Abstract
Objective
To analyse the prognosis and outcomes of SARS-CoV-2 infection in patients with primary SS.
Methods
We searched for patients with primary SS presenting with SARS-CoV-2 infection (defined following and according to the European Centre for Disease Prevention and Control guidelines) among those included in the Big Data Sjögren Registry, an international, multicentre registry of patients diagnosed according to the 2002/2016 classification criteria.
Results
A total of 51 patients were included in the study (46 women, mean age at diagnosis of infection of 60 years). According to the number of patients with primary SS evaluated in the Registry (n = 8211), the estimated frequency of SARS-CoV-2 infection was 0.62% (95% CI 0.44, 0.80). All but two presented with symptoms suggestive of COVID-19, including fever (82%), cough (57%), dyspnoea (39%), fatigue/myalgias (27%) and diarrhoea (24%), and the most frequent abnormalities included raised lactate dehydrogenase (LDH) (88%), CRP (81%) and D-dimer (82%) values, and lymphopenia (70%). Infection was managed at home in 26 (51%) cases and 25 (49%) required hospitalization (five required admission to ICU, four died). Compared with patients managed at home, those requiring hospitalization had higher odds of having lymphopenia as laboratory abnormality (adjusted OR 21.22, 95% CI 2.39, 524.09). Patients with comorbidities had an older age (adjusted OR 1.05, 95% CI 1.00, 1.11) and showed a risk for hospital admission six times higher than those without (adjusted OR 6.01, 95% CI 1.72, 23.51) in the multivariate analysis.
Conclusion
Baseline comorbidities were a key risk factor for a more complicated COVID-19 in patients with primary SS, with higher rates of hospitalization and poor outcomes in comparison with patients without comorbidities.
Keywords: Primary SS, COVID-19, SARS-Cov-2, comorbidities, outcomes
Rheumatology key messages
The estimated frequency of SARS-CoV-2 infection in patients with primary SS is 0.62%.
Primary SS patients with COVID-19 and comorbidities had a higher rate of poor outcomes.
A specific monitoring of comorbidities of patients with primary SS during the pandemic is recommended.
Introduction
Primary SS (SS) is a systemic autoimmune disease overwhelmingly diagnosed in women (>95%) aged between 30 and 60 years in two-thirds of cases [1]. The key clinical feature of primary SS is the development of sicca symptoms, reported by >95% of patients, accompanied in a significant number of cases by a wide variety of systemic manifestations, including the autoimmune damage of internal organs [2]. Primary SS is not a rare disease, affecting around one in every 400 people [3].
A novel coronavirus was identified in January 2020, as the aetiological agent of a cluster of cases of pneumonia detected in Wuhan City (China). The virus was called ‘severe acute respiratory syndrome coronavirus 2’ (SARS-CoV-2) and the lack of prior immunity has resulted in an exponential increase of infected patients across the globe [4], currently with >24 million confirmed worldwide cases and more than 835 000 deaths [5] (14 September 2020). The disease caused by SARS-CoV-2 has a very wide clinical spectrum ranging from asymptomatic cases [6] to severe acute pneumonia with life-threatening systemic multi-organ failure [7].
People with rheumatic and systemic autoimmune diseases are considered at-risk for a severe coronavirus disease 2019 (COVID-19) considering their underlying abnormal immune response and the frequent use of immunosuppressive drugs. Unfortunately, the body of scientific evidence supporting this potential enhanced risk is small, especially for individual diseases. There is no study so far that has evaluated the impact of COVID-19 on primary SS, which have some specific features that could favour an increased risk for developing a severe COVID-19 (pulmonary autoimmune damage, use of immunosuppressive agents, high frequency of lymphoma) [8–10]. Considering the current progression of the COVID-19, having this information could be useful for planning a personalized medical healthcare to the patient with primary SS in a pandemic scenario.
The objective of this study is to analyse the prognosis and outcomes of COVID-19 in patients with primary SS.
Methods
Patients
The Big Data Sjögren Project Consortium is an international, multicentre registry designed in 2014 to take a ‘high-definition’ picture of the main features of primary SS using worldwide data-sharing cooperative merging of pre-existing clinical SS databases from leading centres in clinical research in SS from the five continents (see [1] for additional methodological details). The centres share a harmonized data infrastructure and conduct cooperative online efforts in order to refine already-collected data in each centre, under the coordination of two data scientists (NA-D and BK). Inclusion criteria are the fulfilment of the 2002 classification criteria [11] and/or 2016 ACR/EULAR criteria [12]. Exclusion criteria for considering SS as a primary disease included chronic HCV/HIV infection, previous lymphoproliferative processes, and associated systemic autoimmune diseases other than SS. Diagnostic tests for SS (ocular tests, oral tests and salivary gland biopsy) were carried out according to the recommendations of the European Community Study Group [13]. The study was approved by the Ethics Committee of the Coordinating Centre (Hospital Clinic, Barcelona, Spain, registry HCB/2015/0869).
Design
By the first week of May, all centres included in the Big Data Project were contacted via email by MR-C asking for patients included in the Registry who could be diagnosed with COVID-19 according to the European Centre for Disease Prevention and Control guidelines [14] on the basis of epidemiological criteria (having a close contact with a confirmed COVID-19 case in the 14 days prior to onset of symptoms), clinical criteria (fever, cough, shortness of breath, sudden onset of anosmia, ageusia or dysgeusia, headache, chills, muscle pain, fatigue, vomiting and/or diarrhoea), diagnostic criteria (radiological evidence showing lesions compatible with COVID-19) and microbiological criteria (detection of SARS-CoV-2 nucleic acid in a clinical specimen; a positive result in serological tests was also considered as positive criteria). Due to the key role of laboratory parameters in the diagnosis and prognosis of COVID-19 [15], we enlarged the diagnostic criteria to include a suggestive biological profile of COVID-19 (raised CRP, raised D-dimer, lymphopenia, raised lactate dehydrogenase (LDH), and/or raised ferritin). According to these criteria, patients were classified according to the following case definitions:
Possible case: any person meeting the clinical criterion.
Probable case: any person meeting the epidemiological and clinical criteria, OR any person meeting the enlarged diagnostic criteria (highly suggestive radiological AND biological pictures, after excluding other aetiologies).
Confirmed case: any person meeting the microbiological criteria.
Only probable and confirmed cases were included in the study. We excluded patients presenting with suggestive symptoms without any objective test suggesting COVID-19 (possible cases), patients in whom the results of the diagnostic tests were not available/reachable (and therefore, case definition cannot be applied), concomitant infectious processes (only for cases lacking a microbiological confirmation of COVID-19 infection), and patients diagnosed as probable cases before 1 March 2020.
Data about COVID-19 infection was retrospectively extracted from electronic health records by use of a standardized de-identified data collection form including demographics, comorbidities (obesity (BMI ≥30), chronic cardiovascular, pulmonary, kidney or hepatic diseases, neoplasia), symptoms at the time of SARS-CoV-2 infection diagnosis, COVID-19 pharmacological treatment, and COVID-19 clinical outcomes (including need for hospitalisation/supplemental oxygen, intensive care admission, mechanical ventilation, and death). Clinically, patients with SARS-CoV-2 infection were classified as asymptomatic cases (people presenting with no clinical signs and symptoms from medical interviews and physical examinations), mild symptomatic (no need for hospitalisation/supplemental oxygen), and severe symptomatic cases (need for hospitalisation) [16]. Laboratory results were collected as close to the time of SARS-CoV-2 diagnosis or initial hospital admission as possible. When evaluating the use of COVID-19 treatments, hydroxychloroquine, corticosteroids and tocilizumab were only considered as COVID-19 treatments if they were given for the purpose of COVID-19 treatment. SS-related features were collected following definitions included in previous studies [1, 17, 18].
Statistical analysis
Descriptive data are presented as mean and standard deviation (SD) for continuous variables and numbers and percentages (%) for categorical variables. The χ2 test was used to study the main features related to COVID-19 according to the following dichotomic variables: case classification (confirmed vs probable cases), management of infection (at home vs hospital admission) and comorbidities (presence vs absence). The t test was used to compare the mean age at diagnosis. Logistic multivariate regression models adjusting for age and sex (as the key prognostic markers for a more complicated COVID-19) were constructed to analyse independent factors associated with case classification, management of infection and comorbidities. Age, sex and variables with a P <0.05 in the univariate analysis were included in the models and stepwise model selection by Akaike information criterion (AIC) was used. To handle missing data due to non-evaluated features, ‘available case analysis’ was assumed. All significance tests were two-tailed and values of P <0.05 were considered significant. All analyses were conducted using the R v.3.5.0 for Windows statistical software package (https://www.R-project.org/).
Results
The email requesting for patients with primary SS diagnosed with COVID-19 infection was answered by 39 centres (25 did not identify cases and 14 reported 69 potential cases). By 30 June, we received the data from 59 cases that were evaluated for inclusion in the study: six were excluded after being classified as possible cases and two due to lack of accessibility to diagnostic studies in the absence of a confirmed microbiological diagnosis. Therefore, a total of 51 patients were included in the study (46 women, with a mean age at diagnosis of primary SS of 51.5 years); the frequencies of the main SS-related features were 94.1% for dry eye, 88.2% for dry mouth, 89.7% for abnormal ocular tests, 76.7% for abnormal oral diagnostic tests, 85.7% for positive minor salivary gland biopsy, 82.3% for anti-Ro antibodies and 35.3% for anti-La antibodies. The mean total ESSDAI score was 7.5 (range 0–48). Systemic involvements with the highest frequency of active patients included the articular (52.9%), pulmonary (15.7%) and constitutional (15.7%) ESSDAI domains (Supplementary Table S1, available at Rheumatology online). According to the number of patients with primary SS included by the 39 participating centres (n = 8211), the estimated frequency of SARS-CoV-2 infection was 0.62% (95% CI 0.44, 0.80).
Table 1 summarizes the main features of SARS-CoV-2 infection in the 51 patients with primary SS. Patients were diagnosed at a mean age of 60.4 years (range 37–88); most were retired, housewives or worked in public services (most as health workers). There were three main epidemiological clusters of transmission comprising family, work (mainly in healthcare facilities), and unknown transmission. Comorbidities were reported in 23 (45%) patients, mainly chronic pulmonary diseases, but also chronic cardiovascular diseases and obesity. All patients but two presented with at least one symptom suggestive of COVID-19. The most frequent symptoms were fever (82%), cough (57%), dyspnoea (39%), fatigue/myalgias (27%) and diarrhoea (24%). According to the microbiological studies, 33 (65%) were classified as confirmed infections (positive PCR result in 31, positive serological studies in two) and 18 (35%) as probable infections.
Table 1.
Main features of SARS-CoV-2 infection in the 51 patients with primary SS
| n | % | ||
|---|---|---|---|
| Age, years (mean, range) | 60,35 (37–88) | ||
| Sex | Male | 5 | 9.8 |
| Female | 46 | 90.2 | |
| Country | Spain | 33 | 64.7 |
| Italy | 9 | 17.6 | |
| France | 6 | 11.8 | |
| Brazil | 2 | 3.9 | |
| Mexico | 1 | 2.0 | |
| Current job | Retired | 18 | 35.3 |
| Public servicea | 14 | 27.5 | |
| Housewife | 8 | 15.7 | |
| Others | 7 | 13.7 | |
| Unoccupied | 2 | 3.9 | |
| Unknown | 1 | 2.0 | |
| Comorbidities | Any | 23 | 45.1 |
| Cardiovascular disease | 8 | 15.7 | |
| Chronic pulmonary disease | 17 | 33.3 | |
| Obesity (BMI > 30) | 7 | 13.7 | |
| Other chronic diseases | 4 | 7.8 | |
| Baseline SS-related therapies | Any | 30 | 58.8 |
| Hydroxychloroquine | 19 | 37.3 | |
| Corticosteroids | 9 | 17.6 | |
| Immunosuppressants/IVIG | 9 | 17.6 | |
| Biological therapies | 2 | 3.9 | |
| Positive contact tracing | Family | 19 | 37.3 |
| Workb | 14 | 27.5 | |
| Not identified | 18 | 35.3 | |
| Clinical characteristics | Fever (temperature ≥37.5°C) | 42 | 82.4 |
| Cough | 29 | 56.9 | |
| Dyspnoea | 20 | 39.2 | |
| Fatigue/myalgias | 14 | 27.5 | |
| Diarrhoea | 12 | 23.5 | |
| Myalgias | 9 | 17.6 | |
| Anosmia or dysgeusia | 8 | 15.7 | |
| Headache | 6 | 11.8 | |
| Sore throat | 3 | 5.9 | |
| Thoracic pain | 3 | 5.9 | |
| Nausea or vomiting | 2 | 3.9 | |
| SARS-CoV-2 infection | PCR+ | 31 | 60.8 |
| Serology+ | 2 | 3.9 | |
| Not tested | 18 | 35.3 | |
| Radiological features | No infiltrates | 6/33 | 18.2 |
| Unilateral pulmonary infiltrate | 4/33 | 12.1 | |
| Bilateral pulmonary infiltrates | 23/33 | 69.7 | |
| Laboratory parameters | Haemoglobin value <12 g/l | 8/33 | 24.2 |
| Platelets count <150 000/mm3 | 1/33 | 3.0 | |
| White cells count <4000/mm3 | 3/33 | 9.1 | |
| Lymphocytes count <1000/mm3 | 23/33 | 69.7 | |
| Raised D Dimer levels | 18/22 | 81.8 | |
| Raised LDH levels | 22/25 | 88.0 | |
| Raised ferritin levels | 8/14 | 57.1 | |
| Raised liver enzymes levels | 7/29 | 24.1 | |
| Raised CRP levels | 26/32 | 81.3 | |
| COVID-19 treatment | Any | 30 | 58.8 |
| Hydroxychloroquine | 19 | 37.3 | |
| Azithromycin | 14 | 27.5 | |
| Ritonavir-boosted lopinavir | 15 | 29.4 | |
| Tocilizumab | 2 | 3.9 | |
| Methylprednisolone | 2 | 3.9 | |
| Management | Home | 26 | 51.0 |
| Hospitalization | 25 | 49.0 | |
| Duration of hospital stay, days | 10.8 (1–41) | ||
| Complications during admision | Respiratory failure (suppl O2) | 17/25 | 68.0 |
| HLH | 1/25 | 4.0 | |
| Pulmonary embolism | 1/25 | 4.0 | |
| Intensive care unit admission | 5/25 | 20.0 | |
| Invasive mechanical ventilation | 2/25 | 8.0 | |
| Outcomes | Death | 4 | 7.8 |
| Recovered | 47 | 92.2 |
Including healthcare workers (n = 9) and other works (n = 5).
Including working in healthcare facilities (n = 11) and others (n = 3).
In 33 patients (24 who required hospitalization and 9 that were visited in the Emergency department and that were discharged under hospital at home supervision), results from laboratory and radiological studies could be collected. Chest radiographs showed no pulmonary opacities (18%), unilateral (12%) or bilateral (70%) airspace opacities. Among laboratory parameters, the most frequent abnormalities included raised LDH (88%), CRP (81%) and D-dimer (82%) values, and lymphopenia (70%) (Table 1).
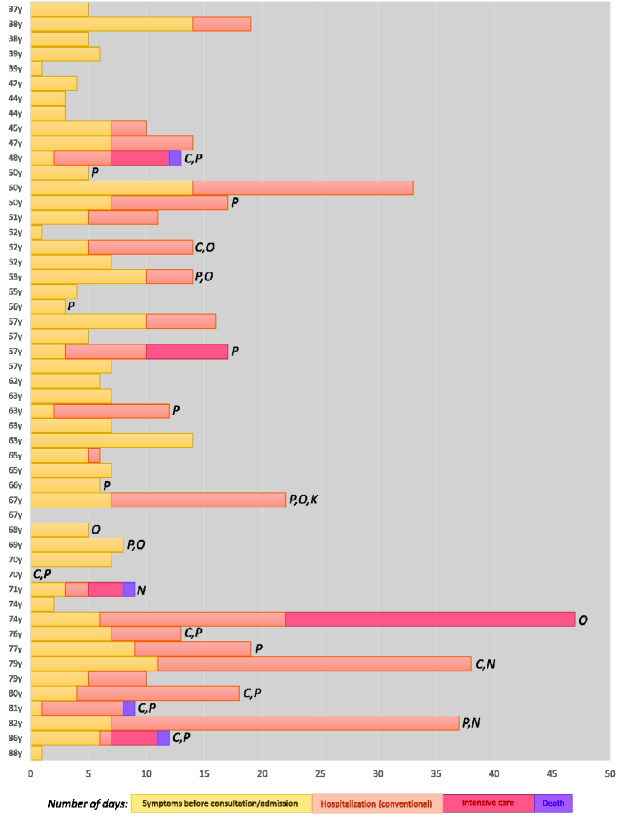
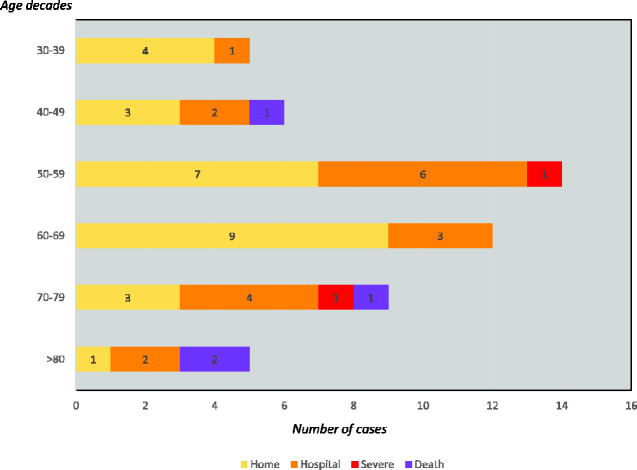
The disease was managed at home in 26 (51%) cases (close follow-up by GPs or by hospital at home programs) and 25 (49%) required hospitalization. Specific COVID-19 treatment was used in 21 patients, including hydroxychloroquine in 19, ritonavir-boosted lopinavir in 15, azithromycin in 14, pulses of methylprednisolone in two and tocilizumab in two patients. Supplemental oxygen was required in 17 (33%) patients. Among the 25 patients who were hospitalized, five (20%) required admission to the intensive care unit because of increasing supplemental oxygen requirements, and two (8%) required mechanical ventilation. No concomitant bacterial or viral infections were detected during admission (except for one patient who developed pneumococcal pneumonia after being treated with tocilizumab), and four patients developed non-infectious complications during the hospitalization (acute kidney failure, pulmonary embolism, post-viral organizing pneumonia and hemophagocytic lymphohistiocytosis (HLH), respectively). Four patients died 5–10 days after hospital admission (three due to progressive respiratory failure, one due to HLH). Figure 1 summarizes the individual outcomes of the 51 patients with primary SS ordered from the youngest to the older age at diagnosis of infection, showing a trend for a progressive increase of hospitalization/ICU requirement the older the patient is, a trend also visible in Fig. 2 that stratifies the distribution of the main outcomes by age decades.
Fig. 1.
Individual outcomes of the 51 patients with primary SS ordered from the youngest to the oldest age at diagnosis of infection
C: cardiovascular disease; K: chronic kidney disease; N: neoplasia; O: obesity; P: chronic pulmonary disease.
Fig. 2.
Distribution of the main outcomes (at home management, hospitalization, intensive care unit, death) by age decades
Demographic, clinical, radiological and laboratory features, and outcomes, were stratified by COVID-19 case definition (probable vs confirmed cases); no statistically significant differences were observed except for a differentiated contact tracing profile (more patients with no identified positive contact represented in cases classified as probable) and the frequency of general manifestations (higher in probable cases) (Table 2). Stratification according to infection management (hospital admission vs at home) showed that patients who required hospitalization had a higher frequency of comorbidities (68% vs 23% in those managed at home, P =0.002), respiratory symptoms (80% vs 50%, P =0.04), pulmonary infiltrates (92% vs 56%, P =0.034), lymphopenia (83% vs 33%, P =0.01) in the univariate analysis. Compared with patients managed at home, those requiring hospitalization had higher odds of having comorbidities (adjusted OR 13.28, 95% CI 1.49, 326.97) and lymphopenia as laboratory abnormality (adjusted OR 21.22, 95% CI 2.39, 524.09) in the logistic multivariate regression model (Table 3). When patients were stratified according to the presence or absence of baseline comorbidities, a higher mean age (65.8 vs 55.9, P =0.01), a lower frequency of ENT features (9% vs 36%, P =0.044) and a higher frequency in the rates of hospitalization (74% vs 29%, P =0.002), requirement of supplemental oxygen (56% vs 14%, P =0.002) and poor outcomes (26% vs 0%, P =0.006) was found in patients with comorbidities in comparison with those without in the univariate analysis. Patients with comorbidities had an older age (adjusted OR 1.05, 95% CI 1.00, 1.11) and showed a risk for requiring hospital admission six times higher than those without (adjusted OR 6.01, 95% CI 1.72, 23.51) in the logistic multivariate regression model (Table 4).
Table 2.
Demographic, clinical, radiological and laboratory features, and outcomes stratified by COVID-19 case definition (probable vs confirmed cases)
| Probable (n = 18) | Confirmed (n = 33) | Bilateral P-value | Multivariate OR [95% CI] | |
|---|---|---|---|---|
| Sex (men) | 1 (5.6) | 4 (12.1) | 0.645 | |
| Age, mean (s.d.), years | 62.5 (13.4) | 59.2 (14.1) | 0.413 | |
| Comorbidities, any | 9 (50) | 14 (42.4) | 0.769 | |
| Positive contact tracing | 0.006 | |||
| Family | 6 (33.3) | 13 (39.4) | Ref | |
| Work | 1 (5.6) | 13 (39.4) | 7.00 [0.87, 152.69] | |
| Not identified | 11 (61.1) | 7 (21.2) | 0.15 [0.02, 0.72] | |
| Baseline therapies | 0.644 | |||
| None | 12 (66.7) | 18 (54.5) | ||
| Hydroxychloroquine | 1 (5.6) | 5 (15.2) | ||
| Immunosuppresants | 5 (27.8) | 10 (30.3) | ||
| Fever | 16 (88.9) | 26 (78.8) | 0.464 | |
| Respiratory symptomsa | 13 (72.2) | 20 (60.6) | 0.543 | |
| Gastrointestinal symptomsb | 5 (27.8) | 9 (27.3) | 1.000 | |
| General symptomsc | 11 (61.1) | 10 (30.3) | 0.042 | 0.11 [0.02, 0.53] |
| ENT symptomsd | 3 (16.7) | 9 (27.3) | 0.502 | |
| Chest radiography (infiltrates) | 8/10 (80) | 19/23 (82.6) | 1.000 | |
| Anaemia (Hb < 12 g/L) | 3/9 (33.3) | 5/24 (20.8) | 0.651 | |
| Leukopenia (<4000/mm3) | 1/9 (11.1) | 2/24 (8.3) | 1.000 | |
| Lymphopenia (<900/mm3) | 5/9 (55.6) | 18/24 (75) | 0.400 | |
| Raised D-Dimer levels | 6/7 (85.7) | 12/15 (80) | 1.000 | |
| Raised LDH levels | 4/5 (80) | 18/20 (90) | 0.504 | |
| Raised ferritin levels | 3/3 (100) | 5/11 (45.5) | 0.209 | |
| Raised CRP levels | 6/9 (66.7) | 20/23 (87) | 0.314 | |
| Hospital admission | 7 (38.9) | 18 (54.5) | 0.382 | |
| Supplemental oxygen requirement | 3 (16.7) | 14 (42.4) | 0.073 | |
| Poor outcomese | 1 (5.6) | 5 (15.2) | 0.405 |
Cough, dyspnoea, thoracic pain.
Diarrhoea, nausea, vomiting.
Fatigue, myalgias.
Anosmia, dysgeusia, sore throat.
Ventilation/ICU requirement, death.
Table 3.
Demographic, clinical, radiological and laboratory features, and outcomes stratified by SARS-CoV-2 infection management
| At home (n = 26) | Hospitalization (n = 25) | Bilateral P-value | Multivariate OR [95% CI] | |
|---|---|---|---|---|
| Sex (men) | 1 (3.8) | 4 (16) | 0.191 | |
| Age, mean (s.d.), years | 56.9 (12.9) | 63.9 (14.2) | 0.071 | |
| Comorbidities, any | 6 (23.1) | 17 (68) | 0.002 | 13.28 [1.49, 326.97] |
| Positive contact tracing | 0.064 | |||
| Family | 8 (30.8) | 11 (44) | ||
| Work | 7 (26.9) | 11 (44) | ||
| Not identified | 11 (42.3) | 3 (12) | ||
| Baseline therapies | 0.919 | |||
| None | 16 (61.5) | 14 (56) | ||
| Hydroxychloroquine | 3 (11.5) | 3 (12) | ||
| Immunosuppresants | 7 (26.9) | 8 (32) | ||
| Fever | 21 (80.8) | 21 (84) | 1.000 | |
| Respiratory symptomsa | 13 (50) | 20 (80) | 0.040 | 7.41 [0.87, 96.97] |
| Gastrointestinal symptomsb | 8 (30.8) | 6 (24) | 0.755 | |
| General symptomsc | 13 (50) | 8 (32) | 0.258 | |
| ENT symptomsd | 10 (38.5) | 2 (8) | 0.019 | |
| COVID-19 definition (confirmed) | 15 (57.7) | 18 (72) | 0.382 | |
| Chest radiography (infiltrates) | 5/9 (55.6) | 22/24 (91.7) | 0.034 | |
| Anaemia (Hb < 12 g/L) | 1/9 (11.1) | 7/24 (29.2) | 0.394 | |
| Leukopenia (<4000/mm3) | 0/9 0 | 3/24 (12.5) | 0.545 | |
| Lymphopenia (<900/mm3) | 3/9 (33.3) | 20/24 (83.3) | 0.010 | 21.22 [2.39, 524.09] |
| Raised D-Dimer levels | 3/3 (100) | 15/19 (78.9) | 1.000 | |
| Raised LDH levels | 3/4 (75) | 19/21 (90.5) | 0.422 | |
| Raised ferritin levels | 1/2 (50) | 7/12 (58.3) | 1.000 | |
| Raised CRP levels | 6/8 (75) | 20/24 (83.3) | 0.625 | |
| Supplemental oxygen requirement | 1 (3.8) | 16 (64) | <0.001 | |
| Poor outcomese | 0 0 | 6 (24) | 0.010 |
Cough, dyspnoea, thoracic pain.
Diarrhoea, nausea, vomiting.
Fatigue, myalgias.
Anosmia, dysgeusia, sore throat.
Ventilation/ICU requirement, death.
Table 4.
Demographic, clinical, radiological and laboratory features, and outcomes stratified according to the presence or absence of comorbidities
| No comorbidities (n = 28) | Comorbidities (n = 23) | Bilateral P-value | Multivariate OR [95% CI] | |
|---|---|---|---|---|
| Sex (men) | 1 (3.6) | 4 (17.4) | 0.162 | |
| Age, mean (s.d.), years | 55.9 (13) | 65.8 (13.1) | 0.010 | 1.05 [1.00, 1.11] |
| Positive contact tracing | 0.084 | |||
| Family | 10 (35.7) | 9 (39.1) | ||
| Work | 11 (39.3) | 3 (13) | ||
| Not identified | 7 (25) | 11 (47.8) | ||
| Baseline therapies | 0.211 | |||
| None | 17 (60.7) | 13 (56.5) | ||
| Hydroxychloroquine | 5 (17.9) | 1 (4.3) | ||
| Immunosuppresants | 6 (21.4) | 9 (39.1) | ||
| Fever | 23 (82.1) | 19 (82.6) | 1.000 | |
| Respiratory symptomsa | 18 (64.3) | 15 (65.2) | 1.000 | |
| Gastrointestinal symptomsb | 7 (25) | 7 (30.4) | 0.757 | |
| General symptomsc | 11 (39.3) | 10 (43.5) | 0.783 | |
| ENT symptomsd | 10 (35.7) | 2 (8.7) | 0.044 | |
| COVID-19 definition (confirmed) | 19 (67.9) | 14 (60.9) | 0.769 | |
| Chest radiography (infiltrates) | 10 (71.4) | 17 (89.5) | 0.363 | |
| Anaemia (Hb < 12 g/L) | 4 (30.8) | 4 (20) | 0.681 | |
| Leukopenia (<4000/mm3) | 0 (0) | 3 (15) | 0.261 | |
| Lymphopenia (<900/mm3) | 9 (69.2) | 14 (70) | 1.000 | |
| Raised D-Dimer levels | 7 (87.5) | 11 (78.6) | 1.000 | |
| Raised LDH levels | 7 (77.8) | 15 (93.8) | 0.530 | |
| Raised ferritin levels | 3 (60) | 5 (55.6) | 1.000 | |
| Raised CRP levels | 9 (75) | 17 (85) | 0.647 | |
| Hospital admission | 8 (28.6) | 17 (73.9) | 0.002 | 6.01 [1.72, 23.51] |
| Supplemental oxygen requirement | 4 (14.3) | 13 (56.5) | 0.002 | |
| Poor outcomese | 0 (0) | 6 (26.1) | 0.006 |
Cough, dyspnoea, thoracic pain.
Diarrhoea, nausea, vomiting.
Fatigue, myalgias.
Anosmia, dysgeusia, sore throat.
Ventilation/ICU requirement, death.
Discussion
The impact of the COVID-19 pandemic on people with rheumatic and systemic autoimmune diseases has been investigated in several (most retrospective) using various methodological approaches (Supplementary Table S2, available at Rheumatology online). Most patients included in these studies have inflammatory arthritis, probably due to their relatively high population frequency [especially for rheumatoid arthritis (RA)] and to the frequent use of biological therapies in these patients. Some studies have reported higher rates of hospitalization [19] or mechanical ventilation [20] in these patients, while in the OPENSAFELY study (the largest cohort study to date analysing clinical risk factors for COVID-19-related death) [21], the age-sex adjusted hazard ratio for death was 1.30 (95% CI 1.21, 1.38) for patients with RA, lupus or psoriasis and 2.06 (95% CI 1.62, 2.61) for patients with other immunosuppressive conditions. Unfortunately, studies focused on individual systemic autoimmune diseases are very limited and mainly focused on small SLE series of <20 patients infected by SARS-CoV-2 [22, 23]. There is no specific study focused on SS, with 35 cases (it is unknown whether primary or associated) included in five studies [24–28] but without a specific description of these patients.
In this study, we have tried to capture the broadest, real-life spectrum of SARS-CoV-2 infection in primary SS patients, including not only hospitalized cases, but also patients diagnosed and followed up in a primary care setting. This approach is irretrievably associated with a lower degree of availability of medical examinations performed (laboratory and imaging studies), an aspect that is reflected, for example, in the percentage of cases confirmed by PCR, a test not available at outpatient levels in those countries hardest hit by the pandemic during March to May 2020 and that was usually realized overwhelmingly in severely ill patients. Despite this, we did not find significant differences between patients with or without confirmed infection by virological studies. We have estimated a frequency of SARS-CoV-2 infection (including both confirmed and probable cases) of 0.62%, a figure that needs to be interpreted cautiously considering the significant risk for bias associated with the very different approaches used to diagnose and follow SARS-CoV-2 infection around the world. Despite this limitation, the estimated infection rate in patients with primary SS is too close to the infection rate estimated by the WHO in the Spanish general population during the same study period (0.53%, 95% CI 0.50, 0.56) [5]. Until now, only one population-based study carried out in Spain has estimated the prevalence of the infection (PCR+) in SS, reporting a high figure (1.85%) in comparison with other diseases or with the reference figure [29]. In fact, when we analyse the frequency of PCR+ patients from only the Spanish centres included in our study, the prevalence is 2.3% (33/1438), a very close figure. The reasons explaining why patients with primary SS may have one of the highest rates of SARS-CoV-2 infection (at least in Spain) with respect to other systemic and rheumatic autoimmune diseases are unknown.
The phenotype of SARS-CoV-2 infection in our patients with primary SS (signs and symptoms at presentation, laboratory results and radiographical abnormalities) is similar to that reported in the largest reported cohorts of infected patients [30, 31], suggesting that primary SS individuals with COVID-19 could be treated with the standard of care that is being applied for the general population. With respect to the prognosis and outcomes, we found that the main baseline features associated with a more complicated infection were similar to that identified in non-SS studies [21] including older age, male sex, chronic comorbidities (pulmonary/kidney diseases, hematological neoplasia), pneumonia (respiratory symptoms and pulmonary infiltrates) and lymphopenia. We did not find an association between baseline SS therapies and hospitalization, probably because of the small sample size. Previous studies in patients with rheumatic diseases have reported increased odds of hospitalization in patients under corticosteroid therapy, lower odds in those treated with biologics in monotherapy, and no significant association with the use of antimalarials [28]. According to a recently published study by Sisó-Almirall et al. [32], autoimmune diseases were an independent risk factor for ICU admission and death.
With respect to the main outcomes of SARS-CoV-2 infection, the figures observed in our patients with primary SS were similar to that reported in patients with rheumatic diseases for hospitalization (44–68%) [19, 20, 28], need for supplemental oxygen (33%) [20], ICU admission (15–21%) [19, 20] and mortality rate (6–9%) [19, 20, 28], and also similar to the figures recently reported in the Spanish general population (hospitalization rate of 49.1% and mortality rate of 5.6%, respectively) [32]. However, when we stratified the outcomes according to the presence or absence of baseline comorbidities, the figures for poor outcomes were significantly higher in primary SS patients and concomitant comorbidities. In fact, among patients with primary SS without baseline comorbidities, none had a poor outcome, suggesting that the development of a complicated COVID-19 seems to be associated more with the existence of pre-infectious comorbidities (most unrelated to the autoimmune disease) than with the primary SS itself.
The overall body of COVID-19 research may be flawed methodologically and underpinned mainly by uncontrolled confounded evidence [33], in most cases related with the rapid pandemic spread and the lack of a homogeneous protocolized management. This may have a significant impact especially in retrospective, observational studies, and methodological limitations should be well acknowledged and explained. First, a selection bias cannot be discarded in our study, considering the great heterogeneity in the accessibility to the status of infection of all SS patients among the participating countries, that may be even very different among regions of the same country. The retrospective approach to data collection places limitations on causal conclusions based on the reported results, and the generalizability of our findings may also be limited because of the contribution of few countries and the small number of patients studied. In addition, the small sample size might have generated underpowered statistical tests.
Second, the estimation of infection rate is probably biased because local recommendations restricted confirmatory testing. The number of PCR-positive cases might have been underestimated because of the low use of tests, especially at the beginning of the epidemic for the less severe cases, as has been reported in previous similar studies [34]. As a result, the frequency of primary SS patients with SARS-CoV-2 infection confirmed by PCR we found was lower than that reported in the largest study focused on patients with rheumatic diseases [28].
Third, because of the observational design, individuals were managed from a heterogeneous clinical point of view, and the appropriate diagnostic tests were not carried out in all cases (especially in the less severe clinical cases), biasing the effect of these variables on outcomes. Strengths of the study are the use of the largest international data-sharing registry of primary SS that has provided the more complete description of the disease in >12 000 patients from 20 countries of the five continents [1, 17, 18], and that all of our cases were resolved or had a known resolution status at the time of manuscript writing, and we gathered complete information on medication use prior to COVID-19 diagnosis and additional historical treatments. As has been advised, principles of open science and raw data sharing may be of greatest importance to allow analysis of data collected during the COVID-19 pandemic, especially in patients with very specific disease conditions [35].
To the best of our knowledge, this is the first study to characterize and evaluate the outcomes of SARS-CoV-2 infection in patients with primary SS. Primary SS-infected individuals seemed to be similarly affected by SARS-CoV-2 compared with the general population in terms of clinical presentation. Notably, baseline comorbidities were risk factors for a more complicated COVID-19 in this population, especially chronic pulmonary disease, not only interstitial lung disease (closely related to poor outcome in primary SS) [36], but also asthma (recently related to a more complicated outcome in SARS-CoV-2 infection) [37]. We found that patients with primary SS and comorbidities had higher rates of hospitalization and poor outcomes (intensive care admission, death) compared with those without baseline comorbidities. These results underscore the need for a specific close monitoring of comorbidities of patients with primary SS during the pandemic.
Supplementary Material
Acknowledgements
Members of the EULAR-SS Task Force Big Data Consortium who contributed to this study: P. Brito-Zerón. C. Morcillo (Autoimmune Diseases Unit, Department of Medicine, Hospital CIMA- Sanitas, Barcelona, Spain); P. Brito-Zerón, A. Flores-Chávez, M. Ramos-Casals (Sjögren Syndrome Research Group (AGAUR), Laboratory of Autoimmune Diseases Josep Font, IDIBAPS-CELLEX, Department of Autoimmune Diseases, ICMiD, University of Barcelona, Hospital Clínic, Barcelona, Spain); N. Acar-Denizli (Department of Statistics, Faculty of Science and Letters, Mimar Sinan Fine Arts University, Istanbul, Turkey); I.F. Horvath, A. Szanto, T. Tarr (Division of Clinical Immunology, Faculty of Medicine, University of Debrecen, Debrecen, Hungary); R. Seror, X. Mariette (Center for Immunology of Viral Infections and Autoimmune Diseases, Assistance Publique – Hôpitaux de Paris, Hôpitaux Universitaires Paris-Sud, Le Kremlin-Bicêtre, Université Paris Sud, INSERM, Paris, France); T. Mandl, P. Olsson (Department of Rheumatology, Malmö University Hospital, Lund University, Lund, Sweden); X. Li, B. Xu (Department of Rheumatology and Immunology, Anhui Provincial Hospital, China); C. Baldini, S. Bombardieri (Rheumatology Unit, University of Pisa, Pisa, Italy); J.E. Gottenberg (Department of Rheumatology, Strasbourg University Hospital, Université de Strasbourg, CNRS, Strasbourg, France); S. Gandolfo, S De Vita (Clinic of Rheumatology, Department of Medical and Biological Sciences, University Hospital ‘Santa Maria della Misericordia’, Udine, Italy); R. Priori, F. Giardina (Department of Internal Medicine and Medical Specialties, Rheumatology Clinic, Sapienza University of Rome, Italy); G. Hernandez-Molina, J. Sánchez-Guerrero (Immunology and Rheumatology Department, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, México City, Mexico); A.A. Kruize, A. Hinrichs (Department of Rheumatology and Clinical Immunology, University Medical Center Utrecht, Utrecht, The Netherlands); V. Valim (Department of Medicine, Federal University of Espírito Santo, Vitória, Brazil); D. Isenberg (Centre for Rheumatology, Division of Medicine, University College London, UK); R. Solans (Department of Internal Medicine, Hospital Vall d'Hebron, Barcelona, Spain); M. Rischmueller, S. Downie-Doyle (Department of Rheumatology, School of Medicine, The University of Western Australia, Crawley, Australia); S-K. Kwok, S-H. Park (Division of Rheumatology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, South Korea); G. Nordmark (Rheumatology. Department of Medical Sciences, Uppsala University, Uppsala, Sweden); Y. Suzuki, M. Kawano (Division of Rheumatology, Kanazawa University Hospital, Kanazawa, Ishikawa, Japan); R. Giacomelli (Clinical Unit of Rheumatology, University of l'Aquila, School of Medicine, L'Aquila, Italy); V. Devauchelle-Pensec, A. Saraux (Rheumatology Department, Brest University Hospital, Brest, France); B. Hofauer, A. Knopf (Otorhinolaryngology/Head and Neck Surgery, Technical University Munich, Munich, Germany); H. Bootsma, A. Vissink (Department of Rheumatology & Clinical Immunology, University of Groningen, University Medical Center Groningen, the Netherlands); J. Morel (Department of Rheumatology, Teaching Hospital and University of Montpellier, Montpellier, France); C. Vollenveider (German Hospital, Buenos Aires, Argentina); F. Atzeni (IRCCS Galeazzi Orthopedic Institute, Milan and Rheumatology Unit, University of Messina, Messina, Italy); S. Retamozo (Instituto De Investigaciones En Ciencias De La Salud (INICSA), Universidad Nacional de Córdoba (UNC), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Instituto Universitario de Ciencias Biomédicas de Córdoba (IUCBC), Córdoba, Argentina); V. Moça Trevisano (Federal University of São Paulo, Sao Paulo, Brazil); B. Armagan, L. Kilic, U. Kalyoncu (Department of Internal Medicine, Hacettepe University, Faculty of Medicine, Ankara, Turkey); S.G. Pasoto (Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina. Universidade de Sao Paulo, Sao Paulo, Brazil); B. Kostov, A. Sisó-Almirall (Primary Healthcare Transversal Research Group, IDIBAPS, Centre d’Assistència Primària ABS Les Corts, CAPSBE, Barcelona, Spain); S. Consani-Fernández (Internal Medicine, Hospital Maciel, Universidad de la República (UdelaR), Montevideo, Uruguay). S. Melchor, P. Carreira (Servicio de Reumatologia, Hospital Universitario 12 de Octubre, Madrid, Spain); F. Carubbi (COVID-19 Medical Unit, San Salvatore Hospital, Department of Medicine, ASL1 Avezzano-Sulmona-L’Aquila, 67100 L’Aquila, Italy); Jose Luis Callejas (Department of Internal Medicine, Hospital San Cecilio, Granada, Spain); M. López-Dupla (Department of Internal Medicine, Hospital Joan XXIII, Tarragona, Spain); R. Pérez-Alvarez (Department of Internal Medicine, Hospital do Meixoeiro, Vigo, Spain); M. Akasbi (Department of Internal Medicine, Hospital Infanta Leonor, Madrid, Spain); P. Guisado-Vasco (Department of Internal Medicine, Hospital Quirón, Madrid, Spain); I. Sánchez (Department of Internal Medicine, Hospital Rey Juan Carlos de Móstoles, Madrid, Spain).
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure statement: None declared.
Data availability statement
External investigators interested in collaboration or using the data from the Sjögren Big Data Project can contact Dr Manuel Ramos-Casals (mramos@clinic.cat). Requests will be considered on a case-by-case basis.
Contributor Information
Members of the EULAR-SS Task Force Big Data Consortium who contributed to this study:
P Brito-Zerón, C Morcillo, P Brito-Zerón, A Flores-Chávez, M Ramos-Casals, N Acar-Denizli, I F Horvath, A Szanto, T Tarr, R Seror, X Mariette, T Mandl, P Olsson, X Li, B Xu, C Baldini, S Bombardieri, J E Gottenberg, S Gandolfo, S De Vita, R Priori, F Giardina, G Hernandez-Molina, J Sánchez-Guerrero, A A Kruize, A Hinrichs, V Valim, D Isenberg, R Solans, M Rischmueller, S Downie-Doyle, S-K Kwok, S-H Park, G Nordmark, Y Suzuki, M Kawano, R Giacomelli, V Devauchelle-Pensec, A Saraux, B Hofauer, A Knopf, H Bootsma, A Vissink, J Morel, C Vollenveider, F Atzeni, S Retamozo, V Moça Trevisano, B Armagan, L Kilic, U Kalyoncu, S G Pasoto, B Kostov, A Sisó-Almirall, S Consani-Fernández, F Carubbi, J L Callejas, M López-Dupla, R Pérez-Alvarez, M Akasbi, P Guisado-Vasco, and I Sánchez
References
- 1. Brito-Zeron P, Acar-Denizli N, Zeher M et al. Influence of geolocation and ethnicity on the phenotypic expression of primary Sjogren’s syndrome at diagnosis in 8310 patients: a cross-sectional study from the Big Data Sjogren Project Consortium. Ann Rheum Dis 2017;76:1042–50. [DOI] [PubMed] [Google Scholar]
- 2. Mariette X, Criswell LA. Primary Sjogren’s Syndrome. N Engl J Med 2018;378:931–9. [DOI] [PubMed] [Google Scholar]
- 3. Sisó-Almirall A, Kostov B, Martínez-Carbonell E et al. The prevalence of 78 autoimmune diseases in Catalonia (MASCAT-PADRIS Big Data Project). Autoimmun. Rev 2020;19:102448. [DOI] [PubMed] [Google Scholar]
- 4. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020;135:2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int (14 September 2020, date last accessed).
- 6. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med 2020;173:362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao Y-M, Xu G, Wang B, Liu B-C. Cytokine storm syndrome in coronavirus disease 2019: a narrative review. J Intern Med 2020. doi: 10.1111/joim.13144. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brito-Zerón P, Kostov B, Fraile G et al. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J Hematol Oncol 2017;10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramos-Casals M, Brito-Zerón P, Bombardieri S et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann Rheum Dis 2020;79:3–18. [DOI] [PubMed] [Google Scholar]
- 10. Sambataro G, Ferro F, Orlandi M et al. Clinical, morphological features and prognostic factors associated with interstitial lung disease in primary Sjögren’s syndrome: a systematic review from the Italian Society of Rheumatology. Autoimmun Rev 2020;19:102447. [DOI] [PubMed] [Google Scholar]
- 11. Vitali C, Bombardieri S, Jonsson R et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiboski CH, Shiboski SC, Seror R et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome. Ann Rheum Dis 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- 13. Vitali C, Bombardieri S, Moutsopoulos HM et al. Preliminary criteria for the classification of Sjögren’s syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum 1993;36:340–7. [DOI] [PubMed] [Google Scholar]
- 14. Eurosurveillance Editorial Team. Updated rapid risk assessment from ECDC on coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Eur Commun Dis Bull 2020;25: 2003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wiersinga WJ, Rhodes A, Cheng AC et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782–93. [DOI] [PubMed] [Google Scholar]
- 16. Tabata S, Imai K, Kawano S et al. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis. Lancet Infect Dis 2020;20:1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brito-Zeron P, Acar-Denizli N, Ng W-F et al. Epidemiological profile and north-south gradient driving baseline systemic involvement of primary Sjogren’s syndrome. Rheumatology 2020;59:2350–9. [DOI] [PubMed] [Google Scholar]
- 18. Brito-Zeron P, Acar-Denizli N, Ng W-F et al. How immunological profile drives clinical phenotype of primary Sjogren’s syndrome at diagnosis: analysis of 10,500 patients (Sjogren Big Data Project). Clin Exp Rheumatol 2018;36(Suppl 1):102–12. [PubMed] [Google Scholar]
- 19. Sanchez-Piedra C, Diaz-Torne C, Manero J et al. Clinical features and outcomes of COVID-19 in patients with rheumatic diseases treated with biological and synthetic targeted therapies. Ann Rheum Dis 2020;79:988–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D’Silva KM, Serling-Boyd N, Wallwork R et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis 2020;79:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williamson EJ, Walker AJ, Bhaskaran K et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bozzalla Cassione E, Zanframundo G, Biglia A et al. COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine. Ann Rheum Dis 2020;79:1382–3. [DOI] [PubMed] [Google Scholar]
- 23. Mathian A, Mahevas M, Rohmer J et al. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis 2020;79:837–9. [DOI] [PubMed] [Google Scholar]
- 24. Zhao J, Pang R, Wu J et al. Clinical characteristics and outcomes of patients with COVID-19 and rheumatic disease in China ‘hot spot’ versus in US ‘hot spot’: similarities and differences. Ann Rheum Dis 2020. doi: 10.1136/annrheumdis-2020-218183. [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25. Ye C, Cai S, Shen G et al. Clinical features of rheumatic patients infected with COVID-19 in Wuhan, China. Ann Rheum Dis 2020;79:1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang Y, Chen Z, Wang Y et al. Clinical characteristics of 17 patients with COVID-19 and systemic autoimmune diseases: a retrospective study. Ann Rheum Dis 2020;79:1163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Emmi G, Bettiol A, Mattioli I et al. SARS-CoV-2 infection among patients with systemic autoimmune diseases. Autoimmun Rev 2020;19:102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gianfrancesco M, Hyrich KL, Al-Adely S et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pablos JL, Abasolo L, Alvaro-Gracia JM et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis 2020;79:1170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borobia AM, Carcas AJ, Arnalich F et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med 2020;9:1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lapostolle F, Schneider E, Vianu I et al. Clinical features of 1487 COVID-19 patients with outpatient management in the Greater Paris: the COVID-call study. Intern Emerg Med 2020;15:813–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sisó-Almirall A, Kostov B, Mas-Heredia M et al. Prognostic factors in Spanish COVID-19 patients: A case series from Barcelona. PLoS ONE 2020;15:e0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alexander PE, Debono VB, Mammen MJ et al. COVID-19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J Clin Epidemiol 2020;123:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Del Amo J, Polo R, Moreno S et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med 2020;173:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gewin V. Six tips for data sharing in the age of the coronavirus. Nature 2020. doi: 10.1038/d41586-020-01516-0. [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36. Nannini C, Jebakumar AJ, Crowson CS et al. Primary Sjogren’s syndrome 1976-2005 and associated interstitial lung disease: a population-based study of incidence and mortality. BMJ Open 2013;3:e003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mahdavinia M, Foster KJ, Jauregui E et al. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract 2020;8:2388–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
External investigators interested in collaboration or using the data from the Sjögren Big Data Project can contact Dr Manuel Ramos-Casals (mramos@clinic.cat). Requests will be considered on a case-by-case basis.