Abstract
The coronavirus disease 2019 (COVID-19) pandemic in Japan is not as disastrous as it is in other Western countries, possibly because of certain lifestyle factors. One such factor might be the seaweed-rich diet commonly consumed in Japan. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which binds to angiotensin-converting enzyme 2 (ACE2) on the cell surface and downregulates ACE2, likely elevating the ratio of angiotensin-converting enzyme (ACE) to ACE2. The overreaction of the immune system, combined with the cytokine storm and ACE dominance, is purported to cause the condition of COVID-19 patients to deteriorate rapidly. Dietary seaweeds contain numerous components, including ACE inhibitory peptides, soluble dietary fibers (eg, fucoidan, porphyran), omega-3 fatty acids, fucoxanthin, fucosterol, vitamins D3 and B12, and phlorotannins. These components exert antioxidant, anti-inflammatory, and antiviral effects directly as well as indirectly through prebiotic effects. It is possible that ACE inhibitory components could minimize the ACE dominance caused by SARS-CoV-2 infection. Thus, dietary seaweeds might confer protection against COVID-19 through multiple mechanisms. Overconsumption of seaweeds should be avoided, however, as seaweeds contain high levels of iodine.
Keywords: COVID-19, Japanese food, marine bioactives, prebiotics, functional food, seaweed
INTRODUCTION
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused the coronavirus disease 2019 (COVID-19) pandemic, with 24 854 140 cumulative confirmed cases and 838 924 cumulative deaths reported globally as of August 30, 2020.1 A comprehensive summary from China indicates that 81% of COVID-19 cases are either asymptomatic or mildly symptomatic. Still, the disease progresses rapidly in the remaining cases, leading to acute respiratory distress syndrome (ARDS) with a cytokine storm.2 Risk factors for COVID-19 include cardiovascular disease, hypertension, and diabetes, but these cannot fully explain the varying levels of severity of the COVID-19 crisis among high-income countries.
Japan was one of the first countries affected by the COVID-19 pandemic, yet the number of deaths per 100 000 population is much lower than in other Western countries, despite the apparent relaxed and slow response by the Japanese government.3 It is unclear why the COVID-19 crisis is less severe in Japan than in other countries. The reasons are likely multifactorial and may include Japanese daily customs, such as removing shoes at home, wearing masks in public, and not shaking hands or hugging as frequently.4,5 Another contributing factor might be the traditional Japanese diet, which is rich in seaweeds. Various seaweeds, including Saccharina spp (kombu), Undaria pinnatifida (wakame), and Porphyra spp (nori), are among the major ingredients in traditional Japanese cuisine. Per capita consumption of dietary seaweed consumption in Japan is among the highest in the world.6 Edible seaweeds have been shown to have various health benefits, which derive from their antihypertensive, anti-inflammatory, and antiviral effects.7–10 This evidence forms the basis for the hypothesis that dietary seaweeds have contributed to the limited severity of the COVID-19 pandemic in Japan. This review summarizes the pathophysiology of COVID-19 and examines the potential protective effects of dietary seaweeds against COVID-19.
COVID-19
SARS-CoV-2 and SARS-CoV
As the nomenclature implies,11 the virological characteristics of SARS-CoV-2 are similar to those of severe acute respiratory syndrome coronavirus (SARS-CoV), which caused the SARS outbreak in China and other countries during 2002–2005, with more than 8000 cases, including almost 800 fatal cases.12 The SARS-CoV-2 genome is 79.6% identical to the SARS-CoV genome.13 Both viruses bind to the cell-surface protein angiotensin-converting enzyme 2 (ACE2) via the coronavirus spike (S) protein and use ACE2 as an entry receptor. The ACE2 protein is widely expressed in various tissues and organs, including the respiratory system (eg, nasopharyngeal epithelial cells and alveolar type 2 pneumocytes) and the gastrointestinal tract, where expression of ACE2 is highest.14–16 In addition to SARS-CoV-2 infection of the respiratory system, intestinal infection with SARS-CoV-2 following potential fecal-oral transmission has been suggested.17,18 The SARS-CoV-2 S protein has a greater affinity for ACE2 than does the SARS-CoV S protein,19 which presumably accounts for the higher transmissibility of SARS-CoV-2.20–22 After entering the cell, both SARS-CoV-2 and SARS-CoV generate 3C-like protease (3CLpro), one of the two proteases encoded by these viruses (96% sequence identity between SARS-CoV-2 and SARS-CoV) and responsible for processing the nonstructural viral proteins.23–25
The clinical features of SARS-CoV and SARS-CoV-2 infections are also similar. As the names imply, both cause have severe acute respiratory syndrome with similar clinical symptoms (respiratory [eg, cough, shortness of breath], inflammatory [eg, fever, myalgia], and gastrointestinal [eg, diarrhea]) and radiological findings. There is also similarity in the laboratory findings of SARS and COVID-19, with lymphocytopenia, increased proinflammatory cytokines (eg, interleukin 6 [IL-6]), and hypercoagulability (eg, D-dimer formation) accompanied by thrombotic events observed in both diseases.26–33 The lung histology of a COVID-19 autopsy case shows diffuse alveolar damage with multinucleated giant (syncytial) cells and viral inclusion bodies, resembling histological findings of SARS.34,35
Several differences between SARS and COVID-19 have been noted. For instance, SARS-CoV-2 infection elicits fewer innate immune responses (induction of interferons and proinflammatory cytokines), which results in higher viral replication in the lung than SARS-CoV infection. This could explain the milder symptoms and lower mortality but higher infectivity of COVID-19 than SARS.36 Still, the overall similarity in the clinical features, laboratory findings, and pathological characteristics of these two diseases indicates similar pathophysiological processes, and previous research findings for SARS-CoV may be applicable to SARS-CoV-2, at least in part.
Immune overreaction
The alarming feature of COVID-19 is the rapid increase in the severity of the disease, which leads to ARDS. The overreaction of the immune response, called the cytokine storm, plays an essential role in this process. In the cytokine storm, overproduction of proinflammatory cytokines causes widespread vascular hyperpermeability and hypercoagulability, leading to multiorgan damage and ARDS, the leading cause of death in SARS and COVID-19 cases.28,30,37 The levels of these proinflammatory cytokines are correlated with the severity of disease. The critical role of the cytokine storm is consistent with the favorable outcome of steroid administration in ARDS patients with SAR-CoV-2 infection,38–40 even though the use of steroids in COVID-19 patients is generally not recommended by the World Health Organization because of concerns over the possible delay in viral clearance and other adverse events.41
The initial event of this immune overreaction seems to be the production of massive amounts of reactive oxygen species in the lung in response to the viral infection. The newly formed reactive oxygen species oxidize phospholipids within pulmonary surfactant to generate oxidized phospholipids. This, in turn, causes the lung macrophages to produce a large amount of the proinflammatory cytokine IL-6 via Toll-like receptor 4, shown to lead to acute lung injury in a mouse model.42 Importantly, the prominent formation of oxidized phospholipids was observed in every SARS-related case of ARDS that the research group evaluated.
Consistent with the findings of this animal study, the levels of IL-6 and other proinflammatory cytokines are elevated in severe cases of SARS and COVID-19.30,43,44 The presumed central role of IL-6 in severe COVID-19 cases provides a scientific rationale for a clinical trial that investigated the use of the interleukin 6 receptor (IL-6R) antagonist tocilizumab in such cases.37,45
Renin-angiotensin system
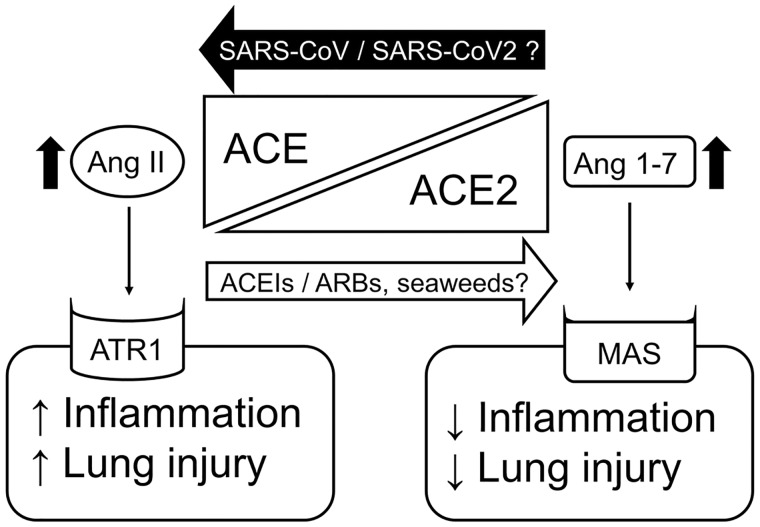
Another major system that regulates acute lung injury is the renin-angiotensin system (RAS). The RAS is generally known as the master regulatory system of the blood pressure in the body, but it also regulates inflammation and fibrosis of organs locally, via paracrine signaling. It is dually regulated by two enzymes, angiotensin-converting enzyme (ACE) and ACE2. ACE is a carboxypeptidase that positively regulates the RAS by producing angiotensin II (Ang II), which causes vasoconstriction and exerts proinflammatory effects via Ang II receptor type 1 (ATR1).46,47 On the other hand, ACE2 is another carboxypeptidase that negatively regulates the RAS by converting Ang II to angiotensin 1-7 (Ang 1-7), which causes vasodilation and exerts anti-inflammatory effects via a G-protein–coupled receptor MAS.48,49 Thus, RAS activity is determined by the balance between the ACE/Ang II/ATR1 and the ACE2/Ang 1-7/MAS axes (Figure 1).
Figure 1.
The roles of ACEIs/ARBs and SARS-CoV/SARS-CoV-2 in the balance between the ACE/Ang II/ATR1 and ACE2/Ang 1-7/MAS axes in regulation of the renin-angiontensin system. Dietary seaweeds are thought to shift the balance toward the ACE2/Ang 1-7/MAS axis through their possible ACE inhibitory effects. Abbreviations: ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; ACEIs, ACE inhibitors; Ang II, angiotensin II; Ang 1-7, angiontensin 1-7; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ARBs, angiotensin II receptor type 1 blockers; ATR1, angiotensin II receptor type 1.
As ACE2 serves as an entry receptor for SARS-CoV-2 and SARS-CoV, it is reasonable to speculate that the levels of ACE2 expression in the lung epithelial cells determine the susceptibility of individuals to these viruses. Since ACE inhibitors (ACEIs) and ATR1 blockers (ARBs) are known to upregulate ACE2 expression, there is a concern that both ACEIs and ARBs might aggravate SARS-CoV-2 infection.50 However, the sudden cessation of ACEIs or ARBs could cause a rebound increase of blood pressure, elevating the cardiovascular risk of patients with COVID-19. Moreover, ACE2 can be organ protective by counteracting the ACE/Ang II/ATR1 axis (Figure 1). Ace2 is protective against severe acute lung injury, whereas Ace promotes severe acute lung injury in mice models. Furthermore, recombinant Ace2 and ARBs are also protective against severe acute lung injury in this study.51 It is currently suggested that neither ACEIs nor ARBs need to be discontinued in patients with COVID-19.52–54
Kuba et al55 also showed that ACE2 downregulation on the surface of lung epithelial cells leads to a dominance of the ACE/Ang II/ATR1 axis in lung tissue, which is crucial in the development of acute lung injury by SARS-CoV infection (Figure 1). In their study, SARS-CoV S protein also produced acute lung injury while downregulating Ace2 on the cell surface and increasing Ang II in the lung tissue, and the lung injury was rescued by ARB administration in wild-type mice.55 Because of the similarity in pathophysiological and clinical features between SARS-CoV infection and SARS-CoV-2 infection, this crucial research finding for SARS-CoV infection is likely applicable to SARS-CoV-2 infection. Thus, ACE2 should also be protective against acute lung injury by SARS-CoV-2 infection as well. Consistent with this speculation, two independent studies showed that both ACEIs and ARBs improved the overall clinical outcomes of COVID-19 patients with hypertension.56,57
SEAWEEDS IN THE TRADITIONAL JAPANESE DIET
The prevention and management of the COVID-19 pandemic requires the minimization of public exposure to SARS-CoV-2 and curtailment of disease progression to ARDS in people previously exposed to the virus. Daily diets can alter inflammatory processes in the body, as indicated by epidemiological, experimental, and clinical studies.58,59 The current review focuses on the traditional diet in Japan, where the number of deaths per 100 000 people is much lower than in other Western countries.60
The traditional Japanese diet is rich in diverse types of seaweeds. The consumption of edible seaweed in Japan reaches 1 kilogram of dry weight per person annually6 or more than 14 wet grams per person per day.61 Dietary seaweeds are classified into three types: brown seaweeds, which include Saccharina (kombu), Undaria pinnatifida (wakame), Cladosiphon okamuranus (mozuku), and Sargassum fusiforme (hijiki); red seaweeds, which include Porphyra spp (nori); and green seaweeds, which include Ulva spp (sea lettuce or aosa).62,63 In Japan, Undaria pinnatifida (wakame) and Porphyra spp (nori) are the two top seaweeds consumed, accounting for 75% of total seaweed consumption.64 South Koreans also consume dietary seaweeds at a level comparable to that observed in the Japanese.65 The number of COVID-19 deaths per 100 000 people in South Korea is now even lower than that in Japan.60
These edible seaweeds contain numerous and diverse bioactive components with various health benefits, including antihypertensive, antioxidant, and anti-inflammatory effects. These effects appear to counteract the immune overreaction and the dominance of the ACE/Ang II/ATR1 axis in patients with COVID-19, as outlined in the next section. However, these components must be sufficiently bioavailable in order to produce beneficial effects. Bioavailability is dictated by many factors, including digestion and absorption in the gastrointestinal system, hepatic and intestinal metabolism, and composition of the gut microbial flora.8,9
NUTRITIONAL COMPONENTS IN DIETARY SEAWEEDS
Protein and peptides
Edible seaweeds are rich in protein. Multiple ACE inhibitory peptides have been isolated from edible seaweeds such as Undaria pinnatifida (wakame), Sargassum fusiforme (hijiki), and Porphyra spp (nori).66–70 Seaweed proteins are digested in the gastrointestinal tract,7,8 and the peptides generated through digestion likely contribute to the blood-pressure-lowering effects of seaweeds through ACE inhibition, at least in part.71 Thus, the peptides in edible seaweeds might function as dietary ACE inhibitors, possibly exerting a protective effect against COVID-19 by reducing the degree of ACE/Ang II/ATR1 axis dominance.
Fucoidan
Dietary fibers are plant-derived carbohydrates that are nondigestible in the gastrointestinal tract. They are classified as either soluble or insoluble fibers.72 One of the soluble fibers found in brown seaweeds is fucoidan, a sulfated polysaccharide found in the extracellular matrix.73 Fucoidan is absorbed through the gut epithelium into the systemic circulation, although its oral bioavailability is low.74.75
Fucoidan exerts anti-inflammatory effects by reducing the production of proinflammatory cytokines.76,77 Orally administered fucoidan reduces the levels of proinflammatory cytokines, including interleukin 1β (IL-1β) and IL-6, in patients with advanced cancer.78 Similarly, orally administered fucoidan has been shown to reduce radiation-induced pneumonitis and lung fibrosis in a mouse model.79 Orally administered fucoidan also exerts antithrombotic effects by inducing biosynthesis of prostacyclin.80 The crude extract of the brown seaweed Cladosiphon okamuranus (mozuku) consistently exerted antithrombotic effects in rats after 8 weeks of oral administration, likely through the fucoidan in the extract.81 Another study shows that low-molecular-weight fucoidan prepared in the laboratory has greater antithrombotic activity and oral bioavailability than middle-molecular-weight fucoidan.82
Fucoidan has also been shown to have antiviral activity against influenza A virus, hepatitis B virus, canine distemper virus, and human immunodeficiency virus (HIV), mainly in vitro.73 One study in human volunteers showed that serum/plasma concentrations of fucoidan following oral ingestion of a Cladosiphon okamuranus (mozuku) extract were sufficient to exert anti-HIV activity.83,84 Furthermore, a recent study by Kwon et al85 showed fucoidan extracted from Saccharina japonica (kombu) to have strong antiviral activity against SARS-CoV-2 in vitro. The activity of fucoidan against SARS-CoV-2 was even greater than that of remdesivir. Thus, fucoidan in dietary brown seaweeds might exert antiviral effects against SARS-CoV-2, at least within the intestine.
Porphyran
Porphyran, another soluble dietary fiber in seaweeds, is a sulfated polysaccharide found in the extracellular matrix of red seaweeds, including Porphyra spp (nori). Porphyran accounts for more than 40% of the dry weight of red seaweeds.86 Porphyran was shown to exert antioxidant and anti-inflammatory effects in vitro87–89 and in vivo after oral administration in a contact hypersensitivity mouse model.90 Orally ingested porphyran is hydrolyzed by porphyranases in Bacteroides plebeius, a bacterium found in the gut microbiota of Japanese and Korean populations.91–93 It is unclear whether the antioxidant and anti-inflammatory properties of orally ingested porphyran are dependent on the microbiota-mediated hydrolysis of porphyran in the gastrointestinal tract.
Omega-3 unsaturated fatty acids
Seaweeds are also rich sources of omega-3 unsaturated fatty acids, which include eicosapentaenoic acid (EPA; 20:5 omega-3) and docosahexaenoic acid (DHA; 22:6 omega-3) (Figure 2A). The high levels of these omega-3 fatty acids in fish derive from marine plants such as algae and plankton, which are consumed by fish and are rich in omega-3 fatty acids.62 Because these fatty acids cannot be synthesized in the human body, they are regarded as essential fatty acids for humans. Epidemiological studies support the beneficial health effects of dietary omega-3 unsaturated fatty acids, which are shown to reduce the risk of cardiovascular disease.94–98 The American Heart Association recommends regular consumption of fish as a way to obtain omega-3 fatty acids via the diet.98
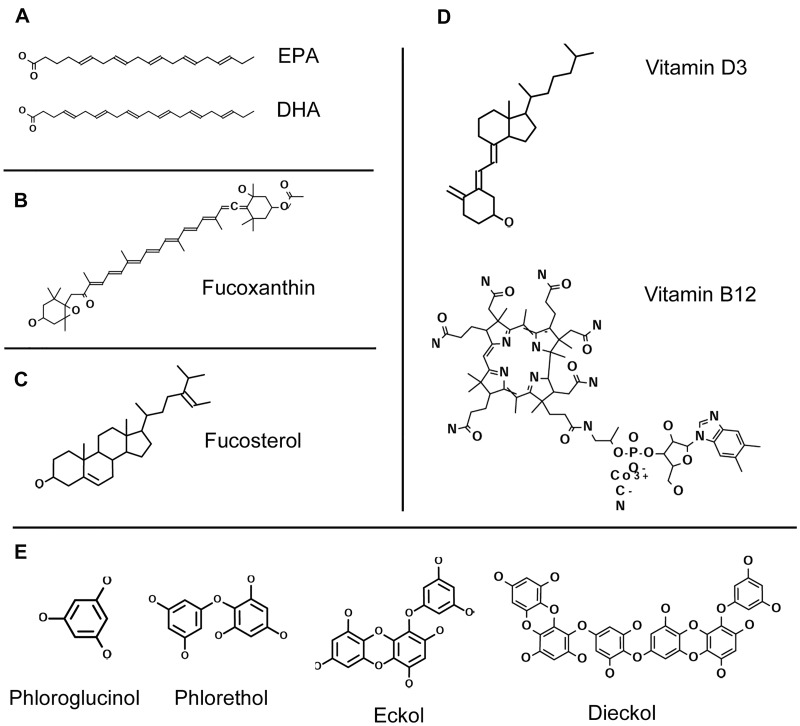
Figure 2.
Chemical structure of seaweed components. (A) omega-3 unsaturated fatty acids, which include eicosapentaenoic acid (EPA; 20:5 omega-3) and docosahexaenoic acid (DHA; 22:6 omega-3); (B) fucoxanthin; (C) fucosterol; (D) vitamin D3 (cholecalciferol) and vitamin B12 (cyanocobalamin); and (E) phlorotannins, phloroglucinol (1,3,5-trihydroxylbenzene), phlorethol, eckol, and dieckol.
The ability of omega-3 unsaturated fatty acids to reduce and resolve inflammation has been demonstrated in both in vitro studies and in animal models of inflammatory disease.99 Several studies, including a meta-analysis, also support the beneficial effects of omega-3 unsaturated fatty acids in patients with sepsis or ARDS,100–103 although one clinical trial found no benefit of omega-3 unsaturated fatty acids in patients with ARDS.104 On the basis of previous studies, the use of omega-3 unsaturated fatty acids is advocated for prophylaxis and treatment of COVID-19.105,106
Fucoxanthin (carotenoid)
Fucoxanthin is a xanthophyll-like carotenoid found abundantly in the chloroplasts of brown seaweeds (Figure 2B). Fucoxanthin gives the characteristic brown hue to brown seaweeds. Orally administered fucoxanthin is rapidly hydrolyzed to an active metabolite, fucoxanthinol, in the gut and is further converted into another active metabolite, amarouciaxanthin A, in the liver. Fucoxanthin also provides diverse beneficial health effects, including potent antioxidant activity and anti-inflammatory effects by scavenging oxidants with its allenic bond and reducing the levels of proinflammatory cytokines such as IL-1β and IL-6.107,108
Fucosterol
Fucosterol, or 24-ethylidene cholesterol, is a characteristic phytosterol present abundantly all brown seaweeds109,110 (Figure 2C). Fucosterol exerts both an anti-inflammatory effect by downregulating the generation of proinflammatory cytokines111,112 and an anti-thrombotic effect by inducing plasminogen activator in endothelial cells in vitro.113 An antiatopic effect of orally administered fucosterol in mice has also been reported.114
Vitamins D3 and B12
Both brown and red seaweeds are rich dietary sources of vitamin D37,8 (Figure 2D). Most of the epidemiological studies conducted thus far indicate anti-inflammatory effects of vitamin D. Some clinical studies also show that oral vitamin D3 supplementation decreases levels of proinflammatory cytokines.115 In vitro studies suggest that vitamin D exerts anti-inflammatory effects by modulating the nuclear factor κB and unfolded protein response pathways.116,117 Animal and human studies show the significant association between vitamin D deficiency and ARDS or sepsis.118,119 One recent study also indicates the lung-protective effect of vitamin D supplementation in a mouse model of lipopolysaccharide (LPS)-induced acute lung injury.120 Interestingly, another animal study shows that vitamin D exerts an organ-protective effect against acute lung injury by minimizing ACE2 downregulation by LPS.121 There is no report about antiviral effects of vitamin D against SARS-CoV or SARS-CoV-2, even though antiviral effects of vitamin D against multiple types of viruses have been reported.122 On the basis of previous studies demonstrating the importance of adequate vitamin D in supporting immune function, the use of vitamin D supplementation is advocated for prophylaxis and treatment of COVID-19.105,123,124
Red and green seaweeds are also rich dietary sources of vitamin B128 (Figure 2D). Interestingly, vitamin B12 is ranked fourth among the compounds with possible inhibitory effects against SARS-CoV-2 3CLpro in virtual screening.24 Another in silico study also indicates that vitamin B12 might be able to inhibit the RNA-dependent RNA polymerase activity of SARS-CoV-2.125
Phlorotannins
Phlorotannins are tannin derivatives or polyphenolics, compounds that account for 3% to 15% of the dry weight of brown seaweeds.126,127 They are phloroglucinol (1,3,5-trihydroxylbenzene) oligomers or polymers. Multiple forms of phlorotannins exist, depending on the degree of polymerization (eg, phlorethol is a phloroglucinol dimer, whereas eckol is a phloroglucinol trimer, and dieckol is a phloroglucinol heptamer)63 (Figure 2E). These compounds exist as either a free form or as a complex form with alginic acid in the physodes (small vesicles) and cell walls to protect the seaweeds from ultraviolet light, herbivores, and bacteria.128
Phlorotannins exert anti-inflammatory effects by reducing levels of proinflammatory cytokines (tumor necrosis factor, IL-1β, IL-6) and reactive oxygen species.63,129 Both direct oxidant-scavenging effects and ACE inhibitory effects are presumed to be the mechanisms underlying the anti-inflammatory action of phlorotannins.130,131
Phlorotannins also exert antiviral effects against the influenza virus, HIV, and porcine epidemic diarrhea coronavirus.132–134 Significantly, with the exception of the monomeric phloroglucinol, phlorotannins inhibit SARS-CoV 3CLpro. Among the phlorotannins, dieckol has the most potent inhibitory activity against SARS-CoV 3CLpro.135 SARS-CoV-2 3CLpro is very similar to SARS-CoV 3CLpro24.25; thus, phlorotannins are expected to inhibit SARS-CoV-2 3CLpro as well.
Phlorotannins undergo enzymatic digestion in the upper gastrointestinal tract and are then fermented by the colonic microbiota to become smaller oligomeric units before being absorbed, predominantly in the colon.136 Their bioavailability after oral administration is limited. It is unclear whether phlorotannins in brown seaweeds can exert antioxidant, anti-inflammatory, or antiviral effects after oral ingestion.
PREBIOTIC EFFECTS OF DIETARY SEAWEEDS
Daily diets affect the composition of the gut microbiota, and the composition of the gut microbiota, in turn, has profound effects on inflammation and the immune system of the host. Microbial imbalance, or dysbiosis, has been implicated in inflammatory diseases through immune dysregulation.137–139 Both probiotics (live microorganisms that produce health-promoting effects after oral ingestion, eg, Lactobacillus or Bifidobacterium) and prebiotics (food ingredients that improve gut microbiota after oral ingestion) correct dysbiosis by inducing the production of short-chain fatty acids (SCFAs), such as butyrate, through fermentation by the gut microbiota. Short-chain fatty acids are speculated to be key mediators of the anti-inflammatory effects of probiotics and prebiotics.140
Soluble dietary fibers provide substrates for the production of SCFAs through fermentation by the gut microbiota. Orally administered fucoidan functions as a prebiotic by increasing the counts of Lactobacillus and Bifidobacterium organisms in the gut. Other soluble dietary fibers in dietary seaweeds, such as agar, alginate, and laminarin, also serve as prebiotics.141–144 Furthermore, the omega-3 unsaturated fatty acids in dietary seaweeds might also serve as prebiotics to increase SCFA-producing bacteria within the gut microbiota.145,146 Together, these findings indicate that dietary seaweeds likely serve as prebiotics.
POTENTIAL RISKS OF DIETARY SEAWEEDS
While seaweed-rich diets may have potential to protect against COVID-19, they are also associated with potential risks.8 Seaweeds are rich in iodine, a trace element required for thyroid hormone synthesis. The Food and Nutrition Board of the Institute of Medicine in the United States has established the recommended iodine intake as 150 µg/d and the tolerable upper limit as 1.1 mg/d,147 but the average iodine intake among the Japanese is 3 mg/d, which is the safe upper limit of iodine intake set by the Ministry of Health, Labour and Welfare in Japan.148 Excessive iodine intake from dietary seaweed consumption can cause both hyper- and hypothyroidism.149–151 The strong tolerance of the Japanese population to higher iodine intake might be attributable to the various soy products (eg, miso, soy sauce, tofu, etc) commonly used in traditional Japanese cuisine. These products have antithyroid effects through soy isoflavones and are commonly coingested with seaweeds in Japan.152–154 In other words, dietary soy products raise the amount of iodine required to avoid hypothyroidism. Interestingly, iodine is also known to optimize the innate immune response in the body; thus, high dietary iodine intake was recently speculated to be protective against COVID-19.155
Another concern about seaweed consumption is the contamination of seaweed with heavy metals such as arsenic or mercury. The arsenic concentration is highest in the seaweeds within the marine food web, although most dietary seaweeds (Sargassum fusiforme [hijiki] being the exception) contain most of their arsenic as organic arsenic compounds such as arsenosugars. Organic arsenic compounds appear to have significantly lower toxicity than inorganic arsenic, the predominant form of arsenic in Sargassum fusiforme (hijiki).156 However, scientific data about organic arsenic compound speciation in seaweeds and in vivo toxicological evaluations of these organic arsenic compounds in humans are limited. Moreover, the effects of food processing and cooking on these organic arsenic compounds have not been evaluated.156
The levels of mercury in blood were mildly associated with the amount of various types of foods consumed, including seaweeds, in a Korean study.157 In a preprint paper, the bioaccumulation of mercury is speculated to be a possible aggravating factor of COVID-19 by inducing hypertension, hypercoagulability, and immune overreaction.158
CONCLUSION
Dietary seaweeds contain numerous components that can exert antioxidant, anti-inflammatory, and antiviral effects, directly and indirectly, by improving the gut microbiota. Specifically, orally ingested seaweeds might exert direct antiviral effects against SARS-CoV-2 within the intestine through fucoidan and other components.85 Several other components of seaweeds might be capable of reducing the degree of ACE/Ang II/ATR1 axis dominance in COVID-19 patients by inhibiting ACE. These potential health benefits of dietary seaweeds might be a contributing factor to the lower severity of the COVID-19 crisis in Japan. The bioavailability of each component after consumption of seaweed varies, and thus each component might not be able to exert these effects alone. It is plausible that these components might work additively or even synergistically, although there is no scientific data to either support or refute this idea.
At this time, it is not possible to propose a global recommendation for the daily dose and frequency of dietary seaweed consumption because the individual tolerance to iodine intake seems to vary, depending on daily dietary habits.152–154 Further toxicological investigations of organic arsenic compounds in dietary seaweeds are warranted.156 Because of concerns about excessive intake of iodine and heavy metals, overconsumption of seaweeds is certainly not recommended. The development of seaweed-derived supplements with reduced levels of iodine and heavy metals might be a logical strategy to allow the consumption of greater amounts of dietary seaweeds without adverse effects.
Acknowledgments
Funding/support. No external funds supported this work.
Declaration of interest. The author has no relevant interests to declare.
Publication History
Initial manuscript received: May 6, 2020
First revision received: July 9, 2020
Second revision received: July 26, 2020
Manuscript accepted for publication: October 5, 2020
Published: December 18, 2020
References
- 1. World Health Organization. Coronavirus disease (COVID-19). Weekly epidemiological update. Published August 31, 2020. http://www.who.int/docs/default-source/coronaviruse/situation-reports/20200831-weekly-epi-update-3.pdf?sfvrsn=d7032a2a_4
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 3. Hayasaki E. Covid-19: how Japan squandered its early jump on the pandemic. BMJ. 2020;369:m1625. [DOI] [PubMed] [Google Scholar]
- 4. Cho-Han C, Cho-Hung C, Cho-Hsien C, et al. The practice of wearing surgical masks during the COVID-19 pandemic. Emerg Infect Dis. 2020;26:1962. 10.3201/eid2608.201498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhen-Dong G, Zhong-Yi W, Shou-Feng Z, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583–1591. [10.3201/eid2607.200885] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naylor J. Production, Trade and Utilization of Seaweeds and Seaweed Products. Food and Agriculture Organization of the United Nations; 1976. [Google Scholar]
- 7. Brown ES, Allsopp PJ, Magee PJ, et al. Seaweed and human health. Nutr Rev. 2014;72:205–216. [DOI] [PubMed] [Google Scholar]
- 8. Cherry P, O’Hara C, Magee PJ, et al. Risks and benefits of consuming edible seaweeds. Nutr Rev. 2019;77:307–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacArtain P, Gill CIR, Brooks M, et al. Nutritional value of edible seaweeds. Nut Rev. 2007;65(12 pt 1):535–543. [DOI] [PubMed] [Google Scholar]
- 10. Pereira L, Critchley AT. The COVID 19 novel coronavirus pandemic 2020: seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J Appl Phycol. 2020;32:1875–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gorbalenya AE, Baker SC, Baric RS, et al. ; Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11:836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qi F, Qian S, Zhang S, et al. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pontén F, Jirström K, Uhlen M. The Human Protein Atlas—a tool for pathology. J Pathol. 2008;216:387–393. [DOI] [PubMed] [Google Scholar]
- 16.ACE2. Human Protein Atlas Summary website. Accessed July 29, 2020. https://www.proteinatlas.org/ENSG00000130234-ACE2
- 17. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luo C, Yao L, Zhang L, et al. Possible transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a public bath center in Huai'an, Jiangsu Province, China. JAMA Netw Open. 2020;3:e204583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281:4085–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kandeel M, Al-Nazawi M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020;251:117627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ul Qamar MT, Alqahtani SM, Alamri MA, Chen LL. Structural basis of SARS-CoV-2 3CL(pro) and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020;10:313–319. [10.1016/j.jpha.2020.03.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. COVID-19 Investigation Team. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med. 2020;26:861–868. [10.1038/s41591-020-0877-5] [DOI] [PubMed] [Google Scholar]
- 28. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. [DOI] [PubMed] [Google Scholar]
- 30. Wong CK, Lam CWK, Wu AKL, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao XY, Xu XX, Yin HS, et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Umapathi T, Kor AC, Venketasubramanian N, et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS). J Neurol. 2004;251:1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chu H, Chan JF-W, Wang Y, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. 2020;71:1400–1409. [10.1093/cid/ciaa410] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. [DOI] [PubMed] [Google Scholar]
- 38. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469–469. [DOI] [PubMed] [Google Scholar]
- 40. Mahase E. Covid-19: demand for dexamethasone surges as RECOVERY trial publishes preprint. BMJ. 2020;369:m2512. [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization. Clinical management of COVID-19: interim guidance. Published May 27, 2019. https://www.who.int/publications/i/item/clinical-management-of-covid-19
- 42. Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. [10.1172/jci137244] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020;71:1937–1942.[10.1093/cid/ciaa449] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang C, Wu Z, Li J-W, et al. Cytokine release syndrome of severe COVID-19 and interleukin-6 receptor antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Husain K, Hernandez W, Ansari RA, et al. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J Biol Chem. 2015;6:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pacurari M, Kafoury R, Tchounwou PB, et al. The Renin-Angiotensin-aldosterone system in vascular inflammation and remodeling. Int J Inflam. 2014;2014:689360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Souza LL, Costa-Neto CM. Angiotensin-(1-7) decreases LPS-induced inflammatory response in macrophages. J Cell Physiol. 2012;227:2117–2122. [DOI] [PubMed] [Google Scholar]
- 49. Tsai HJ, Liao MH, Shih CC, et al. Angiotensin-(1-7) attenuates organ injury and mortality in rats with polymicrobial sepsis. Crit Care. 2018;22:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? [published correction appears in Lancet Respir Med. 2020;8:e21]. Lancet Respir Med. 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. South AM, Tomlinson L, Edmonston D, et al. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020;16:305–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43:648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Meng J, Xiao G, Zhang J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Minihane AM, Vinoy S, Russell WR, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tuohy KM, Fava F, Viola R. ‘ The way to a man’s heart is through his gut microbiota'–dietary pro- and prebiotics for the management of cardiovascular risk. Proc Nutr Soc. 2014;73:172–185. [DOI] [PubMed] [Google Scholar]
- 60.Mortality analyses: mortallty in the most affected countries. Johns Hopkins University Coronavirus Resource Center website. Accessed August 30, 2020. https://coronavirus.jhu.edu/data/mortality
- 61. Fukuda S, Saito H, Nakaji S, et al. Pattern of dietary fiber intake among the Japanese general population. Eur J Clin Nutr. 2007;61:99–103. [DOI] [PubMed] [Google Scholar]
- 62. Collins KG, Fitzgerald GF, Stanton C, et al. Looking beyond the terrestrial: the potential of seaweed derived bioactives to treat non-communicable diseases. Mar Drugs. 2016;14:60. [10.3390/md14030060] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rosa GP, Tavares WR, Sousa PMC, et al. Seaweed secondary metabolites with beneficial health effects: an overview of successes in in vivo studies and clinical trials. Mar Drugs. 2019;18:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Richards-Rajadurai N. Production, marketing and trade of seaweeds. In: Technical Resource Papers Regional Workshop on the Culture and Utilization of Seaweeds Volume II. Food and Agriculture Organization of the United Nations; 1990. [Google Scholar]
- 65. Kim EK, Ju SY. Asthma and dietary intake of fish, seaweeds, and fatty acids in Korean adults. Nutrients. 2019;11:2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cian RE, Martínez-Augustin O, Drago SR. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res Int. 2012;49:364–372. [Google Scholar]
- 67. Suetsuna K. Purification and identification of angiotensin I-converting enzyme inhibitors from the red alga Porphyra yezoensis. J Mar Biotechnol. 1998;6:163–167. [PubMed] [Google Scholar]
- 68. Suetsuna K. Separation and identification of angiotensin I-converting enzyme inhibitory peptides from peptic digest of Hizikia fusiformis protein [in Japanese]. Nippon Suisan Gakkaishi. 1998;64:862–866. [Google Scholar]
- 69. Suetsuna K, Maekawa K, Chen J-R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J Nutr Biochem. 2004;15:267–272. [DOI] [PubMed] [Google Scholar]
- 70. Suetsuna K, Nakano T. Identification of an antihypertensive peptide from peptic digest of wakame (Undaria pinnatifida). J Nutr Biochem. 2000;11:450–454. [DOI] [PubMed] [Google Scholar]
- 71. Hata Y, Nakajima K, Uchida J, et al. Clinical effects of brown seaweed, Undaria pinnatifida (wakame), on blood pressure in hypertensive subjects. J Clin Biochem Nutr. 2001;30:43–53. [Google Scholar]
- 72. Anderson JW, Baird P, Davis RH Jr, et al. Health benefits of dietary fiber. Nutr Rev. 2009;67:188–205. [DOI] [PubMed] [Google Scholar]
- 73. Luthuli S, Wu S, Cheng Y, et al. Therapeutic effects of fucoidan: a review on recent studies. Mar Drugs. 2019;17:487. [10.3390/md17090487] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kadena K, Tomori M, Iha M, et al. Absorption study of mozuku fucoidan in Japanese volunteers. Mar Drugs. 2018;16:254. [10.3390/md16080254] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nagamine T, Nakazato K, Tomioka S, et al. Intestinal absorption of fucoidan extracted from the brown seaweed, Cladosiphon okamuranus. Mar Drugs. 2014;13:48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Asanka Sanjeewa KK, Jayawardena TU, Kim H-S, et al. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr Polym. 2019;224:115195. [DOI] [PubMed] [Google Scholar]
- 77. Park J, Cha J-D, Choi K-M, et al. Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo. Int Immunopharmacol. 2017;43:91–98. [DOI] [PubMed] [Google Scholar]
- 78. Takahashi H, Kawaguchi M, Kitamura K, et al. An exploratory study on the anti-inflammatory effects of fucoidan in relation to quality of life in advanced cancer patients. Integr Cancer Ther. 2018;17:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yu H-H, Chengchuan Ko E, Chang C-L, et al. Fucoidan inhibits radiation-induced pneumonitis and lung fibrosis by reducing inflammatory cytokine expression in lung tissues. Mar Drugs. 2018;16:392, [10.3390/md16100392] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ren R, Azuma Y, Ojima T, et al. Modulation of platelet aggregation-related eicosanoid production by dietary F-fucoidan from brown alga Laminaria japonica in human subjects. Br J Nutr. 2013;110:880–890. [DOI] [PubMed] [Google Scholar]
- 81. Yasuzawa T, Mima A, Ueshima S. Antithrombotic effect of oral administration of mozuku (Cladosiphon okamuranus, brown seaweed) extract in rat. J Nutr Sci Vitaminol (Tokyo). 2019;65:171–176. [DOI] [PubMed] [Google Scholar]
- 82. Zhao X, Guo F, Hu J, et al. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb Res. 2016;144:46–52. [DOI] [PubMed] [Google Scholar]
- 83. Prokofjeva MM, Imbs TI, Shevchenko NM, et al. Fucoidans as potential inhibitors of HIV-1. Mar Drugs. 2013;11:3000–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tokita Y, Nakajima K, Mochida H, et al. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by sandwich ELISA. Biosci Biotechnol Biochem. 2010;74:350–357. [DOI] [PubMed] [Google Scholar]
- 85. Kwon PS, Oh H, Kwon S-J, et al. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Venkatraman KL, Mehta A. Health benefits and pharmacological effects of Porphyra species. Plant Foods Hum Nutr. 2019;74:10–17. [DOI] [PubMed] [Google Scholar]
- 87. Isaka S, Cho K, Nakazono S, et al. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int J Biol Macromol. 2015;74:68–75. [DOI] [PubMed] [Google Scholar]
- 88. Yanagido A, Ueno M, Jiang Z, et al. Increase in anti-inflammatory activities of radical-degraded porphyrans isolated from discolored nori (Pyropia yezoensis). Int J Biol Macromol. 2018;117:78–86. [DOI] [PubMed] [Google Scholar]
- 89. Zhang Z, Zhang Q, Wang J, et al. Regioselective syntheses of sulfated porphyrans from Porphyra haitanensis and their antioxidant and anticoagulant activities in vitro. Carbohydr Polym. 2010;79:1124–1129. [Google Scholar]
- 90. Ishihara K, Oyamada C, Matsushima R, et al. Inhibitory effect of porphyran, prepared from dried “nori”, on contact hypersensitivity in mice. Biosci Biotechnol Biochem. 2005;69:1824–1830. [DOI] [PubMed] [Google Scholar]
- 91. Hehemann JH, Correc G, Barbeyron T, et al. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908–912. [DOI] [PubMed] [Google Scholar]
- 92. Hehemann J-H, Kelly AG, Pudlo NA, et al. Bacteria of the human gut microbiome catabolize red seaweed glycans with carbohydrate-active enzyme updates from extrinsic microbes. Proc Natl Acad Sci USA. 2012;109:19786–19791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Peters BA, Yi SS, Beasley JM, et al. US nativity and dietary acculturation impact the gut microbiome in a diverse US population. ISME J. 2020;14:1639–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ballard-Barbash R, Callaway CW. Marine fish oils: role in prevention of coronary artery disease. Mayo Clin Proc. 1987;62:113–118. [DOI] [PubMed] [Google Scholar]
- 95. Bang HO, Dyerberg J, Hjøorne N. The composition of food consumed by Greenland Eskimos. Acta Med Scand. 2009;200:69–73. [DOI] [PubMed] [Google Scholar]
- 96. Bang HO, Dyerberg J, Nielsen AB. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet. 1971;297:1143–1146. [DOI] [PubMed] [Google Scholar]
- 97. Burr ML. Fish and ischaemic heart disease. World Rev Nutr Diet. 1993;72:49–60. [DOI] [PubMed] [Google Scholar]
- 98. Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. [DOI] [PubMed] [Google Scholar]
- 99. Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–484. [DOI] [PubMed] [Google Scholar]
- 100. Elamin EM, Miller AC, Ziad S. Immune enteral nutrition can improve outcomes in medical-surgical patients with ARDS: a prospective randomized controlled trial. J Nutr Disord Ther. 2012;2:109.[10.4172/2161-0509.1000109] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. [10.1097/00003246-199908000-00001] [DOI] [PubMed] [Google Scholar]
- 102. Langlois PL, D'Aragon F, Hardy G, et al. Omega-3 polyunsaturated fatty acids in critically ill patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Nutrition. 2019;61:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pontes-Arruda A, Martins LF, de Lima SM, et al. Enteral nutrition with eicosapentaenoic acid, γ-linolenic acid and antioxidants in the early treatment of sepsis: results from a multicenter, prospective, randomized, double-blinded, controlled study: the INTERSEPT study. Crit Care. 2011;15:R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Calder PC, Carr AC, Gombart AF, et al. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Messina G, Polito R, Monda V, et al. Functional role of dietary intervention to improve the outcome of COVID-19: a hypothesis of work. Int J Mol Sci. 2020;21:3104. [10.3390/ijms21093104] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bae M, Kim M-B, Park Y-K, et al. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158618. [DOI] [PubMed] [Google Scholar]
- 108. Zhang H, Tang Y, Zhang Y, et al. Fucoxanthin: a promising medicinal and nutritional ingredient. Evid Based Complement Alternat Med. 2015;2015:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Abdul QA, Choi RJ, Jung HA, et al. Health benefit of fucosterol from marine algae: a review. J Sci Food Agric. 2016;96:1856–1866. [DOI] [PubMed] [Google Scholar]
- 110. Zhen X-H, Quan Y-C, Jiang H-Y, et al. Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur J Pharmacol. 2015;768:131–138. [DOI] [PubMed] [Google Scholar]
- 111. Jung HA, Jin SE, Ahn BR, et al. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem Toxicol. 2013;59:199–206. [DOI] [PubMed] [Google Scholar]
- 112. Yoo MS, Shin JS, Choi HE, et al. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-κB and p38 mitogen-activated protein kinase in RAW264.7 macrophages. Food Chem. 2012;135:967–975. [DOI] [PubMed] [Google Scholar]
- 113. Shimonaka M, Hagiwara H, Kojima S, et al. Successive study on the production of plasminogen activator in cultured endothelial cells by phytosterol. Thromb Res. 1984;36:217–222. [DOI] [PubMed] [Google Scholar]
- 114. Hwang E, Park SY, Shin HS, et al. Effect of oral administration of fucosterol from Hizikia fusiformis on DNCB-induced atopic dermatitis in NC/Nga mice. Food Sci Biotechnol. 2014;23:593–599. [Google Scholar]
- 115. Gonçalves de Carvalho CMR, Ribeiro SML. Aging, low-grade systemic inflammation and vitamin D: a mini-review. Eur J Clin Nutr. 2017;71:434–440. [DOI] [PubMed] [Google Scholar]
- 116. Zhou W, Yuan G, Wang Q. Vitamin D attenuates lipopolysaccharide-induced inflammatory response in endothelial cells through inhibition of PI3K/Akt/NF-κB signaling pathway. Pharmazie. 2019;74:412–417. [DOI] [PubMed] [Google Scholar]
- 117. Zhu J, Bing C, Wilding JPH. Vitamin D receptor ligands attenuate the inflammatory profile of IL-1β-stimulated human white preadipocytes via modulating the NF-κB and unfolded protein response pathways. Biochem Biophys Res Commun. 2018;503:1049–1056. [DOI] [PubMed] [Google Scholar]
- 118. Dancer RC, Parekh D, Lax S, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015;70:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Upala S, Sanguankeo A, Permpalung N. Significant association between vitamin D deficiency and sepsis: a systematic review and meta-analysis. BMC Anesthesiol. 2015;15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zheng S, Yang J, Hu X, et al. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition. Biochem Pharmacol. 2020;177:113955. [DOI] [PubMed] [Google Scholar]
- 121. Xu J, Yang J, Chen J, et al. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. 2017;16:7432–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Teymoori-Rad M, Shokri F, Salimi V, et al. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29:e2032. [DOI] [PubMed] [Google Scholar]
- 123. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jakovac H. COVID-19 and vitamin D—is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318:e589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Narayanan N, Nair DT. Vitamin B12 may inhibit RNA-dependent-RNA polymerase activity of nsp12 from the SARS-CoV-2 virus. IUBMB Life. 2020;72:2112–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ragan MA, Craigie JS. Physodes and the phenolic compounds of brown algae. Isolation and characterization of phloroglucinol polymers from Fucus vesiculosus (L.). Can J Biochem. 1976;54:66–73. [DOI] [PubMed] [Google Scholar]
- 127. Shibata T, Kawaguchi S, Hama Y, et al. Local and chemical distribution of phlorotannins in brown algae. J Appl Phycol. 2004;16:291–296. [Google Scholar]
- 128. Arnold TM, Targett NM. To grow and defend: lack of tradeoffs for brown algal phlorotannins. Oikos. 2003;100:406–408. [Google Scholar]
- 129. Bae JS. Barrier protective activities of phloroglucinol on lipopolysaccharide (LPS)-induced barrier disruption in human endothelial cells. Inflammation. 2012;35:920–926. [DOI] [PubMed] [Google Scholar]
- 130. Li Y, Qian Z-J, Ryu B, et al. Chemical components and its antioxidant properties in vitro: an edible marine brown alga, Ecklonia cava. Bioorg Med Chem.. 2009;17:1963–1973. [DOI] [PubMed] [Google Scholar]
- 131. Wijesinghe WA, Ko SC, Jeon YJ. Effect of phlorotannins isolated from Ecklonia cava on angiotensin I-converting enzyme (ACE) inhibitory activity. Nutr Res Pract. 2011;5:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ahn M-J, Yoon K-D, Min S-Y, et al. Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol Pharm Bull. 2004;27:544–547. [DOI] [PubMed] [Google Scholar]
- 133. Artan M, Li Y, Karadeniz F, et al. Anti-HIV-1 activity of phloroglucinol derivative, 6,6'-bieckol, from Ecklonia cava. Bioorg Med Chem. 2008;16:7921–7926. [DOI] [PubMed] [Google Scholar]
- 134. Kwon H-J, Ryu YB, Kim Y-M, et al. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorg Med Chem. 2013;21:4706–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Park J-Y, Kim JH, Kwon JM, et al. Dieckol, a SARS-CoV 3CL(pro) inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg Med Chem. 2013;21:3730–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Corona G, Ji Y, Anegboonlap P, et al. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br J Nutr. 2016;115:1240–1253. [DOI] [PubMed] [Google Scholar]
- 137. Gordon JI. Honor thy gut symbionts redux. Science. 2012;336:1251–1253. [DOI] [PubMed] [Google Scholar]
- 138. Holmes E, Kinross J, Gibson GR, et al. Therapeutic modulation of microbiota–host metabolic interactions. Sci Transl Med. 2012;4:137rv136. [DOI] [PubMed] [Google Scholar]
- 139. Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5–9. [DOI] [PubMed] [Google Scholar]
- 140. Dalile B, Van Oudenhove L, Vervliet B, et al. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. [DOI] [PubMed] [Google Scholar]
- 141. Nguyen SG, Kim J, Guevarra RB, et al. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food Funct. 2016;7:4193–4201. [DOI] [PubMed] [Google Scholar]
- 142. Ramnani P, Chitarrari R, Tuohy K, et al. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe. 2012;18:1–6. [DOI] [PubMed] [Google Scholar]
- 143. Shang Q, Shan X, Cai C, et al. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016;7:3224–3232. [DOI] [PubMed] [Google Scholar]
- 144. Zaporozhets TS, Besednova NN, Kuznetsova TA, et al. The prebiotic potential of polysaccharides and extracts of seaweeds. Russ J Mar Biol. 2014;40:1–9. [Google Scholar]
- 145. Costantini L, Molinari R, Farinon B, et al. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18:2645. [10.3390/ijms18122645] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Watson H, Mitra S, Croden FC, et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. 2018;67:1974–1983. [DOI] [PubMed] [Google Scholar]
- 147. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. The National Academies Press; 2001. 10.17226/10026 [DOI] [PubMed]
- 148. Zava TT, Zava DT. Assessment of Japanese iodine intake based on seaweed consumption in Japan: a literature-based analysis. Thyroid Res. 2011;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Matsubayashi S, Mukuta T, Watanabe H, et al. Iodine-induced hypothyroidism as a result of excessive intake of confectionery made with tangle weed, Kombu, used as a low calorie food during a bulimic period in a patient with anorexia nervosa. Eat Weight Disord. 1998;3:50–52. [DOI] [PubMed] [Google Scholar]
- 150. Miyai K, Tokushige T, Kondo M. Suppression of thyroid function during ingestion of seaweed “Kombu” (Laminaria japonoca) in normal Japanese adults. Endocr J. 2008;55:1103–1108. [DOI] [PubMed] [Google Scholar]
- 151. Nishiyama S, Mikeda T, Okada T, et al. Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid. 2004;14:1077–1083. [DOI] [PubMed] [Google Scholar]
- 152. Doerge DR, Chang HC. Inactivation of thyroid peroxidase by soy isoflavones, in vitro and in vivo. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:269–279. [DOI] [PubMed] [Google Scholar]
- 153. Doerge DR, Sheehan DM. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect. 2002;110(suppl 3):349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16:249–258. [DOI] [PubMed] [Google Scholar]
- 155. Verheesen RH, Traksel RAM. Iodine, a preventive and curative agent in the COVID-19 pandemic? Med Hypotheses. 2020;144:109860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Taylor V, Goodale B, Raab A, et al. Human exposure to organic arsenic species from seafood. Sci Total Environ. 2017;580:266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Park S, Lee BK. Strong positive associations between seafood, vegetables, and alcohol with blood mercury and urinary arsenic levels in the Korean adult population. Arch Environ Contam Toxicol. 2013;64:160–170. [DOI] [PubMed] [Google Scholar]
- 158. Lee TX. Is mercury causing COVID-19 deaths? QEIOS. 2020; OF0L6S.4. Preprint posted September 1, 2020. [10.32388/OF0L6S.4]