Abstract
A 75-year-old woman was admitted to a regional hospital with an acute kidney injury (AKI) and nausea on a background of recent treatment for Staphylococcus aureus bacteraemia secondary to pneumonia. The treatment thereof resulted in a high anion gap metabolic acidosis (HAGMA). The pneumonia was initially treated with intravenous piperacillin and tazobactam and the patient transferred to a tertiary hospital. There, the diagnosis of S. aureus bacteraemia secondary to a pulmonary source was confirmed and treatment was changed to intravenous flucloxacillin and the patient was discharged to hospital in the home (HITH is a service that allows short-term healthcare at home to be provided to people who would otherwise need to be in hospital) to complete the antibiotic course. Five weeks after commencing flucloxacillin, the patient was referred back to hospital with nausea and worsening kidney function with an associated significant HAGMA. The patient has a background of chronic kidney disease and chronic back pain for which she was taking long-term paracetamol. The HAGMA was determined to be due to a pyroglutamic acidosis (PGA), deemed secondary to the combined use of paracetamol and flucloxacillin. This was subsequently confirmed with a plasma pyroglutamic acid concentration level of 7467 µmol/L (reference range 20–50 µmol/L) and a urinary level of 1700 mmol/mol creatinine (<110 mmol/mol creatinine). To our knowledge, this is the highest plasma and urinary levels published to date. Furthermore, considering the common use of paracetamol and penicillins, it is important to recognise HAGMA as a potential complication of co-administration of paracetamol and iso-oxylopenicillin. The HAGMA resolved after cessation of flucloxacillin despite the continuation of paracetamol and without administration of N-acetylcysteine. PGA-related HAGMA appears to be a unique potential side effect of iso-oxylopenicillin rather than other beta-lactams.
Keywords: contraindications and precautions, drug interactions, renal system, metabolic disorders
Background
A high anion gap metabolic acidosis (HAGMA) is a common acid–base derangement resulting from a variety of metabolic changes. The majority of causes are summarised by the acronym GOLD MARK (Glycols, oxoproline, Lactate, D-lactate, Methanol, Aspirin, Renal failure and Ketoacidosis).1
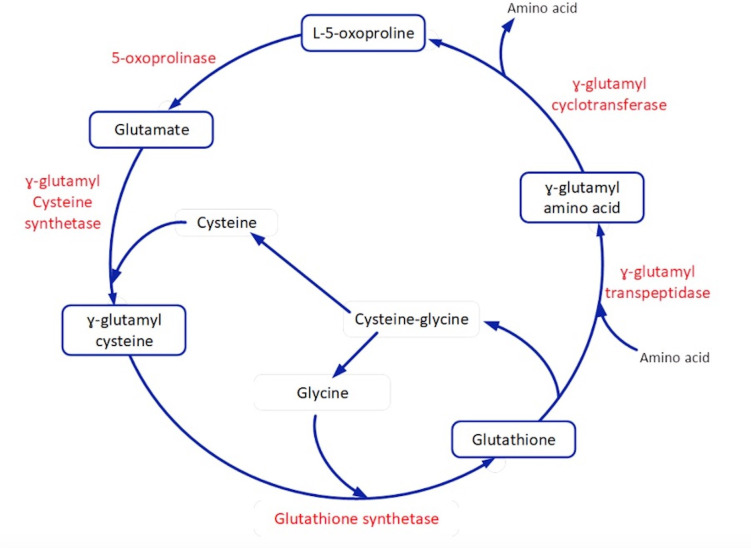
A rarely identified cause of HAGMA is the accumulation of pyroglutamic acid (5-oxoproline), possibly due to being under-recognised and under-reported rather than reflecting a true rare prevalence.2–6 Pyroglutamic acidosis (PGA) can either be congenital or acquired.7 Congenital aetiology involves inborn errors of metabolism that specifically affect enzymes in the γ-glutamyl cycle, such as glutathione synthetase deficiency (refer to figure 1). In addition, PGA can be acquired in the setting of reduced glutathione or reduced cysteine states and as an adverse drug reaction (eg, flucloxacillin, paracetamol). The association of flucloxacillin and paracetamol with HAGMA was first noted in 1989 in a woman with haemolytic anaemia and neurological symptoms.8
Figure 1.
γ-Glutamyl cycle.
The causes of and contributors to HAGMA can be difficult to accurately identify in patients who have multiple comorbidities and especially in the context of polypharmacy. PGA is usually a diagnosis of exclusion supported by the appropriate clinical scenario combined with plasma and/or urine pyroglutamic acid levels. Patients with comorbidities may be prescribed paracetamol and penicillins (isoxazolyl) often concurrently, and it is important to recognise that acquired PGA is likely to be under-reported and have significant sequelae in patients’ clinical course.9
Left unresolved, unmanaged metabolic acidosis can contribute significantly to mortality.2 The consequences of chronic HAGMA in patients with chronic kidney disease may include osteopenia, increased muscle catabolism, secondary hyperparathyroidism, reduced respiratory reserve and increased severity of subsequent infections.10 Therefore, it is important to manage and treat the underlying cause of HAGMA. There is limited literature on the accumulation of pyroglutamic acid resulting in HAGMA, and we report this case to alert clinicians of the need to consider PGA in the differential diagnosis of HAGMA.
Case presentation
A 75-year-old female presented to the emergency department with dyspnoea. She had been treated for recurrent lower respiratory tract infections by the general practitioner (GP) with oral antibiotics (12 cases in the past 14 months). Her relevant medical history included chronic kidney disease stage 3 (baseline creatinine 80–110 µmol/L), polymyalgia rheumatica requiring long-term steroids, asthma and chronic back pain. The pneumonia diagnosis was confirmed by a chest radiograph and CT showing multifocal nodular consolidation in the right lower lobe with cavitations and blood cultures were taken that grew Staphylococcus aureus.
Her medical history otherwise included depression, hypercholesterolaemia, gastro-oesophageal reflux disease, spondylosis, glaucoma, hypertension, lacunar stroke, transient ischaemic attack, vitamin B12 deficiency and vascular dementia.
Her long-term treatment included hydromorphone, paracetamol, topiramate, paracetamol–codeine–doxylamine, doxylamine, colecalciferol, aspirin, duloxetine, pantoprazole, prednisolone, docusate, macrogol, hydroxocobalamin, salbutamol and denosumab.
On presentation, she was initially afebrile, heart rate of 95 bpm, respiratory rate of 25 breaths per minute and oxygen saturation of 96%. On examination, there were crackles on the right base, dual heart sounds with no murmurs and no peripheral stigmata of infective endocarditis. She was subsequently treated for pneumonia and urinary tract infection with intravenous piperacillin–tazobactam. The working diagnosis was S. aureus bacteraemia secondary to a pulmonary source.
The patient was transferred to the closest tertiary hospital as the eventual need for further investigations, including a trans-oesophageal echo, was anticipated in the context of S. aureus bacteraemia. The treatment of her bacteraemia was eventually changed to intravenous flucloxacillin (2 g four times daily) once sensitivities were confirmed. Flucloxacillin was planned to continue for a further 5 weeks via peripherally inserted central catheter (PICC) on an 8 g/24 hours infuser that was monitored via hospital in the home (HITH).
Four weeks into the HITH treatment, the patient developed nausea and was investigated with blood results that showed worsening renal function associated with HAGMA. She was subsequently referred back to the regional hospital. Her heart rate was 105 bpm, respiratory rate of 20 breaths per minute and O2 sat 98% on room air. At that point, HAGMA was attributed to an acute kidney injury (AKI). Following adequate fluid resuscitation, HAGMA and AKI persisted. She was investigated for the AKI with a renal ultrasound which was negative and there were no eosinophils found in the urine. Interstitial nephritis secondary to flucloxacillin use was considered as another reason for her AKI but was deemed unlikely. The treatment plan was to commence sodium bicarbonate 840 mg once daily following consultation with the renal team. Ultimately, further investigations for the AKI were completed at the tertiary hospital. The HAGMA was suspected to be caused by the concurrent use of flucloxacillin and paracetamol. As a result of this, flucloxacillin was changed to cefazolin and the patient transferred again to a tertiary facility pending further investigations as well as suspected pulmonary embolism following reports of chest pain.
Investigations
Baseline before flucloxacillin
The initial working diagnosis was community-acquired pneumonia (pH of 7.38; pCO2 40 mm Hg; pO2 26 mm Hg; bicarbonate 23 mmol/L; anion gap 14 mmol/L). The patient reported having been treated for recurrent chest infections by her GP with oral antibiotics without improvement. On admission to hospital, her initial heart rate was 95 bpm, respiratory rate was 25 breaths per minute (tachypnoea) and O2 sat was 96% on room air. A chest X-ray was performed which showed consolidation in the right lower lobe that was consistent with the clinical presentation of pneumonia. Initially, the patient was treated with broad-spectrum piperacillin–tazobactam following the collection of relevant cultures. Sputum samples before transfer to tertiary facility grew S. aureus sensitive to flucloxacillin, cefazolin, clindamycin and co-trimoxazole. A urine sample showed Klebsiella pneumoniae sensitive to amoxicillin clavulanate, cefazolin, trimethoprim and gentamicin and resistant to ampicillin and nitrofurantoin. Blood cultures grew S. aureus that was sensitive to flucloxacillin and cefazolin while resistant to Penicillin G. It is worthy mentioning that S. aureus was grown on several sputum cultures in the 8 months preceding admission coinciding with the aforementioned recurrent chest infections. As the blood cultures grew S. aureus sensitive to flucloxacillin, intravenous flucloxacillin (2 g four times daily) was then commenced.
The patient was transferred to the closest tertiary hospital where she was reviewed by the infectious disease and respiratory teams. The final working diagnosis was methicillin-sensitive S. aureus (MSSA) bacteraemia secondary to a cavitating lower lobe pneumonia. CT chest and abdomen demonstrated right lower lobe pneumonia with cavitations. During this admission, she underwent transthoracic and trans-oesophageal echocardiograms which did not reveal any evidence of infective endocarditis. An MRI of the spine excluded discitis and osteomyelitis. Ultimately, the long-term steroid use for polymyalgia rheumatica was identified as a major contributing factor to immune suppression and a plan to wean steroids was formulated. Finally, a PICC was inserted and the plan was for ongoing intravenous flucloxacillin 2 g every 4 hours with ongoing infectious disease and respiratory reviews in addition to follow-up imaging in 4 weeks’ time. Flucloxacillin was planned to continue for a further 5 weeks via PICC on an 8 g/24 hours infuser that was monitored via HITH.
HAGMA cause identified
The patient was referred back to the regional hospital due to deteriorating renal function found on blood tests performed for the investigation of nausea while under HITH (table 1). A repeat chest CT scan showed a small focus of right lower lobe consolidation with small pulmonary cavitating nodules which is indicative of a potential secondary atypical infection.
Table 1.
Electrolytes over time
| At baseline before flucloxacillin | PGA confirmed | A day after flucloxacillin ceased | One week after flucloxacillin ceased | Unit | Reference | |
| Sodium | 140 | 137 | 148 | 140 | mmol/L | 135–145 |
| Potassium | 3.9 | 2.8 | 4.2 | 3.8 | mmol/L | 3.5–5.2 |
| Chloride | 104 | 111 | 121 | 110 | mmol/L | 95–110 |
| Bicarbonate | 22 | 9 | 11 | 21 | mmol/L | 22–32 |
| Anion gap | 14 | 17 | 16 | 9 | mmol/L | 4–13 |
| Urea | 12.6 | 6.2 | 6.2 | 5.4 | mmol/L | 2.9–8.2 |
| Creatinine | 106 | 206 | 204 | 147 | µmol/L | 36–73 |
| Urea/creatinine | 119 | 30 | 30 | 37 | 40–100 | |
| eGFR | 44 | 20 | 20 | 30 | mL/min/1.73 m2 | >60 |
eGFR, estimated glomerular filtration rate; PGA, pyroglutamic acidosis.
The venous blood gas showed acidaemia (pH 7.26; pCO2 20 mm Hg; pO2 37 mm Hg; bicarbonate 9 mmol/L) with underlying significant high anion gap metabolic acidosis. At this point, PGA was suspected to be caused by flucloxacillin and paracetamol. The patient was found to have a blood pyroglutamic acid concentration of 7467 µmol/L and urine concentration of 1700 mmol/mol creatinine (<110 mmol/mol creatinine) a day after flucloxacillin was discontinued.
Prior to transfer to the tertiary facility, a chest X-ray was performed to investigate the reported chest pain, but there were no significant findings. A ventilation and perfusion scan was performed at the tertiary hospital and showed no evidence of a pulmonary embolism.
HAGMA resolved after cessation of flucloxacillin
A pyroglutamic acid level was able to be taken at the tertiary facility and the level came back at 7467 µmol/L (normal range is 20–50 µmol/L) and urinary level of 1700 mmol/mol creatinine (<110 mmol/mol creatinine). Cefazolin was adjusted for renal impairment and paracetamol was continued while admitted at the tertiary facility although ideally this should have been ceased.
The patient was then back-transferred to the regional hospital after her HAGMA had started to improve. It was decided to change her antibiotics to oral amoxicillin–clavulanic acid as she was nearing the completion of her intended course for MSSA bacteraemia. Paracetamol was continued for pain management although ideally should have been ceased to help resolve the HAGMA. Her condition continued to improve and HAGMA completely resolved before she was discharged home.
A third CT chest at the regional hospital showed that there was moderate improvement of the multifocal consolidation bilaterally. However, there was a new area of ground-glass calcification within the anterior segment of the right upper lobe which suggested a new focal infection.
A follow-up chest X-ray showed no significant consolidation.
Treatment
The HAGMA was initially of an unknown cause, so the patient was commenced on sodium bicarbonate 840 mg daily. The venous blood gas showed acidaemia (pH 7.26; pCO2 20 mm Hg; pO2 37 mm Hg; bicarbonate 9 mmol/L) indicating significant high anion gap metabolic acidosis (table 1). Usual causes for AKI and HAGMA were considered and excluded. Eventually, PGA was suspected to be caused by flucloxacillin and paracetamol with the risk factors being chronic kidney disease and advanced age.
The flucloxacillin was ceased and the patient was instead treated with amoxicillin and clavulanic acid. This was sufficient to resolve the HAGMA without the cessation of paracetamol or the institution of a NAC infusion (table 2). In retrospect, at the time, paracetamol could also have been ceased to help resolve the HAGMA.
Table 2.
Venous blood gas analysis over time
| At baseline before flucloxacillin | PGA confirmed | After a day flucloxacillin ceased | Unit | Reference | |
| pH | 7.38 | 7.26 | 7.44 | – | 7.35–7.45 |
| pCO2 | 40 | 20 | 16 | mm Hg | 32–48 |
| pO2 | 26 | 37 | 18 | mm Hg | 30–40 |
| Bicarbonate | 23 | 9 | 11 | mmol/L | 22–32 |
PGA, pyroglutamic acidosis.
Outcome and follow-up
The patient was discharged on amoxicillin and clavulanic acid and advised to complete an appropriate course. She was advised to undergo a repeat CT scan and was referred to the respiratory outpatient clinic. She was also advised to present to her GP for further investigations of macrocytic anaemia. For the ensuing months, as an outpatient her bicarbonate levels and kidney function remained at baseline.
Discussion
Pyroglutamic acidosis is a rarely recognised cause of HAGMA. The incidence of PGA is not known although it is likely to be underdiagnosed considering the high prevalence of risk factors. Those include advanced age, sepsis, malnutrition, uncontrolled diabetes, female gender, chronic liver disease, chronic kidney disease, iso-oxylopenicillin use and paracetamol use.11–17
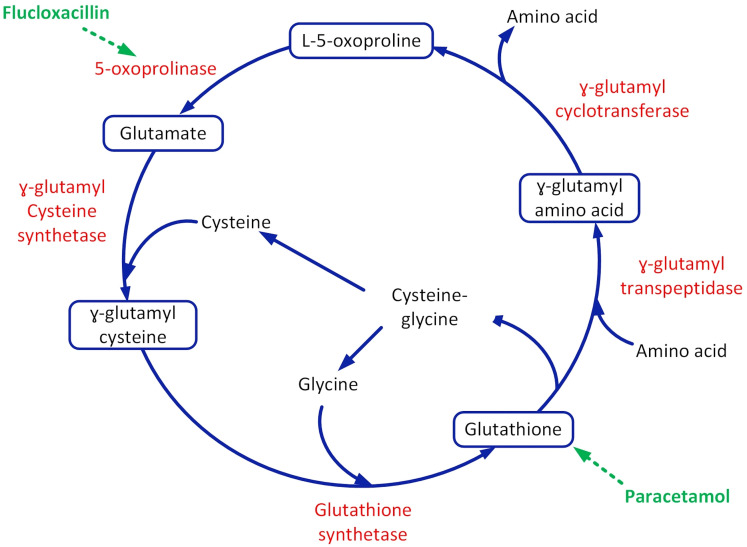
PGA results from decreased glutathione, which causes increased production of pyroglutamic acid, and inhibition of 5-oxoprolinase, which decreases breakdown of pyroglutamic acid. When glutathione levels are depleted, the negative feedback on γ-glutamyl cysteine synthetase is diminished and production of pyroglutamic acid is favoured.16 Paracetamol contributes to cysteine deficiency through direct conjugation and glutathione deficiency via its metabolite N-acetyl benzoquinonemine that binds irreversibly to glutathione.18 Synthetic penicillins (iso-oxylopenicillin) such as flucloxacillin and dicloxacillin inhibit 5-oxoprolinase which prevents the degradation of pyroglutamic acid to glutamate, thereby contributing to pyroglutamic acidosis (refer to figure 2).19 20
Figure 2.
γ-Glutamyl cycle and the effect of long-term paracetamol and flucloxacillin in promoting pyroglutamic acidosis.
In this case, the patient’s blood pyroglutamic acid level was 7467 µmol/L, which is significantly higher than previously detailed in other case reports. The urine pyroglutamic acid was 1700 mmol/mol creat (<110 mmol/mol creat). Nevertheless, it is presently unknown as to how the degree of elevation of PGA correlates to the symptoms or the degree of acidaemia. As such in this case, it was associated with moderate acidaemia.
As with other organic acids, pyroglutamic acid is excreted in the urine, leading to pyroglutamic aciduria.21 The patient had an AKI on the background of chronic kidney disease which decreases the elimination of pyroglutamic acid, further exacerbating the accumulation.
The diagnosis of PGA is often made on the basis of a medication history, arterial and venous acid–base analysis and exclusion of more common causes of HAGMA. Definitive diagnosis is based on plasma or urine pyroglutamic acid levels. However, these specific tests are not performed widely which restricts its implementation into clinical practice.22
The treatment and management of PGA-related HAGMA involves ceasing offending medications and commencing best supportive care. There have been cases where flucloxacillin has been substituted for alternative beta-lactam penicillins, which would support that PGA-related HAGMA occurs secondary to iso-oxylopenicillins and that other beta-lactams are safe to use in those instances.23–26 While flucloxacillin was ceased, in retrospect, paracetamol should have also been ceased. Some reports discuss the use of bicarbonate supplements to stabilise the pH, and NAC which has had some reported efficacy. However, the evidence to support the use of NAC in HAGMA is limited and the potential risks associated with NAC administration remain unclear.27 28 The benefits of NAC are not well established with risks of NAC administrations.29 30 Ultimately, ceasing or substituting the offending medications as well as best supportive cares seem to be sufficient in the treatment of PGA-related HAGMA.
Learning points.
Paracetamol and iso-oxylopenicillins are commonly prescribed medications; therefore, it is important to be aware of adverse effects of co-administration.
Pyroglutamic acidosis (PGA) is a diagnosis of exclusion.
In the appropriate clinical context, definitive diagnosis is attained with a blood or urine level of pyroglutamic acid.
Cessation of flucloxacillin was sufficient in resolving the high anion gap metabolic acidosis (HAGMA) without an N-acetylcysteine infusion in this case.
In line with published literature, we recommend the cessation of both iso-oxylopenicillin and paracetamol. However, in this case, paracetamol was continued but still the HAGMA resolved.
Awareness of PGA is imperative to diagnosing and treating.
Footnotes
Contributors: AZI: conception and planning/design. GB: organisation, conduct, reporting and acquisition of data. BC: analysis and interpretation of data. HG: involved in the writing and proof-reading of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mehta AN, Emmett JB, Emmett M. Gold mark: an anion gap mnemonic for the 21st century. The Lancet 2008;372:892 10.1016/S0140-6736(08)61398-7 [DOI] [PubMed] [Google Scholar]
- 2.Zand Irani A, Almuwais A, Gibbons H. Acquired pyroglutamic acidosis due to long-term dicloxacillin and paracetamol use. BMJ Case Rep 2020;13:e233306. 10.1136/bcr-2019-233306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolleman EJ, Hoorn EJ, Didden P, et al. Guilty as charged: unmeasured urinary anions in a case of pyroglutamic acidosis. Neth J Med 2008;66:351–3. [PubMed] [Google Scholar]
- 4.Tummers S, Oei S, Nooteboom F, et al. Severe metabolic acidosis induced by 5-oxoproline accumulation after paracetamol and flucloxacillin administration. Neth J Crit Care 2019;28. [Google Scholar]
- 5.Osborne W, Chavda A, Katritsis G, et al. Lesson of the month 1: a rare adverse reaction between flucloxacillin and paracetamol. Clin Med 2019;19:127–8. 10.7861/clinmedicine.19-2-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sunethra T High anion gap metabolic acidosis due to pyroglutamic aciduria (5-oxoprolinuria) in an elderly patient. Aust J Med Sci 2015;36:72–4. [Google Scholar]
- 7.Mayatepek E 5-Oxoprolinuria in patients with and without defects in the gamma-glutamyl cycle. Eur J Pediatr 1999;158:221–5. 10.1007/s004310051054 [DOI] [PubMed] [Google Scholar]
- 8.Creer MH, Lau BW, Jones JD, et al. Pyroglutamic acidemia in an adult patient. Clin Chem 1989;35:684–6. 10.1093/clinchem/35.4.684 [DOI] [PubMed] [Google Scholar]
- 9.Berbee JK, Lammers LA, Krediet CTP, et al. Metabolic acidosis caused by concomitant use of paracetamol (acetaminophen) and flucloxacillin? A case report and a retrospective study. Eur J Clin Pharmacol 2017;73:1459–65. 10.1007/s00228-017-2311-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraut JA, Madias NE. Consequences and therapy of the metabolic acidosis of chronic kidney disease. Pediatr Nephrol 2011;26:19–28. 10.1007/s00467-010-1564-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonham JR, Rattenbury JM, Meeks A, et al. Pyroglutamicaciduria from vigabatrin. Lancet 1989;1:1452–3. 10.1016/S0140-6736(89)90158-X [DOI] [PubMed] [Google Scholar]
- 12.Pitt JJ, Hauser S. Transient 5-oxoprolinuria and high anion gap metabolic acidosis: clinical and biochemical findings in eleven subjects. Clin Chem 1998;44:1497–503. 10.1093/clinchem/44.7.1497 [DOI] [PubMed] [Google Scholar]
- 13.Sekhar RV, McKay SV, Patel SG, et al. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 2011;34:162–7. 10.2337/dc10-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitt JJ, Brown GK, Clift V, et al. Atypical pyroglutamic aciduria: possible role of paracetamol. J Inherit Metab Dis 1990;13:755–6. 10.1007/BF01799581 [DOI] [PubMed] [Google Scholar]
- 15.Emmett M Acetaminophen toxicity and 5-oxoproline (pyroglutamic acid): a tale of two cycles, one an ATP-depleting futile cycle and the other a useful cycle. Clin J Am Soc Nephrol 2014;9:191–200. 10.2215/CJN.07730713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey GA, Lyall HJ, Corke CF, et al. Pyroglutamic acidemia: a cause of high anion gap metabolic acidosis. Crit Care Med 2000;28:1803–7. 10.1097/00003246-200006000-00018 [DOI] [PubMed] [Google Scholar]
- 17.Butera L, Feinfeld DA, Bhargava M. Sex differences in the subunits of glutathione-S-transferase isoenzyme from rat and human kidney. Enzyme 1990;43:175–82. 10.1159/000468728 [DOI] [PubMed] [Google Scholar]
- 18.Liss DB, Paden MS, Schwarz ES, et al. What is the clinical significance of 5-oxoproline (pyroglutamic acid) in high anion gap metabolic acidosis following paracetamol (acetaminophen) exposure? Clin Toxicol 2013;51:817–27. 10.3109/15563650.2013.844822 [DOI] [PubMed] [Google Scholar]
- 19.Lanoy C, Bouckaert Y. Metabolic acidosis and 5-oxoprolinuria induced by flucloxacillin and acetaminophen: a case report. J Med Case Rep 2016;10:184. 10.1186/s13256-016-0964-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zand L, Muriithi A, Nelsen E, et al. Severe anion gap metabolic acidosis from acetaminophen use secondary to 5-oxoproline (pyroglutamic acid) accumulation. Am J Med Sci 2012;344:501–4. 10.1097/MAJ.0b013e318259bd45 [DOI] [PubMed] [Google Scholar]
- 21.Heireman L, Mahieu B, Helbert M, et al. High anion gap metabolic acidosis induced by cumulation of ketones, L- and D-lactate, 5-oxoproline and acute renal failure. Acta Clin Belg 2018;73:313–6. 10.1080/17843286.2017.1358504 [DOI] [PubMed] [Google Scholar]
- 22.Myall K, Sidney J, Marsh A. Mind the gap! An unusual metabolic acidosis. Lancet 2011;377:526. 10.1016/S0140-6736(10)61383-9 [DOI] [PubMed] [Google Scholar]
- 23.EH A, Lam K, Umakanthan M, et al. High anion gap metabolic acidosis: a case of pyroglutamic acidosis. Nephrology 2017;22:926–7. [DOI] [PubMed] [Google Scholar]
- 24.Mo L, Liang D, Madden A, et al. A case of delayed onset pyroglutamic acidosis in the sub-acute setting. Intern Med J 2016;46:747–9. 10.1111/imj.13104 [DOI] [PubMed] [Google Scholar]
- 25.Thomas SD High anion gap metabolic acidosis due to pyroglutamic aciduria (5-oxoprolinuria) in an elderly patient. Aust J Sci Med 2015;36:72. [Google Scholar]
- 26.Luyasu S, Wamelink MMC, Galanti L, et al. Pyroglutamic acid-induced metabolic acidosis: a case report. Acta Clin Belg 2014;69:221–3. 10.1179/2295333714Y.0000000022 [DOI] [PubMed] [Google Scholar]
- 27.Raibman Spector S, Mayan H, Loebstein R, et al. Pyroglutamic acidosis as a cause for high anion gap metabolic acidosis: a prospective study. Sci Rep 2019;9:3554. 10.1038/s41598-019-39257-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hundemer GL, Fenves AZ. Acquired 5-oxoproline acidemia successfully treated with N-acetylcysteine. Proc 2017;30:169–70. 10.1080/08998280.2017.11929570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chertoff J N-Acetylcysteine's role in sepsis and potential benefit in patients with microcirculatory derangements. J Intensive Care Med 2018;33:87–96. 10.1177/0885066617696850 [DOI] [PubMed] [Google Scholar]
- 30.Bavakunji RV, Turner JD, Jujjavarapu S, et al. An unusual case of severe high anion gap metabolic acidosis. NDT Plus 2011;4:90–2. 10.1093/ndtplus/sfr009 [DOI] [PMC free article] [PubMed] [Google Scholar]