Abstract
Background
Healthcare workers (HCWs) are commonly infected by SARS-CoV-2 and represent one of the most vulnerable groups. Adequate prevention strategies are necessary to guarantee HCWs’ safety, as well as to prevent dissemination of the infection among patients.
Aims
To describe a case series of SARS-CoV-2-positive HCWs in a large public healthcare organization in Milan (Italy) during the most devastating weeks of the epidemic and analyse the sources, symptoms and duration of SARS-CoV-2 infection.
Methods
This study included 172 SARS-CoV-2-positive HCWs who were infected between the 25th of February and the 7th of April 2020. A nasopharyngeal swab (NPS) and RT-PCR were used to indicate.
Results
Initially, the most common sources of infection were other positive HCWs (49%). Medical doctors and nursing assistants were most frequently infected, with infection rates of 53/1000 and 50/1000, respectively. COVID-19 departments were less affected than internal medicine, surgery, intensive care, or emergency room. The most commonly reported symptom was mild cough, while loss of smell (anosmia) and loss of taste (ageusia) were reported as moderate and severe by 30–40% of HCWs. The time necessary for 50% of workers to recover from the infection was 23 days, while it took 41 days for 95% of HCWs to become virus-free.
Conclusions
HCWs are commonly infected due to close contacts with other positive HCWs, and non-COVID departments were most affected. Most HCWs were asymptomatic or subclinical but contact tracing and testing of asymptomatic HCWs help identify and isolate infected workers.
Keywords: Contact tracing, COVID-19 symptoms, healthcare personnel, infection rate, SARS-CoV-2
Key learning points.
What is already known about this subject:
There have been more than 30 million SARS-CoV-2 cases and a million deaths attributed to COVID-19.
Symptoms include fever, cough, dyspnoea, muscle pain, and many prevention strategies focus on identifying symptoms of infection.
Healthcare workers represent 10% of overall cases, and often more than 10% of hospital personnel get infected.
What this study adds:
The main source of infection for healthcare workers were other healthcare workers.
About 90% of the SARS-CoV-2-infected workers were asymptomatic or had only mild symptoms during the follow-up period.
The median duration of infection was 23 days, and it took 41 days for 95% of the workers to be virus-free.
What impact this may have on practice or policy:
Contact tracing and testing of asymptomatic workers help stop the spread of the infection in the workplace.
Source control should be implemented in hospital settings, even in non COVID-19 departments.
Requiring a double negative NPS before returning to work is questionable, due to the low infectivity.
Introduction
As of September 2020, more than 30 million cases of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and a million deaths have been reported worldwide [1]. Commonly reported symptoms include fever, dry cough, headache, sore throat, and sneezing, while a growing number of reports underline asymptomatic or subclinical subjects, which are difficult to identify, but very likely able to spread the infection [2–5]. Reports published on COVID-19 patients underline that healthcare workers (HCWs) are at high risk of infection and represent one of the most vulnerable groups [6,7]. The safety of HCWs is fundamental as a measure of workers’ health and well-being protection, but also to avoid the dissemination of the infection among patients and other HCWs, which can cause a shortage of personnel in this severe public health crisis.
The World Health Organization’s (WHO) definitions of suspect cases require at least mild symptoms, although contact tracing and adjustment of suspect case definition are encouraged [8]. Inclusion of contact tracing and testing of asymptomatic patients can help identify potential spreaders of SARS-CoV-2 in hospitals but adds a high logistic burden [9]. However, the additional burden might be justified, bearing in mind that around 10% of infected persons are HCWs and that most hospitals report 10% or more of staff getting infected [10]. Challenges are also posed by the removal from workplace and return-to-work procedures which depend on the expected duration of the disease and a negative nasopharyngeal swab (NPS), which can be false negative, as well as local regulation [11]. In a public health crisis such as COVID-19, adequate preventive measures and procedures protecting HCWs are critical.
On January 31st, two Chinese visitors tested positive for SARS-Cov-2 in Italy, while on February 21st, a 38-year-old man from the Northern Lombardy region was the first Italian citizen positive for SARS-CoV-2 [12]. From three confirmed cases on February 21st, the number grew to 4636 cases (1500-fold increase) and 197 deaths by March 7th [13]. By the beginning of April, the number of cases in Italy grew to more than 130 000 and 16 000 deaths, putting enormous pressure on the Italian healthcare system. Almost half of the cases were in the Northern Lombardy region, and more than 11% in the city of Milan, with more than 10% represented by HCWs [14].
The aim of this study was to describe, in a case series of HCWs of a large public healthcare organization which provided healthcare services to patients with SARS-CoV-2, the course of the epidemic of SARS-CoV-2 among HCWs, the sources of their infection, the onset and progression of COVID-19 symptoms, and the duration of infection.
Methods
Azienda Socio Sanitaria Territoriale (ASST) Santi Paolo e Carlo is part of the Italian public (state owned and financed) healthcare system, and is composed of two hospitals and 40 outpatient units. The ASST covers healthcare activities from general practice, health during pregnancy, paediatric care, vaccinations, to emergency and hospital care. The security, catering, cleaning and some HCWs are external staff (‘subcontractors’) and were not the focus of our investigation as they are not covered by occupational health services by our Occupational Health Unit.
As the number of COVID-19 patients in the region of Lombardy grew, first San Paolo (on the 24th of February) and then San Carlo (on the 6th of March) started admitting COVID-19 patients, with the number of beds (fully occupied) going from several dozen to up to 140 and 160 at San Paolo San Carlo hospitals, respectively. Beds were made available by converting various departments (internal medicine, surgery, etc.) into COVID-19 departments which provided three levels of care: intensive care (e.g. intubated patients), sub-intensive care (e.g. patients not intubated but on auxiliary ventilation – helmets), regular care (e.g. patients with severe COVID-19 symptoms, or those recovering from intensive care).
Our case series included all internal HCWs who tested positive for SARS-CoV-2 in the period from the beginning of the pandemic until the 7th of April 2020.
Ethical committee approval was not sought for this study according to Italian national regulation. The data were collected and analysed as part of the health surveillance program carried out by our Occupational Health Unit. Workers were informed about the procedures and have signed informed consent regarding data collection and analysis. The hospital management has approved the use of the data and publishing of the findings.
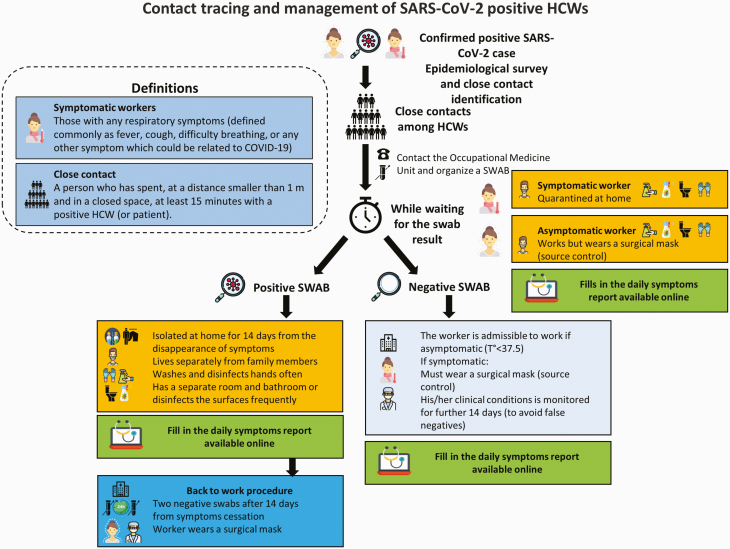
Figure 1 outlines the contact tracing, testing, isolation and return-to-work procedure applied during the SARS-CoV-2 pandemic.
Figure 1.
Contact tracing and management of HCWs.
Once a HCW’s or patient’s NPS was found positive, an epidemiological survey was performed by the infectious diseases office to identify close contacts of this confirmed SARS-CoV-2 case. A ‘close contact’ was defined as a person who had a face-to-face dialogue or who spent at least 15 min in an indoor environment with a COVID-19 patient, without wearing a personal protective device (PPE, e.g. surgical mask). Symptomatic close contacts were quarantined at home pending the NPS result, while asymptomatic ones remained at work wearing a surgical/medical mask as a method of source control. Once the result of the NPS came back, SARS-CoV-2-positive HCWs were isolated at home and were to follow a strict procedure to reduce the possibility of infecting their family members. All close contacts continued monitoring their symptoms for at least 2 weeks using the online daily symptoms report prepared by our Unit.
Additional information regarding the management of the epidemic, use of PPE, epidemiological survey, contact tracing, quarantine and isolation of workers, instructions for home isolation and the return-to-work procedure are available in Supplementary Material 1 (available as Supplementary data at Occupational Medicine Online).
Molecular analysis on NPS
The NPS analysis was performed with two kits: GeneFinderTM COVID-19 PLUS RealAmp Kit by ELITech Group and Roche Modular Wuhan CoV N, RdRP and E gene Kit. A test was considered positive if RdRp and E or N or both genes were detected, and repeated to confirm positive if only RdRP or N was detected [15,16]. The turnaround of tests was up to 48 h at the beginning of the epidemic, while it was reduced to up to 24 h in the middle of March.
If the worker was identified through contact tracing, indicating a confirmed close contact with a SARS-CoV-2-positive case (another HCW or a specific patient), in the absence of an alternative explanation (e.g. if a person had a family member at home who was positive), the HCW or the patient was considered the source of infection.
If the worker was tested because of a positive family member or other confirmed positive cases from their personal life, the source of infection was considered the family.
If the worker was tested because they experienced symptoms, but were unaware of any close contacts with SARS-CoV-2-positive patients, HCWs or family members, the source of infection was considered unknown and they are reported in the figures as ‘Unknown’.
According to Italian law, SARS-CoV-2-positive workers were considered virus-free after the resolution of respiratory infection symptoms and two consecutive negative control NPS for SARS-CoV-2 with 24-h distance [17]. Workers were tested 14 days after the resolution of the respiratory infection symptoms, or, in case of asymptomatic workers, 14 days after the positive NPS. In the case of a positive control NPS, the workers were tested after additional 7 days. The duration of the infection is presented as the time (in days) between the positive and two consecutive negative NPS.
Confirmed SARS-CoV-2-positive HCWs, those waiting for the NPS results, and those identified as close contacts but found negative for SARS-CoV-2 were asked to fill out an online daily symptoms report, which was monitored by our team to identify any changes which would require medical attention.
The daily symptoms reports were centred around the date of the positive swab, making that day’s report the follow-up day 0 (zero). Days following the positive swab are denoted with positive integers (from 1 to 15). The intensity of each symptom was collected as absent, mild, moderate and severe. Categorical variables (i.e. symptom intensity) are presented as the proportion of HCWs reporting each symptom. Data management, processing, analysis and visualization were done using R Language and Environment for Statistical Computing [18].
Results
The two hospitals employ a total of 4200 internal workers (of which around 2000 nurses, 750 doctors, 480 administrative workers and 350 social/nursing assistants), while 1500 HCWs work in the 40 outpatient structures. Out of these 5700 workers, 70% are female, with a mean age of 46 years. We present a case series of all 172 out of 185 SARS-CoV-2-positive HCWs found through contact tracing or reporting to our Unit in the period from February 25th until April 7th, 2020. An additional 13 HCWs found through random sampling were excluded from the analysis due to the different approach used. A total of 2485 NPS were performed on HCWs in our two hospitals and the territory, 2025 based on symptoms or close contacts and the remaining 460 randomly. The positive rate in non-random samples was 10%.
Table 1 shows the characteristics of the HCWs included in the study. Among the study group, the most prevalent job title was nurse (49%), followed by medical doctors (24%), nursing assistants (12%), and administrative workers (5%). Most workers were tested and found positive due to a close contact with a positive colleague (49%), followed by worker-initiated testing due to symptoms (unknown source, total 28%), and a SARS-CoV-2-positive family member (11%). About 10% of SARS-CoV-2-positive HCWs reported contacts with positive patients, mostly at San Paolo hospital.
Table 1.
Characteristics of the study population
| All | San Carlo | San Paolo | Territory | |
|---|---|---|---|---|
| N = 172 | N = 74 | N = 71 | N = 27 | |
| Gender | ||||
| Female | 99 (58) | 46 (62) | 33 (46) | 20 (74) |
| Male | 73 (42) | 28 (38) | 38 (54) | 7 (26) |
| Age (years) | 44 (24–66) | 48 (27–66) | 38 (24–64) | 50 (27–63) |
| Job title | ||||
| Nurse (qualified) | 84 (49) | 46 (62) | 29 (41) | 9 (33) |
| Doctor | 42 (24) | 16 (23) | 23 (3) | 3 (11) |
| Nursing assistant | 20 (13) | 7 (9) | 10 (15) | 3 (11) |
| Intern | 9 (5) | 2 (3) | 7 (10) | 0 (0) |
| Administrative | 9 (5) | 0 (0) | 2 (2) | 7 (26) |
| Psychologist | 5 (3) | 0 (0) | 0 (0) | 5 (19) |
| Laboratory | 1 (0) | 1 (1) | 0 (0) | 0 (0) |
| Worker (general) | 2 (1) | 2 (2) | 0 (0) | 0 (0) |
| Source of infection | ||||
| HCWs | 86 (50) | 51 (69) | 20 (28) | 15 (56) |
| Unknown | 50 (29) | 18 (25) | 24 (34) | 8 (30) |
| Family | 19 (11) | 4 (5) | 12 (17) | 3 (11) |
| Patients | 17 (10) | 1 (1) | 15 (21) | 1 (3) |
| No. of daily symptoms reports | 10 (1–26) | 13 (1–24) | 7 (1–26) | 8 (1–21) |
| Duration of infection (days) | 22 (10–49) | 19 (13–49) | 26 (10–48) | 25 (10–48) |
N (%) shown for categorical variables, and median (min–max) shown for continuous variables.
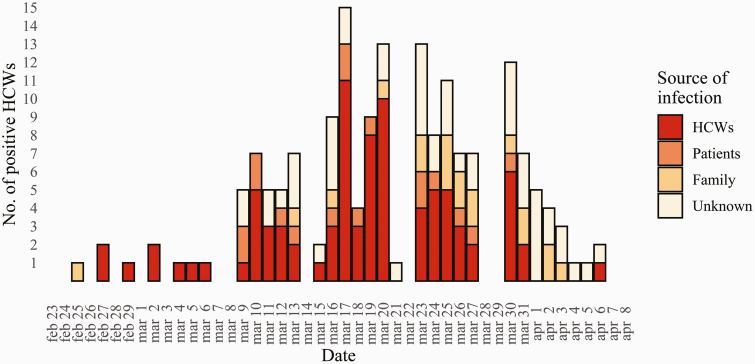
Figure 2 shows the timeline of the positive HCWs identified in our study and the sources of their infection. In the middle weeks of March 2020, most cases (denoted dark red) were tested and found positive due to close contacts with other positive HCWs. The proportion of HCWs infected from close unprotected contacts with colleagues started to decline after the week of March 20th and was replaced by symptomatic HCWs without identified close contact and family-related contacts. Supplementary Material 2 (available as Supplementary data at Occupational Medicine Online) shows the results of contact tracing after the first SARS-CoV-2-positive HCW. The first cluster of cases connected to the index case grew to seven identified cases and there were several hundred close contacts tested. Supplementary Material 3 (available as Supplementary data at Occupational Medicine Online) shows the epidemic timeline divided between the two hospitals and the outpatient units, as well as the individual departments in the two hospitals with the number of infected HCW per department and the source of their infection. In the San Carlo hospital and the outpatient units, the most prevalent source of infection were other HCWs, with the peak during the middle weeks of March 2020. At the San Paolo hospital, positive HCWs were commonly tested due to close contacts with SARS-CoV-2-positive family members or patients (Supplementary Figure 2, available as Supplementary data at Occupational Medicine Online). The most affected departments were those of internal medicine, surgery, intensive care and emergency room, while COVID-19 departments followed (Supplementary Figure 3, available as Supplementary data at Occupational Medicine Online).
Figure 2.
The SARS-CoV-2 epidemic among HCWs in our public healthcare company.
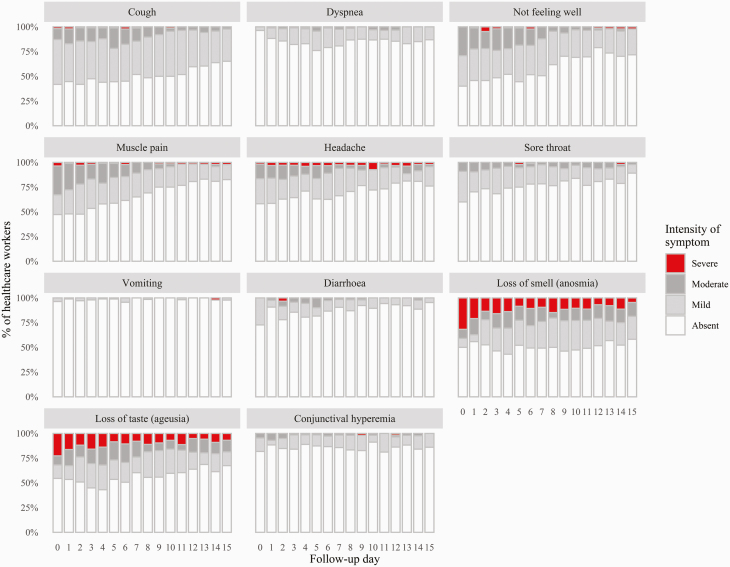
Figure 3 shows the symptoms associated with COVID-19 and their intensity, from absent to severe, as reported by the HCWs during the 15 days following the NPS. The most prevalent specific symptoms were cough (reported by almost 60% of workers) and loss of smell and taste (reported by around 50% of workers). Dyspnoea was reported by only 10–15% of workers. Most prevalent non-specific symptoms were not feeling well (reported by 50–60% of workers), muscle pain (reported by ~50% of workers), headache (reported by ~40% of workers) and sore throat (reported by ~45% of workers). Cough was reported as moderate or severe by only 10–15% of workers, and dyspnoea by less than 2%. General symptoms were reported as moderate and severe by around 30% of workers. Loss of smell and loss of taste were reported as moderate or severe by between 30 and 40% of workers throughout the follow-up period. There was a gradual reduction of most symptoms in the second week, with persisting loss of smell and loss of taste in around 30% of HCWs. Most workers were afebrile during the 15-day follow-up period (Supplementary Material 4, available as Supplementary data at Occupational Medicine Online).
Figure 3.
Symptoms and signs reported by HCWs during the 15-day follow-up.
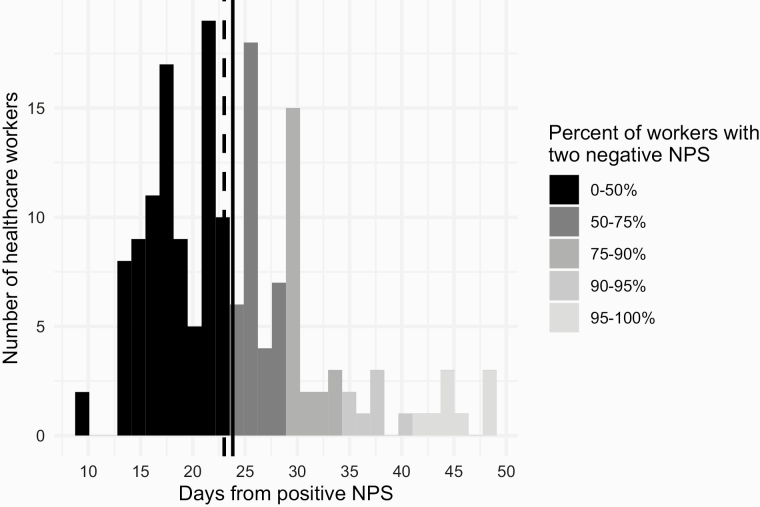
Figure 4 shows the distribution of the time elapsed since the positive swab and the two negative swabs, a prerequisite for the return-to-work by the Italian regulation [17]. The solid and dashed lines denote the mean and median values, respectively, which were both at 23 days. The time needed for 75% of HCWs to return to work was 29 days, 34 days for 90% of HCWs and 41 days for 95% of HCWs. The longest period needed for a worker to become free of respiratory symptoms, have two negative NPS and return to work was 49 days.
Figure 4.
Distribution of the duration of the disease (time from the positive NPS to two negative NPS).
Discussion
The most common source of infection among HCWs at the peak of the epidemic in Milan were other HCWs. The highest infection rate was among doctors (53 per 1000), followed by nursing assistants (50 per 1000), nurses (42 per 1000) and administrative workers (19 per 1000). In case of other HCWs as the source of infection, the different infection rates between doctors, nursing assistants and qualified nurses can be explained by the fact that employees spend more time among their own category of workers. Nevertheless, they also have different roles in caring for the patients: medical doctors spend more time with each individual patient, while nursing assistants, contrary to qualified nurses, are in charge of the hygiene of the patient, transport and manoeuvring, which puts them in closer contact with patients’ bodily fluids and patients themselves.
In our study, all confirmed close contacts were tested, contrary to a large hospital in Madrid, where workers were tested only if presenting with at least mild symptoms [19]. Their results have shown 791 SARS-CoV-2-positive HCW among around 6800 workers (~11%), while our approach resulted in almost four times lower infection prevalence (~3%). This suggests that any ‘symptom-centred’ measure alone does not guarantee full protection of HCWs and patients and could even result in a false sense of safety. In fact, internal medicine, surgery, intensive care and emergency room were departments with the highest number of SARS-CoV-2-positive HCWs in our study, contrary to the common expectation that COVID-19 departments will drive the spread of SARS-CoV-2 among HCWs. HCWs knowingly working directly with COVID-19 patients, at the beginning of the epidemic, were better aware of the risks and managed to protect themselves, while those working at other departments assumed they were not exposed and were thus more vulnerable. In fact, the most reported source of infection in the internal medicine department at the San Paolo hospital were patients, although all (recognized) COVID-19 patients were treated in COVID-19 departments.
The difference between the sources of infection between the two hospitals might be because San Paolo hospital started admitting COVID-19 patients 2 weeks before San Carlo hospital. The authors of the report from Madrid also found no association with so-called ‘high-risk’ areas of the hospital and connect the dynamic of transmission in their hospital to that of the general population [19]. In fact, the important percentage of HCWs infected by an ‘unknown’ source underlines the importance of various contacts which might happen during the travel to work or personal time. In our hospitals, initially, workers wore recommended protection when working with confirmed or suspected SARS-CoV-2-positive patients, but spent time during arrival and departure from work, in changing rooms and during breaks with colleagues without any protection. During March, most workers started wearing surgical masks for source control, although the official recommendation arrived in the beginning of April, which might explain the steep decline in the number of infected HCWs.
Most HCWs in our study reported no or mild symptoms of a respiratory infection, but loss of smell (anosmia) and loss of taste (ageusia/dysgeusia) were commonly reported as moderate or severe and persisted throughout the follow-up period. A recent review article on asymptomatic COVID-19 transmission underlined this risk in the healthcare setting, although concentrating on the risk arising from asymptomatic patients [20]. A recent study has revealed that apparently healthy HCWs might have developed IgG and IgM antibodies, although no association with being exposed to COVID-19 patients was found [21]. These results indicate that relying on symptoms might not be an optimal strategy, and our results put the focus on source control.
It is important to note that the median time between a positive NPS and being virus-free in our study was 23 days, with several cases having positive NPS even after more than 40 days. Most studies suggest that viral nucleic acid can be detected in NPS for a prolonged period of time, although the cultures will remain negative 8–10 days after symptom onset and there is a general consensus that a SARS-CoV-2-positive patient will not be infective 10 days after the positive NPS [22–24]. The Italian policy of keeping the HCWs in isolation until a double negative NPS beyond several weeks from the onset of symptoms is therefore questionable, considering low or no possibility for infecting others and the lack of health personnel during this public health emergency.
One limitation of our study is difficulty of verifying the actual source of infection in HCWs. Nevertheless, the high percent positive rate of more than 10% among tested close contacts compared to 2% among randomly tested individuals supports our assumption. Another limitation is the number of HCWs who have never completed the daily symptoms report, reporting as reasons: not having a smartphone, computer or internet at home; lack of experience with online forms (‘not being technological enough’); and taking care of a sick family member.
Our study has shown that during the peak of the epidemic in Italy, HCWs were commonly infected due to close contact with other positive cases among HCWs and patients. Most reported symptoms were mild and non-specific; therefore, symptom-centred prevention strategies did not provide adequate protection. Departments other than those treating COVID-19 patients might be a high-risk setting during the peak of the epidemic due to lower protection protocols, therefore preventive strategies should address all HCWs in all departments at all times.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the kind help and support from our dear colleagues Rosamaria Bentoglio, Donatella Visentin and all workers of the Occupational Health Unit of the Saints Paolo and Carlo Hospital of Milan without whose selfless effort this work would not be possible.
Competing interest
None declared.
Data Availability
The data underlying this article are available on request. An early report of our findings has been published on medRxiv as a preprint titled ‘Contact tracing and isolation of asymptomatic spreaders to successfully control the COVID-19 epidemic among healthcare workers in Milan (Italy)’ [25].
References
- 1. World Health Organization (WHO). WHO Coronavirus Disease (COVID-19) Weekly Epidemiological Update. 21 September 2020. [Google Scholar]
- 2. Chang D, Xu H, Rebaza A, Sharma L, Dela Cruz CS. Protecting healthcare workers from subclinical coronavirus infection. Lancet Respir Med [Internet] 2020;8:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zou L, Ruan F, Huang M et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020;382:1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eurosurveillance Editorial Team. Updated rapid risk assessment from ECDC on the novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Euro Surveill 2020;25:2003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization (WHO). Global Surveillance for COVID-19 Caused by Human Infection With COVID-19 Virus: Interim Guidance, 20 March 2020. Geneva: World Health Organization, 2020. [Google Scholar]
- 9. Keeling MJ, Hollingsworth TD, Read JM. The efficacy of contact tracing for the containment of the 2019 novel coronavirus (COVID-19). medRxiv. 2020. doi: 10.1101/2020.02.14.20023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gómez-Ochoa SA, Franco OH, Rojas LZ et al. COVID-19 in healthcare workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol [Internet] 2020. doi: 10.1093/aje/kwaa191/5900120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carver C, Jones N. Comparative Accuracy of Oropharyngeal and Nasopharyngeal Swabs for Diagnosis of COVID-19 The Centre for Evidence-Based Medicine, 2020. https://www.cebm.net/covid-19/comparative-accuracy-of-oropharyngeal-and-nasopharyngeal-swabs-for-diagnosis-of-covid-19/ (15 September 2020, date last accessed). [Google Scholar]
- 12. Carinci F. Covid-19: preparedness, decentralisation, and the hunt for patient zero. Br Med J [Internet] 2020;368 10.1136/bmj.m799. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization. The Coronavirus Disease 2019 (COVID-19): Situation Reports—32/39/47/49. Geneva: WHO, 2020. [Google Scholar]
- 14. Istituto Superiore Sanita. Aggiornamento Nazionale: 23 Aprile 2020. Rome: Task Force COVID-19 del Dipartimento Malattie Infettive e Servizio di Informatica, Istituto Superiore di Sanità, 2020. [Google Scholar]
- 15. Tang Y-W, Schmitz JE, Persing DH, Stratton CW. The laboratory diagnosis of COVID-19 infection: current issues and challenges. J Clin Microbiol 2020; 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corman VM, Landt O, Kaiser M et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Consiglio Superiore di Sanita. Parere del Consiglio Superiore di Sanità: definizione di Paziente guarito da Covid-19 e di paziente che ha eliminato il virus SARS-CoV-2. Circolare Ministero della Salute n. 0006607-29/02/2020-DGPRE-DGPRE-P. 2020. [Google Scholar]
- 18. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. http//wwwR-project.org/. [Google Scholar]
- 19. Folgueira MD, Munoz-Ruiperez C, Alonso-Lopez MA, Delgado R. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain, during March 2020. medRxiv 2020. doi: 10.1101/2020.04.07.20055723. [DOI] [Google Scholar]
- 20. Aguirre-Duarte N. Can people with asymptomatic or pre-symptomatic COVID-19 infect others: a systematic review of primary data. medRxiv 2020. doi: 10.1101/2020.04.08.20054023. [DOI] [Google Scholar]
- 21. Sotgiu G, Barassi A, Miozzo M et al. SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm Med 2020;20:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wölfel R, Corman VM, Guggemos W et al. Virological assessment of hospitalized patients with COVID-2019. Nature [Internet] 2020;581:465–469. [DOI] [PubMed] [Google Scholar]
- 23. He X, Lau EHY, Wu P et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med [Internet] 2020;26:672–675. [DOI] [PubMed] [Google Scholar]
- 24. Wiersinga WJ, Rhodes A, Cheng AC et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc 2020;324:782–793. [DOI] [PubMed] [Google Scholar]
- 25. Mandić-Rajčević S, Masci F, Crespi E et al. Contact tracing and isolation of asymptomatic spreaders to successfully control the COVID-19 epidemic among healthcare workers in Milan (Italy). medRxiv. 2020. doi: 10.1101/2020.05.03.20082818. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available on request. An early report of our findings has been published on medRxiv as a preprint titled ‘Contact tracing and isolation of asymptomatic spreaders to successfully control the COVID-19 epidemic among healthcare workers in Milan (Italy)’ [25].