Abstract
Seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies was 10% among the subset of decedents undergoing forensic postmortem examination in June in Maryland. Decedents of motor vehicle crashes had similar seroprevalence compared with those with a natural death (including decedents with SARS-CoV-2 infection). Decedents of motor vehicle crashes may be a sentinel surveillance population.
Keywords: decedents, drug overdose, motor vehicle crash, SARS-CoV-2 antibodies, seroprevalence
Update summary is as follows:Seroprevalence of SARS-CoV-2 antibodies was 10% among decedents undergoing forensic postmortem examination in June in Maryland. Decedents of motor vehicle crashes had similar seroprevalence to those with a natural death (including decedents with SARS-CoV-2 infection) and may be a sentinel surveillance population.
The first cases of coronavirus disease 2019 (COVID-19) in Maryland were confirmed on March 5, 2020. A genomic analysis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients receiving care on March 11–31, 2020 in Maryland, the District of Columbia, and Virginia determined multiple introductions of the virus to the region [1]. The 7-day average number of confirmed cases in Maryland peaked at 1125 on May 25, 2020 and declined to 364 on June 30, 2020 [2]. The daily positivity rate for SARS-CoV-2 ribonucleic acid (RNA) was higher for Latino patients seeking care at the Johns Hopkins Health System between March 11 and May 25, 2020, peaking at 53.4% on May 10, 2020 compared with 16.1% for white and 29.6% for black patients [3]. A survey administered on June 17–28, 2020 to 1030 Marylanders found that 55 (5.2%) had ever tested positive for SARS-CoV-2 RNA [4].
The proportion of individuals who have an immune response to SARS-CoV-2 provides information on the spread of infection. The largest seroprevalence study from 10 geographic sties in the United States (not including Maryland) suggested that 10 times more SARS-CoV-2 infections occurred than the number of confirmed cases [5].
Maryland Statue 5–309 mandates decedents undergo forensic postmortem examination by the Maryland Office of the Chief Medical Examiner (OCME) to determine cause and manner of death for those who died suddenly and unexpectedly, from nonnatural causes, in police custody or with suspicious activity, with no physician attendant, and those who are homeless at death [6]. Individuals who survive long enough after a motor vehicle crash to have a description of injuries documented by medical professional may be waived from postmortem examination if no charge is pending. Decedents undergoing this examination may be underrepresented in general population seroprevalence studies. The objective of this study was to estimate the seroprevalence of SARS-CoV-2 antibodies among decedents examined by the Maryland OCME from May 24 to June 30, 2020.
METHODS
The study population included all decedents undergoing forensic postmortem examination (including toxicology for drugs and alcohol) by the OCME from May 24 to June 30, 2020 from whom 5 mL whole blood could be collected. The Johns Hopkins Bloomberg School of Public Health Internal Review Board (IRB) determined that the study is not human subjects research, and the Maryland Health Department IRB provided “exemption” status.
In April 2020, the Maryland OCME implemented a protocol that included questioning living relatives or acquaintances about symptoms in the decedent or recent contact with possible COVID-19-infected person before death. Reverse-transcription polymerase chain reaction (RT-PCR) SARS-CoV-2 testing is performed by the medical examiner if there is suspicion of infection in the decedent.
Severe acute respiratory syndrome coronavirus-2 immunoglobulin (Ig)M and IgG antibodies were detected using the CoronaChek serologic lateral flow assay (purchased from CLIAwaived and made by Hangzhou Biotest Bioctech Co., Ltd.). Studies of CoronaChek on positive and negative control specimens from Maryland showed good sensitivity (95%; 95% confidence interval [CI], 83%–99% in convalescent plasma donors >28 days after symptom onset; 100% [95% CI, 89%–100%] in COVID-19-confirmed hospitalized individuals 15 days after symptom onset) and specificity (100% [95% CI, 94%–100%] in patients infected with rhinoviruses and other coronaviruses before the COVID-19 pandemic) [7].
Age at death, sex, race, Hispanic ethnicity, homelessness, and cause and manner of death were abstracted from the OCME electronic database. Covariates were compared by SARS-CoV-2 antibody status using Student’s t tests and Fisher’s exact χ 2 tests for differences in means and proportions, respectively. The prevalence of SARS-CoV-2 antibodies was estimated for each week during the study period. The 7-day average number of new COVID-19 cases and the positivity rate in Maryland were plotted over the same period [2, 8]. Log-binomial regression models estimated adjusted prevalence ratios (aPRs) and 95% CIs; adjusted models include age (<60 and ≥60 years, sex (male and female), race (white, black, and Hispanic), homeless status, and manner and cause of death (natural, homicide, suicide, accidental illicit drug intoxication, accidental motor vehicle crash, other accident, undetermined, and manner is still pending).
RESULTS
Of the 720 decedents examined, 500 had a viable whole blood specimen for testing. There were 2 Asian decedents, 5 of other race, and 1 with unknown race, none of whom were seropositive. Among the 500 decedents, 19 were SARS-CoV-2 IgM or IgG antibody indeterminate due to hemolysis of the sample and were classified as negative. The seroprevalence of SARS-CoV-2 IgG or IgM antibodies was 10% (50 of 500; 95% CI, 9%–11%); 15 were IgG positive only, 10 were IgM positive only, and 25 were both IgG and IgM positive.
Comparing antibody positive to negative decedents, there was no difference in age (mean age 45.2 vs 45.2 years, P = .99) or homeless status (14% vs 11%, P = .64), but there was a greater proportion of antibody-positive decedents among Hispanic men (20% vs 5%, respectively, P < .01) and among those who died in a motor vehicle crash (18% vs 7%, respectively, P < .01) and a lower proportion among decedents of an accidental illicit drug intoxication (8% vs 15%, P = .28), and decedents with an undetermined manner and cause of death (10% vs 23%, P < .03). Twelve of the 500 decedents were RT-PCR positive for SARS-CoV-2 via the OCME protocol (10 of whom were antibody positive); all 12 were classified as natural deaths.
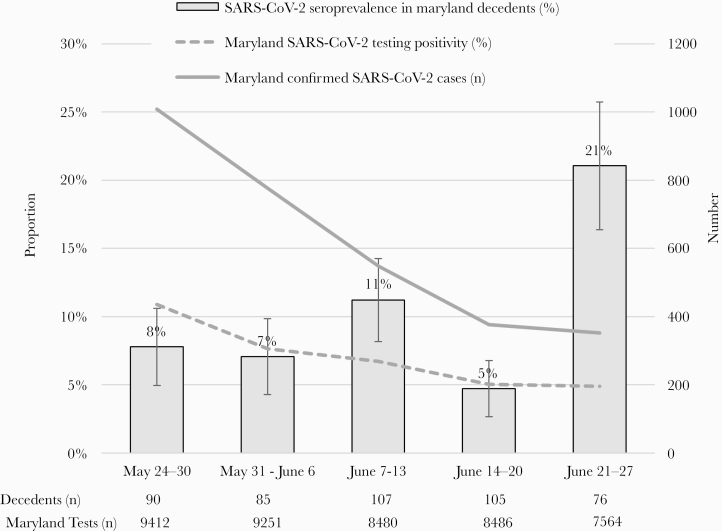
The weekly seroprevalence of SARS-CoV-2 antibodies ranged from 5% (95% CI, 3%–7%) to 21% (95% CI, 16%–26%) per week (Figure 1). During the same period, the 7-day average of the number of confirmed cases (SAR-CoV-2 RT-PCR positive) in the State of Maryland decreased from 1008 on May 30, 2020 to 364 cases on June 30, 2020; the 7-day average testing positivity rate also decreased from 10.9% to 4.7%.
Figure 1.
Weekly seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies among decedents examined by the Maryland Office of the Chief Medical Examiner, and the 7-day average number of cases and testing positivity in Maryland, May 24–June 30, 2020. The 7-day average (average of the specified day and the previous 6 days) number of confirmed cases in Maryland was estimated by The New York Times coronavirus database, available at https://www.nytimes.com/interactive/2020/us/maryland-coronavirus-cases.html. The 7-day average testing positivity in Maryland was from the Maryland Department of Health, available at https://coronavirus.maryland.gov/.
Correlates of antibodies included an approximately 4-fold increase in prevalence in Hispanic compared with non-Hispanic white decedents (aPR = 3.88; 95% CI, 1.65–9.11); there was no difference in the prevalence in black decedents (aPR = 0.93; 95% CI, 0.51–1.69). The 21% increase in prevalence in homeless individuals (vs housed individuals) was not statistically significant (aPR = 1.21; 95% CI, 0.52–2.81). Motor vehicle crash decedents had a similar prevalence of SARS-CoV-2 antibodies compared with those who died of natural causes (aPR = 1.15; 95% CI, 0.44–3.04). Accidental illicit drug intoxication decedents had a 71% decrease in the prevalence of antibodies compared with those who died of natural causes (aPR = 0.29; 95% CI, 0.10–0.86); a decrease was also found in homicide decedents (aPR = 0.37; 95% CI, 0.12–1.19), those who died from suicide (aPR = 0.60; 95% CI, 0.18–2.02), those who died from accidents unrelated to illicit drug intoxication or motor vehicle crashes (aPR = 0.40; 95% CI, 0.8–2.13), and those who have an undetermined manner of death (aPR = 0.51; 95% CI, 0.22–1.20), although these differences were not statistically significant.
DISCUSSION
Although the population undergoing forensic postmortem examination by the OCME may differ in lifestyle from those in general population seroprevalence studies, SARS-CoV-2 seroprevalence in this population reflected ethnicity patterns of COVID-19 burden reported in healthcare settings and surveys of testing in Maryland [3, 4].
Our reported seroprevalence of 10% is higher than that reported in seroprevalence studies before June 1 in the United States outside of New York City [5, 9–11]. There may be a higher seroprevalence in decedents undergoing forensic examination due to shared risk factors for nonnatural causes of death and COVID-19, such as socioeconomic disadvantage at the individual- and neighborhood-level [12, 13].
Decedents undergoing forensic investigation provide insight into populations that may be underrepresented in general population seroprevalence studies. We found a lower prevalence of SARS-CoV-2 antibodies in accidental illicit drug intoxication decedents compared with those who died of natural causes. Network studies among drug users have demonstrated that smaller and stable personal networks (compared with larger networks) are protective against other infectious diseases, such as human immunodeficiency virus [14]. Because overdose deaths may be increasing during the US COVID-19 epidemic, data describing SARS-CoV-2 infection in drug users and the epidemic’s impact on their networks are needed to understand syndemic potential [15]. Conversely, we found 23% (9 of 39) seroprevalence of SARS-CoV-2 antibodies in those who died from motor vehicle crashes; this seroprevalence was similar to those with a natural death (28% or 14 of 69), which included the 12 decedents who were found to be SARS-CoV-2 infected at the time of death via the OCME protocol. Given their high prevalence, motor vehicle crashes decedents may be a unique sentinel population for SARS-CoV-2 infection.
Limitations to our study include 96 decedents whose cause and manner of death are still pending. Of those with an undetermined manner of death, approximately half are illicit drug-related deaths; the lower prevalence of SARS-CoV-2 antibodies among accidental illicit drug intoxication decedents is reflected in the undetermined group. Finally, other antibody tests (eg, enzyme-linked immunosorbent assay [ELISA]) may have produced different results. The rapid flow assay utilized has validated high sensitivity and specificity and is relatively cheap and easy to perform and has been compared with numerous ELISAs for comparison of our results to forthcoming seroprevalence studies that utilize ELISAs [7].
CONCLUSIONS
Our study demonstrates 10% SARS-CoV-2 seroprevalence in decedents undergoing forensic postmortem examination and reflects ethnicity patterns of acute disease burden. These decedents provided timely information and allowed for inclusion of those who may be underrepresented in general population seroprevalence studies. Our approach could be replicated among decedents in other locations.
Acknowledgments
Author contributions. K. N. A., L. L., and S. B. C. had access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. K. N. A., L. L., J. M., W. C. S., J. E., F. D. T., L. A. G., and Y. C. M. contributed to the concept and design. K. N. A., L. L., R. L., O. L., E. K., O. R. B., T. C. Q., S. B. C., and Y. C. M. contributed to acquisition, analysis, and interpretation of the data. K. N. A. contributed to drafting of the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. K. N. A. and S. B. C. contributed to statistical analysis. K. N. A., J. M., W. C. S., J. E., F. D. T., L. A. G., T. C. Q., and Y. C. M. obtained funding. K. N. A., O. L., E. K., O. R. B., J. M., W. C. S., J. E., Z. A., and Y. C. M. contributed to administrative, technical, or material support. K. N. A., L. L., Z. A., O. L., T. C. Q., and Y. C. M. supervised the work.
Financial support. This study was performed in conjunction with a study of drug prevalence among fatally injured roadway users presenting to the Maryland Office of the Chief Medical Examiner, which is funded by the United States Department of Transportation, National Highway Traffic Safety Administration. Additional funding and support were provided by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) (UM1 AI068613), a Bloomberg American Health Initiative COVID-19 Impact grant, and a Johns Hopkins University Catalyst award.
Potential conflicts of interest. K. N. A. reports personal fees from the All of Us Study (NIH) and TrioHealth Inc. during the conduct of this study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Thielen P, Wohl S, Mehoke T, et al. Genomic diversity of SARS -CoV-2 during early introduction to the United States National Capital Region [preprint]. medRxiv 2020. doi: 10.1101/2020.08.13.20174136. [DOI] [Google Scholar]
- 2. Maryland coronavirus map and case count. The New York Times. Available at: https://www.nytimes.com/interactive/2020/us/maryland-coronavirus-cases.html. Accessed 9 August 2020.
- 3. Martinez DA, Hinson JS, Klein EY, et al. SARS-CoV-2 positivity rate for Latinos in the Baltimore-Washington, DC Region. JAMA 2020; 324:392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clipman SJ, Wesolowski AP, Gibson DG, et al. Rapid real-time tracking of nonpharmaceutical interventions and their association with SARS-CoV-2 positivity: the COVID-19 pandemic pulse study. Clin Infect Dis 2020; ciaa1313. doi: 10.1093/cid/ciaa1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med 2020. doi: 10.1001/jamainternmed.2020.4130. [DOI] [PubMed] [Google Scholar]
- 6. Maryland Statue 5–309. United States: Annotated Code of Maryland. Health General. Title 5. Death Available at https://health.maryland.gov/bom/pdf/TITLE_5_HEALTH_GENERAL.pdf. Accessed 15 September 2020.
- 7. Conklin S, Martin K, Manabe Y, et al. Evaluation of serological SARS-CoV-2 lageral flow assays for rapid point of care testing. J Clin Microbiol 2020. doi: 10.1101/2020.07.31.20166041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coronavirus disease 2019 (COVID-19) outbreak 2020. Available at: https://coronavirus.maryland.gov/. Accessed 9 August 2020.
- 9. Sood N, Simon P, Ebner P, et al. Seroprevalence of SARS-CoV-2 -specific antibodies among adults in Los Angeles County, California, on April 10–11, 2020. JAMA 2020; 323:2425–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stadlbauer D, Tan J, Jiang K, et al. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature 2020. doi: 10.1038/s41586-020-2912-6. [DOI] [PubMed] [Google Scholar]
- 11. Sood N, Simon P, Ebner P, et al. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles County, California, on April 10–11, 2020. JAMA 2020. doi: 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harper S, Charters TJ, Strumpf EC. Trends in socioeconomic inequalities in motor vehicle accident deaths in the United States, 1995–2010. Am J Epidemiol 2015; 182:606–14. [DOI] [PubMed] [Google Scholar]
- 13. Arasteh K. Prevalence of comorbidities and risks associated with COVID-19 among black and Hispanic populations in New York City: an Examination of the 2018 New York City Community Health Survey. J Racial Ethn Heal Disparities 2020. doi: 10.1007/s40615-020-00844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costenbader EC, Astone NM, Latkin CA. The dynamics of injection drug users’ personal networks and HIV risk behaviors. Addiction 2006; 101:1003–13. [DOI] [PubMed] [Google Scholar]
- 15. Alter A, Yeager C. COVID-19 impact on US National Overdose Crisis. Ovedose Detection Mapping Application Program Available at: http://odmap.org/Content/docs/news/2020/ODMAP-Report-May-2020.pdf. Accessed 15 September 2020.