Abstract
Purpose
To compare the intraocular pressure (IOP) after an intravitreal triamcinolone acetonide (IVTA) between vitrectomised and non-vitrectomised eyes in patients with diabetes and diabetic macular oedema (DME).
Design
Retrospective comparative study
Methods
Medical records of 157 patients (157 eyes) with type 2 diabetes who received IVTA for DME were reviewed, and the best-corrected visual acuity, IOP and optical central retinal thickness (CRT) were compared preoperatively, at 1, 4, 12 and 24 weeks after IVTA between the vitrectomised and non-vitrectomised groups.
Results
IOP significantly increased at 1 (p<0.0001), 4 (p<0.0001), 8 (p<0.0001), 12 (p=0.0019), 16 (p=0.0006) and 20 weeks (p=0.0191) in the non-vitrectomised group, whereas a significant increase was only observed at 1 (p=0.0003) and 4 weeks (p=0.0006) in the vitrectomised group. ΔIOP, IOP changes from baseline, in the non-vitrectomised group was significantly higher than that in the vitrectomised group at 4 (p=0.0014), 8 (p=0.0081), 12 (p=0.0032) and 16 weeks (p=0.0038). No significant difference was observed in logMAR and CRT at any time point after IVTA between the two groups.
Conclusions
After an initial IVTA, increased IOP and ΔIOP from the baseline IOP were significantly more frequently observed in the non-vitrectomised than that in the vitrectomised group. IVTA is a safer and more effective treatment option for DME in vitrectomised than that in non-vitrectomised eyes.
Keywords: treatment medical
Key messages.
What is already known about this subject?
Injection of intravitreal triamcinolone acetonide (IVTA) is known as an effective treatment for diabetic macula oedema (DME), but it has a risk of intraocular pressure (IOP) elevation.
What are the new findings?
This study shows that after an initial IVTA, increased IOP and ΔIOP from the baseline IOP were significantly more frequently observed in the non-vitrectomised than that in the vitrectomised group.
How might these results change the focus of research or clinical practice?
IVTA is a safer and more effective treatment option for DME in vitrectomised eyes than that for non-vitrectomised eyes.
Introduction
Diabetic macular oedema (DME) is the leading cause of visual impairment in the working-age population in association with the increasing incidence of type 2 diabetes. DME therapy primarily aimed to resolve retinal thickening and maintain or improve retinal function. Intravitreally administered antivascular endothelial growth factor (VEGF) agents1–3 and corticosteroids are the two major medication categories currently used to treat DME.4 5
As VEGF inhibitors reduce the excess vascular permeability by acting on VEGF, corticosteroids act on several pathways involved in the DME pathogenesis to inhibit the inflammatory cytokines including VEGF.6 Corticosteroids are non-specific anti-inflammatory agents and have also been reported to antagonise the action of VEGF.7 8 The effect of three potent corticosteroids, that is, triamcinolone acetonide (TA), fluocinolone acetonide and dexamethasone, was found as treatments for DME. Only injection of intravitreal triamcinolone acetonide (IVTA) has been clinically available in Japan, and some researches have demonstrated IVTA to improve DME.9–11
IVTA is typically effective for about 3 months in a non-vitrectomised eyes, and repeated injections may be necessary to maintain its treatment effect.12 IVTA injection has potential risks including endophthalmitis, lens opacifications and intraocular pressure (IOP) elevation. Transient IOP elevation is the most commonly reported side effect of IVTA. Previous studies have reported that some variables including younger age, high baseline IOP and male gender were significant risk factors for IOP elevation after an IVTA in age-related macular degeneration, retinal vein occlusion (RVO), uveitis and other conditions.13 14 However, no studies compared the IOP after the IVTA only for DME between vitrectomised and non-vitrectomised eyes. Therefore, this study aimed to compare the IOP after IVTA between vitrectomised and non-vitrectomised eyes in patients with diabetes with DME.
Materials and methods
This retrospective comparative clinical research adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Fukui and University of Mie and registered to the University Hospital Medical Information Network Clinical Trials Registry (UMIN) in Japan (R000043655).
Patient selection
Patients or the public were involved in the design, or conduct, or reporting or dissemination plans of our research. Medical records of patients with type 2 diabetes who received IVTA for DME were reviewed. Data of cases that received IVTA between 1 April 2012 and 31 October 2018, at the university of Fukui and University of Mie were included in this study. The exclusion criteria were as follows: (1) patients who had vitreous haemorrhage or endophthalmitis after IVTA, (2) those with missing post-IVTA follow-up data for shorter than 6 months and (3) those who were inappropriately assessed by the investigator. Phakic eyes were excluded to avoid the progression of cataract after IVTA.
IVTA injection
All injections were performed using topical anaesthetic drops (2% of lidocaine hydrochloride). Then, 4 mg of TA (MaQaid; Wakamoto pharmaceutical Co, Ltd, Tokyo, Japan) was injected into the vitreous cavity using a 30 G needle through the pars plana. Eyes that required multiple injections were injected at a minimum interval of 3 months. Retreatment was based on clinical or optical coherence tomography (OCT)-based evidence for persistent macular oedema or decline in the visual acuity. As this was a retrospective study, the choice of retreatment was at the discretion of retina specialists.
Ophthalmic examinations
Preoperatively, at 1, 4, 12 and 24 weeks after IVTA, all the patients had ophthalmic examinations, including Goldman applanation tonometry, best-corrected visual acuity (BCVA) and optical central retinal thickness (CRT) using a map mode of the OCT (SPECTRALIS: Heidelberg Engineering, Vista, California, USA).
BCVA was converted into logMAR and then analysed. Eyes with IOP of ≥24 mm Hg were treated with IOP-lowering drugs (topical or oral).
Statistical analyses
Statistical analyses were performed using JMP V.14 (SAS institute Inc, Tokyo, Japan). The significant differences in ΔIOP between the groups was analysed using the Mann-Whitney test. Bartlett’s test was used to examine equal variances across samples. IOP, CRT or BCVA among the different time points were compared by using the Wilcoxon signed-rank test. ΔIOP and ΔCRT was defined as the amount of the change from baseline. The relationship between ΔIOP and ΔCRT was analysed by simple regression test. P values of <0.05 were considered statistically significant.
Results
Patient characteristics
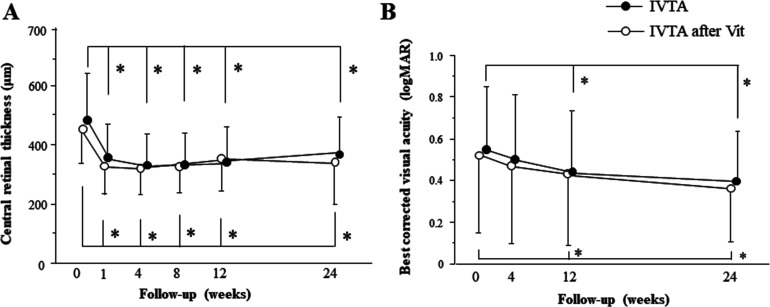
A total of 157 eyes of 157 patients (100 men; 57 women) were included in this study (73 in the vitrectomised and 84 in the non-vitrectomised group). Basic patient characteristics are demonstrated in table 1. In the vitrectomised group, 21 patients needed IVTA, 4 needed anti-VEGF therapy and 2 needed additional vitrectomy. In the non-vitrectomised group, 17 patients needed IVTA, 6 needed anti-VEGF therapy and 2 needed additional vitrectomy. All patients had pseudophakic eyes. Before the initial IVTA treatment, the difference in haemoglobin A1C, serum creatinine and duration of diabetes mellitus was not statistically significant between the two groups. Furthermore, no significant differences in CRT, logMAR and IOP were observed between the two groups. In both non-vitrectomised and vitrectomised groups, the CRT at 1 (p<0.0001 and p=0.0003), 4 (p<0.0001 and p<0.0001), 12 (p<0.0001 and p<0.0001) and 24 weeks (p<0.0001 and p=0.0218) was significantly lower than that in the baseline (figure 1A). BCVA significantly improved at 12 (p=0.0484 and p=0.0428) and 24 weeks (p=0.0218 and p=0.0221) in the non-vitrectomised and vitrectomised groups, respectively (figure 1B). The difference in logMAR and CRT was not significant between the two groups at any time point.
Table 1.
Baseline characteristics
| IVTA group* (n=84) | IVTA after VIT group† (n=73) | P value | |
| Age (years) | 62.3±7.2 | 64.9±8.1 | 0.41* |
| Gender (male/female) | 51/33 | 47/26 | 0.63† |
| Duration of DM (years) | 11.2±2.5 | 12.4±2.8 | 0.24* |
| Haemoglobin A1c (%) | 7.2±0.2 | 7.3±0.3 | 0.56* |
| Insulin therapy | 36 (42.8 %) | 31 (42.5%) | 0.62† |
| Serum creatinine | 2.19±0.32 | 2.31±0.42 | 0.41* |
*Mann-Whitney test.
†χ2 test.
DM, diabetes mellitus; IVTA, intravitreal triamcinolone acetonide; VIT, vitrectomy.
Figure 1.
Changes of central retinal thickness (A) and best-corrected visual acuity (B) at baseline and follow-up. *p<0.05. IVTA, intravitreal triamcinolone acetonide; Vit, vitrectomy.
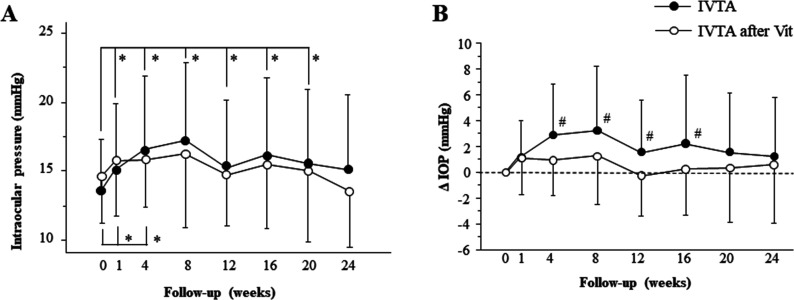
After the initial IVTA, IOP in the non-vitrectomised group significantly increased at 1 (p<0.0001), 4 (p<0.0001), 8 (p<0.0001), 12 (p=0.0019), 16 (p=0.0006) and 20 weeks (p=0.0191, figure 2A). Conversely, in the vitrectomised group, the significant IOP increase was only observed at 1 (p=0.0003) and 4 weeks (p=0.0006). ΔIOP, the IOP changes from baseline, in the non-vitrectomised group was significantly larger than that in the vitrectomised group at 4 (p=0.0014), 8 (p=0.0081), 12 (p=0.0032) and 16 weeks (p=0.0038, (figure 2B). The number of patients who underwent additional IVTA was 16 (19.0 %)/84 and 16 (21.9 %)/73 in the non-vitrectomised and vitrectomised groups, respectively. Seven (8.3 %) and 5 (6.8 %) patients in the non-vitrectomised and vitrectomised groups, respectively, required topical eye drop medication due to an elevated IOP of >24 mm Hg during the observational periods.
Figure 2.
Changes of IOP (A) and ΔIOP (the IOP changes from baseline) (B) at baseline and follow-up. *p<0.05, #p<0.05. IOP, intraocular pressure; IVTA, intravitreal triamcinolone acetonide; Vit, vitrectomy.
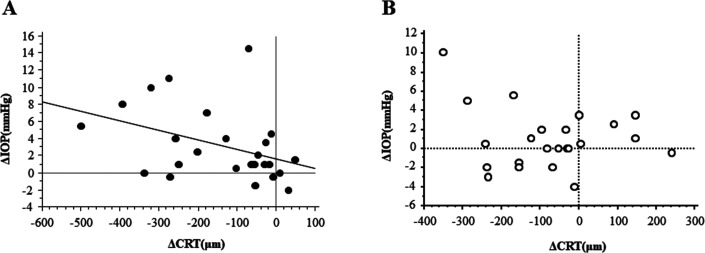
We analysed the correlation between ΔIOP and ΔCRT at 8 weeks (figure 3). In the non-vitrectomised eye, the significant correlation was found at 8 weeks (p=0.0429), but not at any other time period. On the other hand, in the vitrectomised eye, no significant relationship was found at any time point.
Figure 3.
Correlation between ΔIOP and ΔCRT. There was a significant relationship between ΔIOP and ΔCRT at 8 weeks in the non-vitrectomised eye (A), p=0.0429, R2=0.159, but not in the vitrectomised eye (B). CRT, central retinal thickness; IOP, intraocular pressure.
Discussion
In this study, the IOP after IVTA were compared between vitrectomised and non-vitrectomised eyes only in patients with diabetes with DME. After the initial IVTA, IOP and ΔIOP in the non-vitrectomised group were significantly more frequently higher than that in the vitrectomised group. Conversely, no significant differences in BCVA and CRT were observed between the two groups at any time point after IVTA. In this study, the significant relationship was noticed in the non-vitrectomised eye at 8 weeks, the timing showing the peak of IOP elevation after initial IVTA. This finding was consistent with previous reports.15 16 These data indicate that early anatomical response induced by IVTA was correlated with IOP change. While, this correlation was not found in the vitrectomised eyes. This reason may be that the levels of the IOP elevation was relatively small in the vitrectomised group.
Transient IOP elevation is the most commonly reported side effect of IVTA, and several studies have evaluated the risk factors for increased IOP after IVTA.13 14 17 Hirano et al reported that in patients with DME, age-related macular degeneration, RVO, myopic choroidal neovascularisation, uveitis, or other conditions, higher baseline IOP, younger age and simultaneous sub-Tenon capsule and intravitreal injections are risk factors for IOP elevation.14 Sonmez et al reported that in patients with RVO, diabetic retinopathy and uveitis who had received IVTA, higher baseline IOP, younger age and male gender were significant risk factors for IOP elevation after IVTA.13 However, no study has compared the IOP after IVTA only for DME between vitrectomised and non-vitrectomised eyes. To the best of our knowledge, our data first demonstrated that increased IOP and ΔIOP from the baseline IOP in the vitrectomised group were significantly less frequent than that in the non-vitrectomised group after IVTA.
Studies in rabbits have shown that IVTA more rapidly decreased in the vitrectomised eye than that in the non-vitrectomised eye.18 19 Beer et al reported that the mean elimination half-life was shorter in vitrectomised eyes (3.2 days) than that in non-vitrectomised eyes (18.6 days) after a single intravitreal triamcinolone injection.20 In the present study, IOP in the non-vitrectomised group significantly increased at 1, 4, 8, 12, 16 and 20 weeks after the initial IVTA, whereas the significant increase was only observed at 1 and 4 weeks in the vitrectomised group. ΔIOP from the baseline IOP was significantly larger in the non-vitrectomised group than that in the vitrectomised group at 4, 8, 12 and 16 weeks after IVTA. Pharmacokinetic changes after vitrectomy affecting the elimination via the anterior or posterior routes or differences in triamcinolone crystal dissolution rates due to the free flow of the fluid in the vitreous cavity cause faster TA clearance, leading to faster recovery from IOP elevation, in vitrectomised eyes than those in non-vitrectomised eyes.12 18–24
Mason et al reported that the concentration of IVTA can be detected until 2.75 months after a single intravitreal injection in non-vitrectomised eyes; however, TA cannot be detected in the vitreous humour at >3 months after IVTA.12 Theoretically, undetectability of TA is faster in vitrectomised eyes than that in non-vitrectomised eyes. However, the logMAR and CRT in this study were improved in the vitrectomised group after IVTA, and no significant difference was observed in both logMAR and CRT for up to 24 weeks between the vitrectomised group and non-vitrectomised group, despite faster recovery from IOP elevation of non-vitrectomised group. For this reason, the residual TA at the periphery and behind the lens capsule or TA trapped by residual vitreous body may improve the logMAR and CRT,10 and therefore, the concentration of the residual TA might not be high enough to cause IOP elevation. IOP elevation at 16 weeks is still considered as an effect of additional IVTA.
Sub-tenon TA injection (STTA) is a major therapeutic tool for DME to deliver the steroids through the anterior route. For better understanding of the pharmacokinetics, the investigation of the IOP alteration after STTA in the vitrectomised and non-vitrectomised eyes may be informative. Also, STTA may have an advantage to reduce the risk of IOP increase and the incidence of endophthalmitis compared with IVTA, as previously reported.14 To clarify this issue, further study is necessary. The limitation of this study is its retrospective, non-randomised design. The retrospective design with a relatively irregular follow-up may actually have led to an underestimation of IOP incidence as patients might have experienced an undocumented IOP elevation.
Conclusion
After an initial IVTA, increased IOP and ΔIOP from the baseline IOP were significantly more frequently observed in the non-vitrectomised group than that in the vitrectomised group. Conversely, the difference in logMAR and CRT was not significant between the two groups at any time point after IVTA. IVTA is a safer and more effective treatment option for DME in vitrectomised eyes than that for non-vitrectomized eyes.
Footnotes
Contributors: YO, MG, Yoshihiro T, MS and MI were responsible for study design. YO, MG, Yoshihiro T, MM, YY, TM, MS and MI were involved in data acquisition. YO, MG and Yoshihiro T conducted data analysis. YO, MG and Yoshihiro T drafted and wrote the manuscript. All authors approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Obtained.
Ethics approval: The Institutional Review Board of the University of Fukui.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon request.
References
- 1.Brown DM, Nguyen QD, Marcus DM, et al. Long-Term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: rise and ride. Ophthalmology 2013;120:2013–22. 10.1016/j.ophtha.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 2.Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (bolt) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol 2012;130:972–9. 10.1001/archophthalmol.2012.393 [DOI] [PubMed] [Google Scholar]
- 3.Cunningham ET, Adamis AP, Altaweel M, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112:1747–57. 10.1016/j.ophtha.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 4.Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology 2012;119:2125–32. 10.1016/j.ophtha.2012.04.030 [DOI] [PubMed] [Google Scholar]
- 5.Boyer DS, Yoon YH, Belfort R, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014;121:1904–14. 10.1016/j.ophtha.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 6.Golan S, Loewenstein A. Steroids and the management of macular edema. Ophthalmologica 2010;224 Suppl 1:31–40. 10.1159/000315159 [DOI] [PubMed] [Google Scholar]
- 7.Ayalasomayajula SP, Ashton P, Kompella UB. Fluocinolone inhibits VEGF expression via glucocorticoid receptor in human retinal pigment epithelial (ARPE-19) cells and TNF-alpha-induced angiogenesis in chick chorioallantoic membrane (cam). J Ocul Pharmacol Ther 2009;25:97–104. 10.1089/jop.2008.0090 [DOI] [PubMed] [Google Scholar]
- 8.Nauck M, Roth M, Tamm M, et al. Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. Am J Respir Cell Mol Biol 1997;16:398–406. 10.1165/ajrcmb.16.4.9115750 [DOI] [PubMed] [Google Scholar]
- 9.Costa JF, Sousa K, Marques JP, et al. Efficacy and safety of postvitrectomy intravitreal triamcinolone therapy for diabetic macular edema. Eur J Ophthalmol 2016;26:485–90. 10.5301/ejo.5000768 [DOI] [PubMed] [Google Scholar]
- 10.Watanabe A, Tsuzuki A, Arai K, et al. Efficacy of intravitreal triamcinolone acetonide for diabetic macular edema after vitrectomy. J Ocul Pharmacol Ther 2016;32:38–43. 10.1089/jop.2015.0045 [DOI] [PubMed] [Google Scholar]
- 11.Takamura Y, Shimura M, Katome T, et al. Effect of intravitreal triamcinolone acetonide injection at the end of vitrectomy for vitreous haemorrhage related to proliferative diabetic retinopathy. Br J Ophthalmol 2018;102:1351–7. 10.1136/bjophthalmol-2017-311377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason JO, Somaiya MD, Singh RJ. Intravitreal concentration and clearance of triamcinolone acetonide in nonvitrectomized human eyes. Retina 2004;24:900–4. 10.1097/00006982-200412000-00009 [DOI] [PubMed] [Google Scholar]
- 13.Sonmez K, Ozturk F. Complications of intravitreal triamcinolone acetonide for macular edema and predictive factors for intraocular pressure elevation. Int J Ophthalmol 2012;5:719–25. 10.3980/j.issn.2222-3959.2012.06.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano Y, Ito T, Nozaki M, et al. Intraocular pressure elevation following triamcinolone acetonide administration as related to administration routes. Jpn J Ophthalmol 2009;53:519–22. 10.1007/s10384-009-0692-5 [DOI] [PubMed] [Google Scholar]
- 15.Chae JB, Joe SG, Yang SJ, et al. An increase in intraocular pressure after intravitreal steroid injection facilitates reduction of macular edema. Eye 2012;26:479–80. 10.1038/eye.2011.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KT, Lee H, Kim JY, et al. Association between early anatomic response and intraocular pressure change after intravitreal dexamethasone implant: an optical coherence tomography study. J Clin Med 2020;9:2692 10.3390/jcm9092692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Day RF, Barthelmes D, Zhu M, et al. Intraocular pressure rise is predictive of vision improvement after intravitreal triamcinolone acetonide for diabetic macular oedema: a retrospective analysis of data from a randomised controlled trial. BMC Ophthalmol 2014;14:123. 10.1186/1471-2415-14-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin H-S, Park T-S, Moon Y-S, et al. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina 2005;25:556–60. 10.1097/00006982-200507000-00002 [DOI] [PubMed] [Google Scholar]
- 19.Schindler RH, Chandler D, Thresher R, et al. The clearance of intravitreal triamcinolone acetonide. Am J Ophthalmol 1982;93:415–7. 10.1016/0002-9394(82)90130-1 [DOI] [PubMed] [Google Scholar]
- 20.Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology 2003;110:681–6. 10.1016/S0161-6420(02)01969-3 [DOI] [PubMed] [Google Scholar]
- 21.Kakinoki M, Sawada O, Sawada T, et al. Effect of vitrectomy on aqueous VEGF concentration and pharmacokinetics of bevacizumab in macaque monkeys. Invest Ophthalmol Vis Sci 2012;53:5877–80. 10.1167/iovs.12-10164 [DOI] [PubMed] [Google Scholar]
- 22.Ahn J, Kim H, Woo SJ, et al. Pharmacokinetics of intravitreally injected bevacizumab in vitrectomized eyes. J Ocul Pharmacol Ther 2013;29:612–8. 10.1089/jop.2013.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang-Lin J-E, Burke JA, Peng Q, et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci 2011;52:4605. 10.1167/iovs.10-6387 [DOI] [PubMed] [Google Scholar]
- 24.Yanyali A, Aytug B, Horozoglu F, et al. Bevacizumab (Avastin) for diabetic macular edema in previously vitrectomized eyes. Am J Ophthalmol 2007;144:124–6. 10.1016/j.ajo.2007.02.048 [DOI] [PubMed] [Google Scholar]