Abstract
Introduction
Occurrence of inaccurate or delayed diagnoses is a significant concern in patient care, particularly in emergency medicine, where decision making is often constrained by high throughput and inaccurate admission diagnoses. Artificial intelligence-based diagnostic decision support system have been developed to enhance clinical performance by suggesting differential diagnoses to a given case, based on an integrated medical knowledge base and machine learning techniques. The purpose of the study is to evaluate the diagnostic accuracy of Ada, an app-based diagnostic tool and the impact on patient outcome.
Methods and analysis
The eRadaR trial is a prospective, double-blinded study with patients presenting to the emergency room (ER) with abdominal pain. At initial contact in the ER, a structured interview will be performed using the Ada-App and both, patients and attending physicians, will be blinded to the proposed diagnosis lists until trial completion. Throughout the study, clinical data relating to diagnostic findings and types of therapy will be obtained and the follow-up until day 90 will comprise occurrence of complications and overall survival of patients. The primary efficacy of the trial is defined by the percentage of correct diagnoses suggested by Ada compared with the final discharge diagnosis. Further, accuracy and timing of diagnosis will be compared with decision making of classical doctor–patient interaction. Secondary objectives are complications, length of hospital stay and overall survival.
Ethics and dissemination
Ethical approval was received by the independent ethics committee (IEC) of the Goethe-University Frankfurt on 9 April 2020 including the patient information material and informed consent form. All protocol amendments must be reported to and adapted by the IEC. The results from this study will be submitted to peer-reviewed journals and reported at suitable national and international meetings.
Trial registration number
DRKS00019098.
Keywords: accident & emergency medicine, adult gastroenterology, adult surgery
Strengths and limitations of this study.
This is the first prospective study to examine the diagnostic accuracy of an app-based diagnostic tool in an emergency room and the impact on clinical outcomes.
The study will be conducted in a real-life setting to investigate the performance in a high stress environment and to provide rationale for routine clinical application.
The double-blinded design will avoid bias regarding research findings.
The primary limitation of an observational design is that only associations can be described, not causal relationships.
Introduction
Diagnostic errors, comprising inaccurate, delayed or missed diagnoses, are one of the major challenges in public healthcare.1 In the recent ‘Patient Safety Fact File’, WHO outlines 10 crucial facts about patient safety.2 Accordingly, adverse events are among the 10 leading causes of death and disability, contributing to approximately 10% of patients harmed during hospitalisation. Of note, 10%–20% of adverse events have been quoted to be particularly related to diagnostic failure, causing more harm to patients than medication or treatment errors.3–5 Further, false or delayed diagnoses are reported to be the most common reason for medical malpractice litigation.6 Graber et al estimated that diagnostic failures occurred in 5%–15% of cases, depending on the medical specialty with higher percentages assumed in primary care and emergency medicine.7 Various reasons have been identified to contribute to false diagnoses. Graber concluded that cognitive slips, primarily resulting from faulty information processing and verification, and misguided situational confidence occur most frequently.8 9
This is especially evident in ER settings, which often have to deal with high throughputs, fast decision making and incomplete clinical information in a disruptive environment. In particular, emergency room (ER) overcrowding has been identified as a serious threat to patient safety, resulting in poor clinical outcome and a significant increase in mortality.10
Previous studies have revealed that more than 40% of admission diagnoses at first presentation to the ER are not concordant with the final diagnosis of the patient.11–13 That means, that throughout the hospital stay, the patient experiences a change in diagnosis based on a variety of additional diagnostics and reevaluation of initial assumptions, finally leading to the correct diagnosis. In particular, approximately 30% of patients with abdominal pain, being one of the leading causes for visiting the ER, exhibit a discrepancy in diagnosis.14 15 In particular, misdiagnosis rate of acute appendicitis, the most frequent reason for acute abdominal pain, has largely remained unchanged over time and is still associated with a high ratio of negative appendectomies.16 Inaccurate diagnosing in ERs has been shown to be further associated with increased length of hospital stay, rate of consultations, healthcare cost, and risk for mortality and morbidity, contributing to a serious concern to patient safety.11 13 17 18 Thus, a high degree of diagnostic accuracy can lead to an improvement in quality of patient care. Correct admission diagnoses are crucial for a reliable triage and process management and critically influence the initial evaluation in that ER and subsequent clinical course of the patient.19
Digital technologies and artificial intelligence (AI)-based methods have recently emerged as impressively powerful tools to empower physicians in clinical decision making and improve healthcare quality. More specifically, diagnostic decision support systems (DDSS) have demonstrated to facilitate assessment of clinical data input by using an extensive medical knowledge base.20 21 One version of DDSS is Ada, an app-based AI-machine learning system that incorporates patients’ symptoms and other findings into its knowledge base and intelligent technology to deliver effective healthcare.22 23 Based on an algorithmic pathway and driven by chief complaints, the app-based system generates a set of differential diagnoses for a given clinical case. Several studies have reported that DDSS have the potential to increase diagnostic performance, obtaining an accuracy rate of 70%–96%.24 25 In particular, a retrospective study of rare diseases has demonstrated that Ada suggests accurate diagnoses earlier than clinical diagnoses in more than half of all cases.23
However, application of the Ada app has not been investigated in a real-life setting, particularly in ERs, which has to deal with a high stress environment and heavy time constraints. This app-based method may be a valuable companion in triaging patients and support clinicians in making decisions more accurate and sooner by simultaneously reducing risk for medical errors. Therefore, in the present study, we aim to evaluate the diagnostic ability of Ada in ER settings and examine the impact on timing of diagnosis.
Methods and analysis
The eRadaR-trial is designed as a prospective, double-blinded, observational study evaluating the diagnostic accuracy of the Ada-App in the ER of the Department of General, Visceral and Transplant Surgery of the Frankfurt University Hospital, Germany. The trial protocol is written in accordance with the current Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT 2013). The SPIRIT checklist is given in online supplemental Additional file 1.
bmjopen-2020-041396supp001.pdf (77KB, pdf)
The Ada-App specifications and rationale to use the software
The Ada-App is a class I medicinal product certified in accordance with the DIN ISO 13485. Ada is a free-downloadable certified medicinal product and has been validated in different studies by the marketing authorisation holder and developer team. It has shown a higher accuracy (73%) in comparison to other apps (38%) when compared with the correctness of symptom checking. The App was superior to other apps when the hitlist of the five most probable diagnoses were compared (84% vs 51%).22 26–32
The evidence shows that the algorithm is superior to other solutions on the market, it has been validated by the company, and the data were the basis for the certification as a medicinal product class I (CE-mark in accordance with DIN ISO 13485), supporting our rationale to test the potentially most beneficial and promising software on the market.
Study population and eligibility criteria
All patients presenting to the ER with abdominal pain will form the study population and be screened for trial eligibility. Notably, patients presenting with abdominal pain as part of multiple chief complaints (eg, chest pain and abdominal pain) will also be included in the study. Moreover, patients, who will be immediately discharged from the ED on the same day and patients, who will be admitted to the hospital after presenting to the ER will be both included in the study and followed up in an intention-to-treat fashion. Inclusion criteria comprise: (1) adults aged ≥18 years, (2) patients presenting with abdominal pain to the ER and (3) patients willing to participate and able to provide written informed consent. The criteria of exclusion are: (1) intubated patients, (2) unstable patients or (3) patients with severe injuries requiring immediate medical treatment, (4) patients unwilling or incapable of providing informed consent. Eligible patients are asked for their participation in the trial and written informed consent will be obtained from themselves. All reasons for exclusion of patients will be recorded in the trial screening log and analysed accordingly.
Description of study visits and assessment schedule
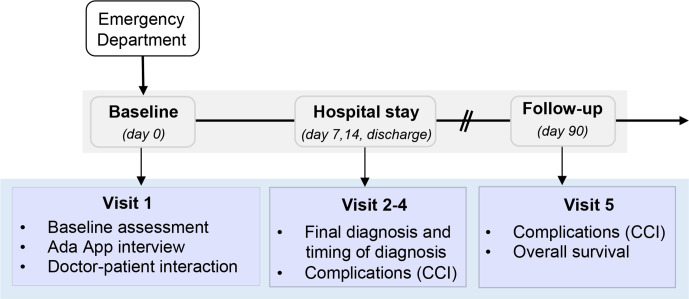
Eligible patients will be interviewed by the study team with the Ada-App based on an algorithmic pathway of questions relating to the symptoms. The Ada-App will only obtain data about patient demographics, patient history and information about current complaints. Patient’s name and date of birth will be pseudonymised using an individual identification code, as described in the section ‘data management and data safety’. Throughout the study, the patient, the study team and the physician treating the patient will be blinded regarding the list of proposed diagnoses by the app. The patient will subsequently be diagnosed by classical doctor-patient interaction and decision making. The clinical course of the patient will be followed until day 90 after initial contact in the ER. Detailed information about outline of the study and assessment schedule are displayed in table 1 and figure 1.
Table 1.
Schedule of study visits and assessments of the eRadaR study
| Baseline | Hospital stay* | Discharge | 90 days FU | ||
| Visits | V1 (Day 0) | V2 and 3 (Days 7, 14) | V4 | V5 (Day 90) | |
| Informed consent | X | ||||
| Eligibility criteria | X | ||||
| Demographic data: | X | X | |||
| (A) CCI | X | ||||
| (B) RAI-C score | |||||
| Ada diagnosis list | X | ||||
| ICD-10 diagnoses | X | X | X | ||
| Symptoms | X | ||||
| Diagnostics† | X | X | |||
| Therapy and OPS-code | X | X | |||
| Rate of consultations | X | ||||
| Complications (CCI) | X | X | X | ||
| Length of hospital stay | X | ||||
| Overall survival | X | ||||
(A) Routine blood samples (C reactive protein, white cell count, haemoglobin, platelets, sodium, potassium, creatinine, albumin, bilirubin, International normalized ratio (INR) (B) instrumental diagnostics (ultrasound, chest/abdominal CT/MRI, ECG, endoscopy).
*Visit 2 or 3 is left out, if the patient is discharged before.
†Diagnostics include.
CCI, Comprehensive Complication Index; FU, follow-up; ICD, International Classification of Diseases; OPS, operations and procedures; RAI-C, Risk Analysis C score; V, visit.
Figure 1.
Study flow chart of the eRadaR study. CCI, Comprehensive Complication Index.
Patient presenting to the ED (visit 1)
After enrolment in the trial, a structured interview with the Ada-App will be conducted and baseline data will be assessed including demographic data according to the Carlson Comorbidity Index and the Risk Analysis Index-C score (RAI-C score), the patients’ symptoms and International Classification of Diseases 10th Revision (ICD-10) diagnoses list.33–35 Participants are then diagnosed and treated according to the standard of care by the attending physician of the ER. As this is a double-blinded study to patients and treating physicians, Ada-App diagnoses lists will be randomly allocated to a study-ID and then manually transferred into the electronic case report forms (eCRF). The trial personnel will be blinded until the end of the study to avoid bias regarding subsequent diagnoses and treatment of the patient, except of the interim analysis, which is mentioned in the section of statistical analysis.
Hospital stay (visit 2, day 7)
This visit is performed on day 7, after the patient is admitted to the hospital. Data about diagnostics and therapies are assessed comprising laboratory results (ie, C reactive protein, white cell count, platelets, haemoglobin, bilirubin, creatinine, sodium and potassium, albumin, INR), computer-assisted diagnostics (ie, ultrasound, chest/abdominal CT/MRI, ECG, endoscopy), type of therapy (conservative, interventional or surgery), OPS code of therapies and complications according to the Comprehensive Complication Index (CCI) together with the date of occurrence.36 If the patient has not been admitted to the hospital or is discharged before day 7, visit 2 is left out.
Hospital stay (visit 3, day 14)
Visit 3 is performed on day 14 after patient’s admission and assessment schedule is equivalent to visit 2. If the patient has not been admitted to the hospital or is discharged before day 14, visit 3 is left out.
Discharge (visit 4)
At discharge, data including the final ICD-10 diagnosis and the timing of diagnosis will be recorded to subsequently analyse the accuracy and the timing of the Ada-App compared with the classical doctor–patient encounter. Further data items include diagnostics (laboratory, instrumental), OPS codes and type of therapies, complications according to CCI, length of hospital stay, overall health cost, rate of consultation.
Follow-up (visit 5)
The follow-up will be performed as a structured telephone interview or in person on day 90 and will encompass following data items: demographic data according to the RAI-C score, complication assessment according to CCI and overall survival.
Interventions
As this is an observational, double-blinded, prospective study, no experimental or control interventions are conducted.
Endpoints
Primary endpoint
The primary endpoint of this study is to evaluate the diagnostic accuracy of the Ada-App by comparing the decision making of the classical doctor–patient interaction with the diagnoses proposed by the app-based algorithm.
Secondary endpoint
Secondary endpoints of this study consist of the following: timing of final discharge diagnosis and time to treatment during hospital stay, comparing accurate diagnoses with discharge diagnoses as descriptive assessments, the occurrence of complications according to the CCI, total length of stay in hospital from initial contact in the ER until discharge, patient morbidity and mortality at day 90, overall health cost analysis and consultation rate. Further endpoints are displayed in the description of assessment schedule (table 1).
Measurement methods
For data capture, following measurement methods will be used:
Primary outcome measurement will be performed using the Ada-App which will deliver a set of differential diagnoses to a given clinical case.23 Based on an algorithmic questionnaire and machine learning technologies, the Ada chatbot assesses symptoms of the patient, similar to the anamnestic techniques and clinical reasoning of physicians. Patients’ data are integrated into an extensive knowledge base, which has been specifically designed by medical doctors by incorporating validated disease models and comprehensive medical literature. Then, differential diagnoses are generated and ranked in order considering two features: the probability, based on epidemiological data and the best match between the diagnosis and the given symptoms. Through AI-based methods and multiple feedback loops, the Ada knowledge base grows after each interaction and diagnostic ability improves continuously.
The occurrence of complications as secondary outcomes will be evaluated and analysed according to the CCI.36 The CCI represents the standard assessment of postoperative morbidity and comprises all complications occurring during a patient’s course based on the Clavien-Dindo classification (CDC). Compared with the CDC, which ranks complications based on the severity of the therapeutic consequence and grades them in five levels, the CCI uses a formula to integrate all complications, ranging them from 0 (‘no complication’) to 100 (‘death’).37 This advanced approach enables comparison of patients harbouring more than one complication and takes more subtle differences into consideration.
For assessment of comorbid diseases and frailty-associated risk in a surgical population, we will use the Charlson Comorbidity Index and the RAI-C score.
Risk–benefit assessment
This is an observational, non-interventional study and does not comprise any specific risk for the patient, as data obtained with the app are not used in the ER standard of care. Therefore, there is no special need for additional safety management. A delay in the diagnosis and treatment of patients presenting to the ER is not expected, as the app-based interview will not require more than 10 min and will exclusively be performed in the waiting zone of the ER by the study team. Baseline assessment (during visit 1) will directly be conducted after patient has been registered at the ER and given informed consent. Besides that, unstable patients requiring immediate medical care are excluded from the study beforehand.
Data management and data safety
The investigators will design and produce eCRF for protocol-required data collection. All information will be entered into these eCRFs by authorised and trained members of the study team and systematically checked for accuracy and completeness. Staff members with responsibilities for data collection or those, having access to the database will be enrolled in a delegation log. Patients’ data collected during the trial will be recorded in pseudonymised form by solely using individual identification codes.
For data assessment using the Ada-App, a specified iPad will be provided, which will be registered at the Frankfurt University Hospital and will be exclusively used for the purpose of this trial. Clinical data will be documented pseudonymously by using a combination of a random number from 1 to 450 and the patient’s year of birth. Participants are then asked to answer the questionnaire of the Ada-App preferably by themselves or otherwise assisted by the study team. The diagnoses will be manually transferred into the eCRF of the related patient after trial completion and unblinding.
All trial data obtained will be integrated into a statistical analysis software and analysed by the Institute of Biostatistics and Mathematical Modelling Frankfurt.
Ethics and dissemination
The eRadaR trial will be conducted in accordance with the Declaration of Helsinki and the international conference of harmonisation good clinical practice guidelines. After a patient has been identified to meet eligibility criteria, the patient will be informed about the aim, outline and individual risk of the study and informed consent will be given. After a sufficient period, the patient can then sign informed consent and will receive a signed copy.
The results of this trial will be submitted for publication in a peer-reviewed journal in a summarised anonymised manner. The study is scientifically supported by the Barmer health insurance company. Barmer will act as a scientific advisor regarding the conduct of the study, will be involved in the process of interpreting the data and in the publication and public distribution process of the study after trial completion. However, there will be no raw data sharing or financial support from the institution.
Statistical analysis
Interim analysis
One formal unblinded interim analysis of the trial data is planned to be performed after enrolment of about 200 patients to evaluate the diagnostic accuracy of the Ada-App with 90 days follow-up information. Statistical analysis will be performed by the responsible study biometrician using a significance level of alpha=0.001 and a subsequent report will be written. These results will be discussed with the investigators and the study team in a staff meeting and the continuation of the trial will be considered.
Sample size calculation and study duration
The assumptions that were made, was that more than 30% of the admission diagnoses are not consistent with the final discharge diagnosis and hypothesised that the Ada-App will increase the diagnostic accuracy from 70% to a rate of 85%. Providing a power of 90% and a two-sided significance level of alpha 5%, a target sample size of N=405 patients has to be recruited to detect the targeted effect. With an estimated dropout rate of 10%, we plan to recruit N=450 patients in this trial. Furthermore, we expect the width of the confidence intervals for the diagnostic accuracy to be 0.1 at maximum (0.09 with an estimated diagnostic accuracy of 0.7, 0.07 with an estimated diagnostic accuracy of 0.85).
This trial is anticipated to start in September 2020 and the duration of patient’s participation is 3 months including follow-up. To achieve the required sample size of patients, trial completion is expected to be in 12 months (August 2020).
Patient and public involvement
Patients were not involved in the development of the research question or study design. They will, however, be involved in visit 1 and will be interviewed by the study team using the Ada-App. Further, the follow-up (visit 5) will be performed as a telephone interview or in person with the patients for data assessment.
Discussion
Diagnostic errors have been identified as a serious threat to patient safety, leading to preventable adverse events, particularly in ERs with a disruptive environment. AI-based tools and algorithms have the potential to substantially reduce diagnostic failures, achieving high rates of diagnostic accuracy, which rivals the capability of clinicians.
A previous study provides an overview of the main types of existing tools, which are classified into categories related to the targeted step of diagnostic processing.25 Over the past few decades, a number of computerised DDSS have been developed, exhibiting promising diagnostic efficacy. Bond et al evaluated four current DDSS using clinical cases from the New England Journal of Medicine, demonstrating that Isabel and Dxplain achieve the strongest performance.38 Compared with former programmes, second-generation DDSS are far more powerful, providing more accurate suggestions with increasing complexity, while concomitantly requiring less time for diagnosing.21 24 This is primarily essential in an era of ER crowding, where fast and accurate triaging is necessary to prioritise critically ill patients and to optimise resource allocation.8 Stewart et al recently summarised various fields of AI application becoming relevant in emergency medicine, including imaging, decision-making, and outcome prediction.39 In terms of triaging, a machine learning-based tool efficiently predicts critical patient outcome, equivalent to the classically used Emergency Severity Index.40 In a prospective, multicentre study, the DDSS Isabel achieved high accuracy in diagnosing patients presenting to the ER, suggesting the final discharge diagnosis in 95% of cases.41 Another clinical decision support system has been evaluated in patients presenting with acute abdominal pain aiming to identify high-risk patients for acute appendicitis.42 Based on automated methods and an integrated risk calculator, patient data was assessed from the electronic health record (EHR) and management strategies suggested according to the risk level. Incorporation into EHR represents one of the most recent advances in the development of DDSS using ‘natural language processing’ techniques, which matches entered clinical data with the underlying knowledge base.43 This might facilitate assessment of larger volumes of data, save more time, and might increase acceptance of DDSS in clinical workflow.
However, in most of these trials using clinical support systems, impact on patient outcome, or on healthcare costs were not assessed. Although diagnoses suggested by DDSS mostly contained the correct diagnosis and achieved high level of users’ satisfaction, relevance and specificity of extensive lists were low.20 25 38 Long lists may lead to distraction or to unnecessary diagnostic with increased risk for iatrogenic injuries and costs. In general, despite the given potential efficacy of DDSS, widespread acceptance for implementation of DDSS into the routine clinical practice is evolving scarcely.44 Studies focusing on AI-based diagnostic tools are generally designed heterogeneously and are often of poor quality, making it difficult to recommend widespread evidence-based clinical application.21 25 While most of the current trials demonstrated high diagnostic accuracy in retrospective and simulated cases, only few studies evaluated their performance in real clinical settings, particularly in high stress environments like ERs. Thus, further validations in prospective studies are required to investigate the diagnostic efficiency and utility of DDSS and their impact on routine clinical decision-making and patient outcome.
Supplementary Material
Acknowledgments
We would like to thank Rene Wendel, Doreen Giannico, Dr Johannes Masseli, Professor Stefan Zeuzem and the whole administrative and medical team of the central emergency room of the Frankfurt University Hospital for their support.
We further are grateful for our established scientific support with the Barmer health care insurance company.
Footnotes
Correction notice: This article has been corrected since it first published. The provenance and peer review statement has been included.
Contributors: SFF-U-Z wrote the manuscript, involved in writing the protocol and was the clinical lead surgeon for the trial. NF performed the sample size calculation for the trial and was involved in drafting the protocol and the manuscript. MvW supported the trial as chief medical informatics officer (CMIO) of the hospital, and was involved in drafting the protocol and the manuscript. CD and DM were involved in creating the idea and drafting the protocol and the manuscript and prepared the CRF logistics. UK is the quality manager of the surgical department and supervised implementation of the project in the ER. UM is the BARMER health insurance representative and is an external advisor to the trial. She drafted the protocol and the manuscript. LA, PB, PS are medical students triaging the patients and putting up logistics in the ER. They all were involved in creating the idea and shaping the project. WOB gave valuable input into the project, supports it majorly as chair of the department, and creates a culture for innovative projects. He further drafted the protocol and manuscript. AAS had the idea for the project, led the trial group and links all parties involved. He is the responsible principal investigator for the trial.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Balla J, Heneghan C, Goyder C, et al. Identifying early warning signs for diagnostic errors in primary care: a qualitative study. BMJ Open 2012;2. 10.1136/bmjopen-2012-001539. [Epub ahead of print: 13 09 2012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Patient safety and risk management service delivery and safety.. Available: https://www.who.int/features/factfiles/patient_safety/en/ [Accessed Sept 2019].
- 3.Leape LL, Brennan TA, Laird N. The nature of adverse events in hospitalized patients. Results of the Harvard medical practice study II. N Engl J Med 1991;324:377–84. 10.1056/NEJM199102073240605 [DOI] [PubMed] [Google Scholar]
- 4.Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Washington (DC), 2000. [PubMed] [Google Scholar]
- 5.Bhasale AL, Miller GC, Reid SE, et al. Analysing potential harm in Australian general practice: an incident-monitoring study. Med J Aust 1998;169:73–6. 10.5694/j.1326-5377.1998.tb140186.x [DOI] [PubMed] [Google Scholar]
- 6.Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med 2006;354:2024–33. 10.1056/NEJMsa054479 [DOI] [PubMed] [Google Scholar]
- 7.Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. Am J Med 2008;121:S2–23. 10.1016/j.amjmed.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 8.Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med 2005;165:1493. 10.1001/archinte.165.13.1493 [DOI] [PubMed] [Google Scholar]
- 9.Hautz SC, Schuler L, Kämmer JE, et al. Factors predicting a change in diagnosis in patients hospitalised through the emergency room: a prospective observational study. BMJ Open 2016;6:e011585. 10.1136/bmjopen-2016-011585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morley C, Unwin M, Peterson GM, et al. Emergency department crowding: a systematic review of causes, consequences and solutions. PLoS One 2018;13:e0203316. 10.1371/journal.pone.0203316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernhard M, Raatz C, Zahn P, et al. [Validity of admission diagnoses as process-driving criteria : influence on length of stay and consultation rate in emergency departments]. Anaesthesist 2013;62:617–23. 10.1007/s00101-013-2207-5 [DOI] [PubMed] [Google Scholar]
- 12.Ben-Assuli O, Sagi D, Leshno M, et al. Improving diagnostic accuracy using EHR in emergency departments: a simulation-based study. J Biomed Inform 2015;55:31–40. 10.1016/j.jbi.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 13.Eames J, Eisenman A, Schuster RJ. Disagreement between emergency department admission diagnosis and hospital discharge diagnosis: mortality and morbidity. Diagnosis 2016;3:23–30. 10.1515/dx-2015-0028 [DOI] [PubMed] [Google Scholar]
- 14.Chiu HS, Chan KF, Chung CH, et al. A comparison of emergency department admission diagnoses and discharge diagnoses: retrospective study. Hong Kong Journal of Emergency Medicine 2003;10:70–5. 10.1177/102490790301000202 [DOI] [Google Scholar]
- 15.Macaluso CR, McNamara RM. Evaluation and management of acute abdominal pain in the emergency department. Int J Gen Med 2012;5:789. 10.2147/IJGM.S25936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kryzauskas M, Danys D, Poskus T, et al. Is acute appendicitis still misdiagnosed? Open Med 2016;11:231–6. 10.1515/med-2016-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McWilliams A, Tapp H, Barker J, et al. Cost analysis of the use of emergency departments for primary care services in Charlotte, North Carolina. N C Med J 2011;72:265–71. [PubMed] [Google Scholar]
- 18.Johnson T, McNutt R, Odwazny R, et al. Discrepancy between admission and discharge diagnoses as a predictor of hospital length of stay. J Hosp Med 2009;4:234–9. 10.1002/jhm.453 [DOI] [PubMed] [Google Scholar]
- 19.Mistry B, Stewart De Ramirez S, Kelen G, et al. Accuracy and Reliability of Emergency Department Triage Using the Emergency Severity Index: An International Multicenter Assessment. Ann Emerg Med 2018;71:581–7. 10.1016/j.annemergmed.2017.09.036 [DOI] [PubMed] [Google Scholar]
- 20.Riches N, Panagioti M, Alam R, et al. The effectiveness of electronic differential diagnoses (DDX) generators: a systematic review and meta-analysis. PLoS One 2016;11:e0148991. 10.1371/journal.pone.0148991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Middleton B, Sittig DF, Wright A. Clinical decision support: a 25 year retrospective and a 25 year vision. Yearb Med Inform 2016;1:103–16. 10.15265/IYS-2016-s034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronicke S, Hirsch MC, Türk E, et al. Can a decision support system accelerate rare disease diagnosis? Evaluating the potential impact of ADA DX in a retrospective study. Orphanet J Rare Dis 2019;14:69. 10.1186/s13023-019-1040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jungmann SM, Klan T, Kuhn S, et al. Accuracy of a Chatbot (ADA) in the diagnosis of mental disorders: comparative case study with lay and expert users. JMIR Form Res 2019;3:e13863. 10.2196/13863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Kareh R, Hasan O, Schiff GD. Use of health information technology to reduce diagnostic errors. BMJ Qual Saf 2013;22 Suppl 2:ii40–51. 10.1136/bmjqs-2013-001884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graber ML, Mathew A. Performance of a web-based clinical diagnosis support system for internists. J Gen Intern Med 2008;23 Suppl 1:37–40. 10.1007/s11606-007-0271-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montazeri M, Multmeier J, Novorol C. The potential for digital patient symptom recording through symptom assessment applications to optimize patient flow and reduce waiting times in urgent care centers: a simulation study, 2020. [Google Scholar]
- 27.Miller S, Gilbert S, Virani V, et al. Patients' utilization and perception of an artificial Intelligence-Based symptom assessment and advice technology in a British primary care waiting room: exploratory pilot study. JMIR Hum Factors 2020;7:e19713. 10.2196/19713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehl A, Bergey F, Cawley C. Syndromic surveillance insights from a symptom assessment APP before and during COVID-19 measures in Germany and the United Kingdom: results from repeated cross-sectional analyses, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knitza J, Callhoff J, Chehab G, et al. Positionspapier Der Kommission Digitale Rheumatologie Der Deutschen Gesellschaft für Rheumatologie E. V.: Aufgaben, Ziele und Perspektiven für eine moderne Rheumatologie. Z Rheumatol 2020;79:562–9. 10.1007/s00393-020-00834-y [DOI] [PubMed] [Google Scholar]
- 30.Hirsch MC, Ronicke S, Krusche M, et al. Rare diseases 2030: how augmented AI will support diagnosis and treatment of rare diseases in the future. Ann Rheum Dis 2020;79:annrheumdis-2020-217125. 10.1136/annrheumdis-2020-217125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert S, Mehl A, Baluch A. Original research: how accurate are digital symptom assessment apps for suggesting conditions and urgency advice?: a clinical vignettes comparison to GPs, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ceney A, Tolond S, Glowinski A, et al. Accuracy of online symptom checkers and the potential impact on service utilisation, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245:1245–51. 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 34.Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg 2017;152:175. 10.1001/jamasurg.2016.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirsch JA, Leslie-Mazwi TM, Nicola GN, et al. The ICD-10 system: a gift that keeps on taking. J Neurointerv Surg 2015;7:619–22. 10.1136/neurintsurg-2014-011321 [DOI] [PubMed] [Google Scholar]
- 36.Clavien P-A, Vetter D, Staiger RD, et al. The Comprehensive Complication Index (CCI®): Added Value and Clinical Perspectives 3 Years "Down the Line". Ann Surg 2017;265. [DOI] [PubMed] [Google Scholar]
- 37.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–96. 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 38.Bond WF, Schwartz LM, Weaver KR, et al. Differential diagnosis generators: an evaluation of currently available computer programs. J Gen Intern Med 2012;27:213–9. 10.1007/s11606-011-1804-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart J, Sprivulis P, Dwivedi G. Artificial intelligence and machine learning in emergency medicine. Emerg Med Australas 2018;30:870–4. 10.1111/1742-6723.13145 [DOI] [PubMed] [Google Scholar]
- 40.Levin S, Toerper M, Hamrock E, et al. Machine-Learning-Based Electronic Triage More Accurately Differentiates Patients With Respect to Clinical Outcomes Compared With the Emergency Severity Index. Ann Emerg Med 2018;71:565–74. 10.1016/j.annemergmed.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 41.Ramnarayan P, Cronje N, Brown R, et al. Validation of a diagnostic reminder system in emergency medicine: a multi-centre study. Emerg Med J 2007;24:619–24. 10.1136/emj.2006.044107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ekstrom HL, Kharbanda EO, Ballard DW. Development of a clinical decision support system for pediatric abdominal pain in emergency department settings across two health systems within the HCSRN. 7. EGEMS (Wash DC), 2019. 10.5334/egems.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang H, Tsui BY, Ni H, et al. Evaluation and accurate diagnoses of pediatric diseases using artificial intelligence. Nat Med 2019;25:433–8. 10.1038/s41591-018-0335-9 [DOI] [PubMed] [Google Scholar]
- 44.Shortliffe EH, Sepúlveda MJ. Clinical decision support in the era of artificial intelligence. JAMA 2018;320:2199. 10.1001/jama.2018.17163 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041396supp001.pdf (77KB, pdf)