C.E.L. reports grants from the Medical Research Council (MR/K501256/1) and Versus Arthritis (21605), outside of the submitted work. D.P.-A. reports grants and other support from Amgen; grants, non-financial support and other support from UCB Biopharma; grants from Les Laboratoires Servier, outside the submitted work; public–private partnerships within Innovative Medicines Initiative (IMI) including European Health Data and Evidence Network and European Medical Information Framework (EMIF) consortia and Synapse Management Partners have supported training programmes organized by D.P.-A.’s department and open tor external participants. J.W., J.H., G.A.R., A.G.S., A.S. and P.R. are employees of Janssen Research and Development and shareholders of Johnson & Johnson. G.H. reports grants from the US National Library of Medicine, during the conduct of the study and grants from Janssen Research, outside the submitted work. B.S.K.-H. reports grants from Innovation Fund Denmark (5153-00002B) and Novo Nordisk Foundation (NNF14CC0001), outside the submitted work. S.K. reports employment with AstraZeneca, outside the submitted work. M.d.W. and M.M. report grants from the Innovative Medicines Initiative. D.R.M. is supported by a Wellcome Trust Clinical Research Development Fellowship (grant 214588/Z/18/Z) and reports funding support from the NIHR, Chief Scientist Office and Tenovus Scotland for research unrelated to this work. F.N. reports employment with AstraZeneca, outside the submitted work. A.P.-U. reports grants from Fundacion Alfonso Martin Escudero and the Medical Research Council, outside the submitted work. P.R. reports grants from the Innovative Medicines Initiative and Janssen Research and Development during the conduct of the study. H.M.-S. and K.K. are employees of IQVIA, outside of the submitted work. M.A.S. reports grants from the US National Science Foundation, US National Institutes of Health and IQVIA and personal fees from Janssen Research and Development during the conduct of the study. D.V. is an employee of Bayer, outside the submitted work. S.C.Y. reports grants from the Korean Ministry of Health & Welfare and Korean Ministry of Trade, Industry & Energy during the conduct of the study. All other authors declare no competing interests.
Ethical approval
All data partners received IRB approval or waivers in accordance with their institutional governance guidelines.
| Database | Statement |
|---|---|
| AmbEMR | This is a retrospective database study on de-identified data and is deemed not human subject research. Approval is provided for OHDSI community studies. |
| CCAE | New England Institutional Review Board (IRB) and was determined to be exempt from broad IRB approval, as this research project did not involve human subject research. |
| CPRD | Approval for CPRD was provided by the Independent Scientific Advisory Committee (ISAC). |
| This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. The protocol for this study ( 20_059R) was approved by the Independent Scientific Advisory Committee (ISAC). | |
| DA Germany | This is a retrospective database study on de-identified data and is deemed not human subject research. Approval is provided for OHDSI community studies. |
| IMRD | The present study is filed and under review for Scientific Review Committee for institutional adjudication. Due to the public health imperative of information related to these data, approval is provided for this publication. |
| IPCI | The present study was approved by the Scientific and Ethical Advisory Board of the IPCI project (project number: 4/2020). |
| JMDC | New England Institutional Review Board (IRB) and was determined to be exempt from broad IRB approval, as this research project did not involve human subject research. |
| MDCD | New England Institutional Review Board (IRB) and was determined to be exempt from broad IRB approval, as this research project did not involve human subject research. |
| MDCD | New England Institutional Review Board (IRB) and was determined to be exempt from broad IRB approval, as this research project did not involve human subject research. |
| Open Claims | This is a retrospective database study on de-identified data and is deemed not human subject research. Approval is provided for OHDSI community studies. |
| Clinformatics | New England Institutional Review Board (IRB) and was determined to be exempt from broad IRB approval, as this research project did not involve human subject research. |
| Optum EHR | New England Institutional Review Board (IRB) and was determined to be exempt from broad IRB approval, as this research project did not involve human subject research. |
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.Yao X, Ye F, Zhang M. et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020;71:732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferner RE, Aronson JK.. Chloroquine and hydroxychloroquine in covid-19. BMJ 2020;369:m1432. [DOI] [PubMed] [Google Scholar]
- 3.Sepriano A, Kerschbaumer A, Smolen JS. et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2020;79:760–70. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Request for emergency use authorization for use of chloroquine phosphate or hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of 2019 coronavirus disease. https://www.fda.gov/media/136534/download (22 November 2020, date last accessed).
- 5.European Medicines Agency. COVID-19: chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programmes. https://www.ema.europa.eu/en/documents/press-release/covid-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes_en.pdf 22 November 2020, date last accessed).
- 6.Colson P, Rolain J-M, Lagier J-C, Brouqui P, Raoult D.. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents 2020;55:105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Cao R, Xu M. et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigerust DJ, Shepherd VL.. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol 2007;15:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Cao R, Zhang L. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao J, Hu S.. Update on use of chloroquine/hydroxychloroquine to treat coronavirus disease 2019 (COVID-19). Biosci Trends 2020;14:156–8. [DOI] [PubMed] [Google Scholar]
- 11.Randomised Evaluation of COVID-19 Therapy (RECOVERY). https://clinicaltrials.gov/ct2/show/NCT04381936 (12 June 2020, date last accessed).
- 12.Boulware DR, Pullen MF, Bangdiwala AS. et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020;383:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina JM, Delaugerre C, Goff JL. et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect 2020;50:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gautret P, Lagier JC, Parola P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020;56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Mahévas M, Tran VT, Roumier M. et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ 2020;369:m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Tian Z, Yang X.. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020;14:72–3. [DOI] [PubMed] [Google Scholar]
- 17.No clinical benefit from the use of hydroxychloroquine in hospitalised patients with COVID-19. Press release. https://www.ox.ac.uk/news/2020-06-05-no-clinical-benefit-use-hydroxychloroquine-hospitalised-patients-covid-19 (22 November 2020, date last accessed).
- 18.World Health Organization. “Solidarity” clinical trial for COVID-19 treatments. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments (12 June 2020, date last accessed).
- 19.Phillips-Howard PA, ter Kuile FO.. CNS adverse events associated with antimalarial agents. Fact or fiction? Drug Saf 1995;12:370–83. [DOI] [PubMed] [Google Scholar]
- 20.Mascolo A, Berrino PM, Capuano A. et al. Neuropsychiatric clinical manifestations in elderly patients treated with hydroxychloroquine: a review article. Inflammopharmacology 2018;26:1141–9. [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Mano T, Iwata A, Toda T.. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends 2020;14:139–43. [DOI] [PubMed] [Google Scholar]
- 22.European Medicines Agency. COVID-19: reminder of the risks of chloroquine and hydroxychloroquine. https://www.ema.europa.eu/en/news/covid-19-reminder-risks-chloroquine-hydroxychloroquine (22 November 2020, date last accessed).
- 23.Agencia Española de Medicamentos y Productos Sanitarios. Cloroquina/hidroxicloroquina: precauciones y vigilancia de posibles reacciones adversas en pacientes con COVID-19. https://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2020/docs/NI_MUH_FV-7-2020-Hidroxicloroquina.pdf?x50414 22 November 2020, date last accessed).
- 24.Schrezenmeier E, Dörner T.. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 2020;16:155–66. [DOI] [PubMed] [Google Scholar]
- 25.Fedele AO, Proud CG.. Chloroquine and bafilomycin A mimic lysosomal storage disorders and impair mTORC1 signalling. Biosci Rep 2020;40:BSR20200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staretz-Chacham O, Choi JH, Wakabayashi K, Lopez G, Sidransky E.. Psychiatric and behavioral manifestations of lysosomal storage disorders. Am J Med Genet B Neuropsychiatr Genet 2010;153B:1253–65. [DOI] [PubMed] [Google Scholar]
- 27.Gurbuz-Ozgur B, Aslan-Kunt D, Sevincok L.. Psychotic symptoms related to hydroxychloroquine. Klinik Psikofarmakoloji Bulteni 2014;24(Suppl 1):S160. [Google Scholar]
- 28.Manzo C, Gareri P, Castagna A.. Psychomotor agitation following treatment with hydroxychloroquine. Drug Saf Case Rep 2017;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sapp OL. Toxic psychosis due to quinarcrine and chloroquine. JAMA 1964;187:373–5. 3rd. [DOI] [PubMed] [Google Scholar]
- 30.Bogaczewicz A, Sobow T, Bogaczewicz J. et al. Chloroquine-induced subacute paranoid-like disorder as a complication of dermatological treatment. Int J Dermatol 2016;55:1378–80. [DOI] [PubMed] [Google Scholar]
- 31.Pinho de Oliveira Ribeiro N, Rafael de Mello Schier A, Ornelas AC. et al. Anxiety, depression and suicidal ideation in patients with rheumatoid arthritis in use of methotrexate, hydroxychloroquine, leflunomide and biological drugs. Compr Psychiatry 2013;54:1185–9. [DOI] [PubMed] [Google Scholar]
- 32.Fish DR, Espir ML.. Convulsions associated with prophylactic antimalarial drugs: implications for people with epilepsy. BMJ 1988;297:526–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward WQ, Walter-Ryan WG, Shehi GM.. Toxic psychosis: a complication of antimalarial therapy. J Am Acad Dermatol 1985;12(Pt 1):863–5. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Nieto JA, Costa-Juan E.. Psychiatric symptoms induced by hydroxychloroquine. Lupus 2015;24:339–40. [DOI] [PubMed] [Google Scholar]
- 35.Hsu W, Chiu N, Huang S.. Hydroxychloroquine-induced acute psychosis in a systemic lupus erythematosus female. Acta Neuropsychiatr 2011;23:318–9. [DOI] [PubMed] [Google Scholar]
- 36.European Medicines Agency. ENCePP Guide on Methodological Standards in Pharmacoepidemiology. http://www.encepp.eu/standards_and_guidances/methodologicalGuide.shtml 2(22 November 2020, date last accessed).
- 37.European Medicines Agency. EU PAS registration: Hydroxychloroquine safety and potential efficacy as an antiviral prophylaxis in light of potential wide-spread use in COVID-19: a multinational, large-scale network cohort and self-controlled case series study. http://www.encepp.eu/encepp/viewResource.htm%3Fid=34498 22 November 2020, date last accessed).
- 38.Observational Health Data Sciences and Informatics. ATLAS open science analytics platform. https://atlas.ohdsi.org/#/home (25 June 2020, date last accessed).
- 39.Observational Health Data Sciences and Informatics. The Book of OHDSI. https://ohdsi.github.io/TheBookOfOhdsi/ (22 November 2020, date last accessed).
- 40.Suchard MA, Schuemie MJ, Krumholz HM. et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet 2019;394:1816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voss EA, Boyce RD, Ryan PB. et al. Accuracy of an automated knowledge base for identifying drug adverse reactions. J Biomed Inform 2017;66:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA.. Robust empirical calibration of p-values using observational data. Stat Med 2016;35:3883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuemie MJ, Ryan PB, DuMouchel W, Suchard MA, Madigan D.. Interpreting observational studies: why empirical calibration is needed to correct p-values. Stat Med 2014;33:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 45.Suchard MA, Simpson SE, Zorych I, Ryan P, Madigan D.. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul 2013;23:10.1145/2414416.2414791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nerurkar L, Siebert S, McInnes IB, Cavanagh J.. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry 2019;6:164–73. [DOI] [PubMed] [Google Scholar]
- 47.Yao H, Chen JH, Xu YF.. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry 2020;7:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stuckler D, Basu S, Suhrcke M, Coutts A, McKee M.. The public health effect of economic crises and alternative policy responses in Europe: an empirical analysis. Lancet 2009;374:315–23. [DOI] [PubMed] [Google Scholar]
- 49.O’Connor RC, Kirtley OJ.. The integrated motivational-volitional model of suicidal behaviour. Philos Trans R Soc Lond B Biol Sci 2018;373: 20170268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercuro NJ, Yen CF, Shim DJ. et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.American College of Cardiology. Ventricular arrhythmia risk due to hydroxychloroquine-azithromycin treatment for COVID-19. https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19 (25 June 2020, date last accessed).
- 52.Thomas KH, Davies N, Metcalfe C. et al. Validation of suicide and self-harm records in the Clinical Practice Research Datalink. Br J Clin Pharmacol 2013;76:145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Electronic Medicines Compendium. Salazopyrin. https://www.medicines.org.uk/emc/product/3838/smpc (25 June 2020, date last accessed).
- 54.Jajić Z, Jajić I.. [Acute psychoses in patients with psoriatic arthritis during treatment with sulfasalazine]. Reumatizam 1998;46:43–4. [PubMed] [Google Scholar]
- 55.Gunnell D, Appleby L, Arensman E. et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7:468–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horby P, Lim WS, Emberson J. et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv 2020; 10.1101/2020.06.22.20137273. [DOI]
- 57.Luo M, Hu Q, Guirong X. et al. Data mining and safety analysis of drugs for novel coronavirus pneumonia treatment based on FAERS: chloroquine phosphate. Herald Med 2020;39:505–13. [Google Scholar]
- 58.Roden DM, Harrington RA, Poppas A, Russo AM.. Considerations for drug interactions on QTc in exploratory COVID-19 (coronavirus disease 2019) treatment. Circulation 2020;141: e906–7. [DOI] [PubMed] [Google Scholar]
- 59.Lane JCE, Weaver J, Kostka K. et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. medRxiv 2020; 10.1101/2020.04.08.20054551. [DOI]
- 60.National Institutes of Health. NIH halts clinical trial of hydroxychloroquine. https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine (25 June 2020, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.