Abstract
Background
During the coronavirus disease 2019 (COVID-19) pandemic, many countries experienced infection in health care workers (HCW) due to overburdened health care systems. Whether infected HCW acquire protective immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is unclear.
Methods
In a Norwegian prospective cohort study, we enrolled 607 HCW before and after the first COVID-19 wave. Exposure history, COVID-19–like symptoms, and serum samples were collected. SARS-CoV-2–specific antibodies were characterized by spike-protein IgG/IgM/IgA enzyme-linked immunosorbent and live-virus neutralization assays.
Results
Spike-specific IgG/IgM/IgA antibodies increased after the first wave in HCW with, but not in HCW without, COVID-19 patient exposure. Thirty-two HCW (5.3%) had spike-specific antibodies (11 seroconverted with ≥4-fold increase, 21 were seropositive at baseline). Neutralizing antibodies were found in 11 HCW that seroconverted, of whom 4 (36.4%) were asymptomatic. Ninety-seven HCW were tested by reverse transcriptase polymerase chain reaction (RT-PCR) during follow-up; 8 were positive (7 seroconverted, 1 had undetectable antibodies).
Conclusions
We found increases in SARS-CoV-2 neutralizing antibodies in infected HCW, especially after COVID-19 patient exposure. Our data show a low number of SARS-CoV-2–seropositive HCW in a low-prevalence setting; however, the proportion of seropositivity was higher than RT-PCR positivity, highlighting the importance of antibody testing.
Keywords: health care workers, COVID-19, SARS-CoV-2, spike protein, antibody characterization, IgG, IgM, IgA, neutralizing antibody, seroconversion
Low numbers of SARS-CoV-2–seropositive HCW were found in Norway and 1.8% of HCW seroconverted with neutralizing antibodies. Seropositivity was higher than RT-PCR positivity in HCW with 36% asymptomatic, representing a risk of infection within the health care setting.
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) emerged in Wuhan, China in late December 2019 [1]. Cases increased dramatically in January 2020 before a lockdown was implemented, which brought the outbreak under control. However, the virus had spread to other countries and Europe became the epicenter of the pandemic in March 2020. The high numbers of cases and associated mortality overwhelmed health care services in Italy [2, 3], leading many countries to implement lockdowns to reduce the spread of SARS-CoV-2 and protect their citizens.
Health care workers (HCW) in contact with COVID-19 patients are at higher risk of occupational infection, up to 11.6-fold, compared to other HCW and in the community [4–6]. The overwhelming hospital admission rates of severely ill patients, exhausting the health care resources, have globally resulted in thousands of infected HCW [5–9] and hundreds of deaths [10–12].
Current testing for SARS-CoV-2 relies on amplification of the viral genome using reverse transcriptase-polymerase chain reaction (RT-PCR) during the acute infection, whereas serological assays can determine infection over a longer period. In China, 17% of HCW were seropositive after exposure to COVID-19 patients, despite testing negative by RT-PCR [13]. Serological assays detecting antibodies to the SARS-CoV-2 spike protein and its receptor-binding domain (RBD) provide an important tool for examining infection in health care settings [14].
COVID-19 cases in Norway were first detected on 26 February 2020 [15] and increased rapidly before a lockdown was implemented on 12 March 2020, with the pandemic peaking on 26 March 2020. The local epidemic period is well defined with early and prioritized RT-PCR testing of HCW with COVID-19 patient exposure or COVID-19–like symptoms. This enabled detailed follow-up of infected HCW and determination of the extent of undiagnosed asymptomatic or mild illness, which can fuel the spread of virus in hospitals and the community. In this study, we enrolled 607 HCW before their exposure to COVID-19 patients to investigate antibody responses to SARS-CoV-2, infection rates, and associated risk factors during the first COVID-19 pandemic wave in Bergen, Norway.
METHODS
Study Design
We conducted a prospective cohort study of HCW working in health care facilities testing and treating COVID-19 patients (Bergen Municipality Emergency Room, Haukeland University and Haraldsplass Deaconess Hospitals), which was approved by the Western Norway Ethics committee (No. 118664). Inclusion criteria were HCW with or without present/potential future exposure to COVID-19 patients, and working from 6 March to 9 April 2020. Exclusion criteria were HCW in quarantine or RT-PCR confirmed SARS-CoV-2 infection at recruitment. All HCW provided written informed consent before inclusion and serum samples were collected at baseline and 6–10 weeks later (before and after the peak of the first COVID-19 pandemic wave) (Supplementary Figure 1). Sera were coded with a unique identification number, aliquoted, and stored at –80°C, and heat-inactivated for 1 hour at 56°C before use.
Case Report Form
A cloud-based case report form was developed using REDCap electronic data capture tools [16] to collect relevant clinical and demographic data, such as recent travel history, contact with suspected or confirmed COVID-19 patients, use of personal protective equipment (PPE), intercurrent illnesses including respiratory disease (fever, dry cough, difficulty breathing, sore throat, myalgia, malaise, and any other relevant symptoms), and RT-PCR results (Table 1).
Table 1.
Demographics, Clinical, and Serological Characteristics of Health Care Workers
| Characteristic | Total (n = 607) |
High Riska (n = 383) |
Low Riska (n = 224) |
Crude Pb |
Adjusted Pc |
|---|---|---|---|---|---|
| Age median ± SD | 39 ± 12.6 | 36 ± 12.6 | 42.5 ± 12.7 | ||
| Age group, y | .0096 | .8382 | |||
| 20–50 | 446 (73.5) | 295 (77.0) | 151 (67.4) | ||
| 51–78 | 161 (26.5) | 88 (23.0) | 73 (32.6) | ||
| Sex | .0138 | .6269 | |||
| Female | 468 (77.1) | 283 (73.9) | 185 (82.3) | ||
| Male | 139 (22.9) | 100 (26.1) | 39 (17.4) | ||
| Workplace | <.0001 | .0010 | |||
| Haukeland University Hospital | 471 (77.6) | 247 (64.5) | 224 (100) | ||
| Haraldsplass Deaconess Hospital | 54 (8.9) | 54 (14.1) | … | ||
| Bergen Municipality Emergency | 82 (13.5) | 82 (21.4) | … | ||
| Profession | <.0001 | .0347 | |||
| Physician | 174 (28.7) | 126 (32.9) | 48 (21.4) | ||
| Nurse | 286 (47.1) | 200 (52.2) | 86 (38.4) | ||
| Other | 147 (24.2) | 57 (14.9) | 90 (40.2) | ||
| Travel history in 2020 at baseline | .2271 | .9452 | |||
| International | 133/606 (21.9) | 91/382 (23.8) | 42/224 (18.7) | ||
| Domestic | 38/606 (6.3) | 26/382 (6.8) | 12/224 (5.3) | ||
| None | 435/606 (71.8) | 265/382 (69.2) | 170/224 (75.9) | ||
| Occupational exposure at baseline | <.0001 | .0094 | |||
| Confirmed patient contact | 37/597 (6.2) | 37/379 (9.8) | 0/218 (0) | ||
| Suspected patient contact | 74/597 (12.4) | 74/379 (19.5) | 0/218 (0) | ||
| No patient contact | 486/597 (81.4) | 268/379 (70.7) | 218/218 (100) | ||
| Occupational exposure at follow-up | <.0001 | <.0001 | |||
| With PPE | 263/605 (43.5) | 263/381 (69.0) | 0/224 (0) | ||
| Without PPE | 10/605 (1.7) | 10/381 (2.6) | 0/224 (0) | ||
| No contact | 332/605 (54.9) | 108/381 (28.3) | 224/224 (100) | ||
| Community exposure at follow-up | 17/604 (2.8) | 15/380 (3.9) | 2/224 (0.9) | .0283 | .4295 |
| Symptoms at follow-up | .0920 | .3229 | |||
| Any symptom | 116/606 (19.1) | 81/382 (21.1) | 35/224 (15.6) | ||
| Dry cough | 59/113 (52.2) | 40/78 (51.2) | 19/35 (54.3) | ||
| Fever | 29/113 (25.7) | 22/79 (27.9) | 7/34 (20.1) | ||
| Dyspnea | 22/113 (19.5) | 20/79 (25.3) | 2/34 (5.9) | ||
| SARS-CoV-2 RT-PCR test at follow-up | .9719 | .7291 | |||
| Positive | 8 (1.3) | 5 (1.3) | 3 (1.3) | ||
| Negative | 89 (14.7) | 62 (16.2) | 27 (12.1) | ||
| Been in quarantine at follow-up | 108/598 (18.1) | 74/377 (19.6) | 34/221 (15.4) | .1928 | .1808 |
| Screening anti-RBD SARS-CoV-2 antibodies at baseline | .4987 | .2401 | |||
| Negative | 550 (90.6) | 343 (89.6) | 207 (92.4) | ||
| Intermediate | 46 (7.6) | 32 (8.3) | 14 (6.2) | ||
| Positive | 11 (1.8) | 8 (2.1) | 3 (1.4) | ||
| Screening anti-RBD SARS-CoV-2 antibodies at follow-up | .5362 | .2400 | |||
| Negative | 542 (89.3) | 338 (88.3) | 204 (91.1) | ||
| Intermediate | 47 (7.7) | 33 (8.6) | 14 (6.2) | ||
| Positive | 18 (3.0) | 12 (3.1) | 6 (2.7) |
Data are No. (%) unless otherwise specified. Bold values are statistically significant values with P < .05.
Abbreviations: COVID-19, coronavirus disease 2019; PPE, personal protective equipment; RBD, receptor-binding domain; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aHigh-risk group that tested and treated COVID-19 patients includes emergency, infectious diseases, and intensive care unit departments at Haukeland University Hospital, Haraldsplass Deaconess Hospital, and Bergen Municipality Emergency Room. Low-risk group that did not treat COVID-19 patients includes other clinical departments and laboratories.
bCrude P value was determined by χ 2 test (associations between exposure groups and characteristics) in R.
cAdjusted P value was calculated by generalized linear mixed-effects model including all demographic, clinical, and serological characteristics in R.
Antigens and Viruses
The SARS-CoV-2 RBD and spike proteins were produced and purified as previously described [14]. The live hCoV-19/ Norway/Bergen-01/2020 (GISAID accession ID EPI_ISL_541970) virus was isolated in house from the throat swabs of a Norwegian RT-PCR–confirmed patient and propagated in Vero cells before use in neutralization assays.
Enzyme-linked Immunosorbent Assay
Sera were tested in a 2-step enzyme-linked immunosorbent assay (ELISA) process: screening ELISA for high-throughput detection of RBD-reactive samples followed by a confirmatory spike protein ELISA, with minor modifications [14]. Paired baseline and follow-up sera (diluted 1:100) were tested in duplicates in 96-well plates to detect total immunoglobulins (Sigma-Aldrich) binding to the RBD protein using 3,3´,5,5´-tetramethylbenzidine (TMB; BDbiosciences). A negative control panel of prepandemic sera (n = 128) and a positive control panel of RT-PCR–confirmed COVID-19 patient sera (n = 43) were used to define the negative and positive cutoffs, respectively, based upon the optical density (OD) at 450/620nm (Supplementary Figure 2). Positive (OD > 0.708) or intermediate (OD > 0.430) sera detected by the screening ELISA were further titrated, starting from 1:100, to detect IgG (Sigma-Aldrich) binding to the RBD and spike proteins. A prepandemic sera pool, a hospitalized patient serum, and the human monoclonal antibody reactive to both SARS-CoV-1 and 2 (CR3022) were used as controls. The mean end point titer was calculated for each sample. Positive sera with spike-specific IgG end point titers above 3 standard deviations of the mean of prepandemic negative control pool (>485) were further titrated to detect spike-specific IgM and IgA (Sigma-Aldrich). Samples with undetectable antibodies were assigned an end point titer of 50 for calculation purpose. HCW were defined as seroconverters if they had ≥4-fold increase in antibody titers from baseline to follow-up.
Neutralization Assays
Paired baseline and follow-up sera with positive or intermediate results by RBD screening ELISA were tested in the microneutralization (MN) assay and positive MN samples were further tested in the virus neutralization (VN) assay, performed in a certified biosafety level 3 laboratory using the live hCoV-19/Norway/Bergen-01/2020 virus.
MN Assay
Briefly, sera were serially diluted, starting from 1:20, and mixed with 100 tissue culture infectious dose 50% (TCID50) virus in 96-well plates. The mixtures were incubated for 1 hour at 37°C before transferring to 96-well plates preseeded with Vero cells for 24-hour incubation at 37°C. Cells were fixed and permeabilized with methanol and 0.6% H2O2 and incubated with the anti-SARS-CoV-2 nucleoprotein rabbit monoclonal IgG (Sino Biological), then anti-rabbit biotinylated goat IgG (H+L) (Southern Biotech), extravidin-peroxidase (Sigma-Aldrich) and substrate o-phenylenediamine dihydrochloride (Sigma-Aldrich). The MN titer was the reciprocal of the serum dilution giving 50% inhibition of virus infectivity.
VN Assay
The VN assay was conducted as for the MN assay, except that the serum/virus/cells were incubated for 4–5 days at 37°C. All wells were examined under a microscope for cytopathic effect. The VN titer was determined as the reciprocal of the highest serum dilution giving 100% inhibition of virus infectivity (no cytopathic effect). Titers <20 were assigned a value of 10 for calculation purpose.
Statistics
ELISA end point titers and MN titers were calculated using Prism version 8.4.2 (GraphPad). Demographic, clinical characteristics, and serological data of occupationally exposed groups were examined using χ 2 tests and adjusted for confounding variables in generalized mixed-effect models. The odds ratio (OR) with 95% confidence interval (CI) was calculated for HCW having SARS-CoV-2–specific antibodies. Serological data were log-transformed and compared between time points in mixed-effects models with adjustment for subject variance and confounding variables. All statistical analyses were performed in R version 4.0.2 and visualized in Prism version 8.4.2. P values < .05 were considered statistically significant.
RESULTS
The pandemic period was well defined in Bergen, Norway due to early rigorous centralized RT-PCR testing of suspected COVID-19 cases, with the first detection of confirmed cases on 28 February 2020, providing a unique opportunity to study the impact of the pandemic on HCW.
Study Population
HCW (n = 607, 77.1% female and median age 39 years, range 20–78 years) were enrolled from Bergen’s main health care institutions testing and treating COVID-19 patients, including 286 nurses (47.1%) and 174 physicians (28.7%) (Table 1). Recruitment started from 6 March 2020 before the first hospitalizations (9 March 2020) and the first death (23 March 2020) from COVID-19 (Figure 1A). HCW were followed up throughout the first pandemic wave in Bergen (6–10 weeks).
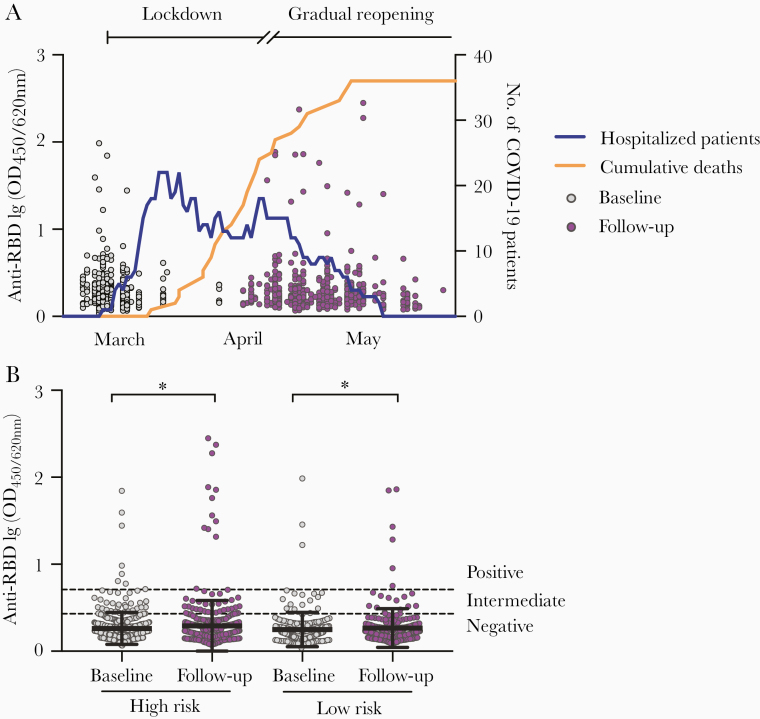
Figure 1.
Screening for SARS-CoV-2 RBD-specific antibodies in HCW before and after COVID-19 patient admissions. A, HCW (n = 607) were recruited between 6 March 2020 and 9 April 2020, and followed up after 6–10 weeks. Each circle represents 1 HCW and their anti-RBD antibodies measured in the screening ELISA as OD at 450/620 nm (left y-axis). The numbers of hospitalized COVID-19 patients (blue line) and cumulative deaths (orange line) in Bergen, Norway are plotted on the right y-axis. Lockdown was initiated in Norway on 12 March 2020 and a gradual reopening starting on 20 April 2020. B, HCW were grouped into high risk (testing facility, COVID-19–designated wards, and intensive care unit wards) and low risk (no known exposure to COVID-19 patients) of occupational exposure to SARS-CoV-2 according to their working department and information in their case report forms. Dotted lines are cutoffs for negative screening results (OD < 0.430) and positive screening results (OD ≥ 0.708) (see Supplementary Figure 1 for further information). Horizontal lines represent mean with standard deviation. OD values were log-transformed and compared between time points in mixed-effects models with adjustment for subject variation, age, sex, and other relevant demographic factors. *P < .05. Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; HCW, health care workers; OD, optical density; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
HCW were grouped by their occupational exposure: high-risk (n = 383, 63.1%) working at the testing facility or on COVID-19–designated wards, and low-risk (n = 224, 36.9%) with no COVID-19 patient exposure (Table 1). We found no significant differences in age, sex, or recent travel history between the 2 groups, although there were more doctors and nurses in the high-risk (85.1%) than the low-risk group (59.8%) (P = .035). HCW with COVID-19–like illness were prioritized for RT-PCR testing and 97 (16%) HCW were tested during the follow-up period. Only 8 HCW tested positive for SARS-CoV-2 (5 high-risk and 3 low-risk HCW).
Serology Results
The SARS-CoV-2 virus attaches to the host cells through RBD on its spike protein, therefore antibodies binding to these proteins have the potential to block viral entry. Spike- and RBD-specific antibodies were used in this study to define infection, as they do not cross-react with other human coronaviruses [14].
RBD- and Spike-Specific Antibodies
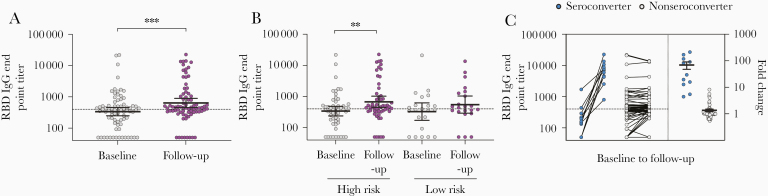
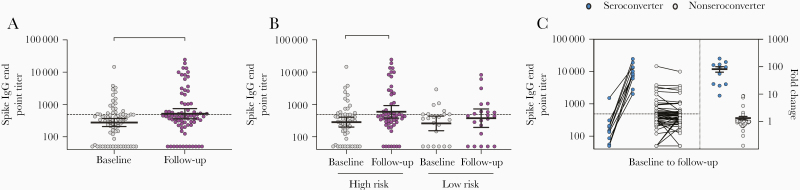
Using screening ELISA, we found that the majority of HCW had undetectable antibodies to RBD before and after the first pandemic wave (90.6% at baseline and 89.3% at follow-up; Table 1 and Figure 1A), although the RBD-binding antibody OD values were significantly higher at follow-up than at baseline in both high-risk (P = .027) and low-risk (P = .034) groups (Figure 1B). The RBD-specific IgG levels were measured for HCW with positive or intermediate results by screening ELISA (n = 76, 12.5%), which were also confirmed by spike protein IgG ELISA (Supplementary Figure 3A and 3B). We observed a significant increase in RBD-specific IgG geometric mean end point titers (GMT) after the first pandemic period (P < .001), from 336 at baseline to 637 at follow-up (Figure 2A). This increase was significant in the high-risk group (P = .002) but not in the low-risk group (Figure 2B). In agreement, a significant increase in spike-specific IgG GMT was observed after the first pandemic wave (P = .002), from 277 at baseline to 518 at follow-up, which was only significant in the high-risk group (P = .012; Figure 3A and 3B and Supplementary Figure 3B). Forty-four HCW (7.2%) had RBD-specific IgG above the positive cutoff of 400; 14 were positive at follow-up (11 seroconverted with ≥4-fold increase and 3 had <2.5-fold increase in titers) and 30 were positive at both baseline and follow-up (Figure 2C). Of these, 32 HCW (5.3%) were confirmed seropositive in the spike IgG ELISA with end point titers above the positive cutoff of 485; 11 seroconverted at follow-up (9/11 in the high-risk group) and 21 were positive at baseline (Figure 3C). Notably, 5 HCW (4/5 in the high-risk group) had >2-fold increase in IgG titers at follow-up but remained below the positive cutoff.
Figure 2.
SARS-CoV-2 RBD-specific IgG antibodies in HCW before and after COVID-19 patient admissions. A, The RBD-specific IgG end point titers were measured for HCW with positive or intermediate RBD screening results (n = 76) by ELISA. B, RBD-specific IgG end point titers in high-risk and low-risk HCW groups. C, HCW were divided into seroconverters (blue circle) who were seropositive and had ≥4-fold increase in IgG titers at follow-up and nonseroconverters (gray circle) who were either seronegative or had <4-fold increase in IgG titers at follow-up. The fold changes are plotted on the right y-axis with horizontal lines representing the mean with standard error of the mean. Dotted lines represent cutoffs for positive results, calculated as 3 standard deviations above the mean of the prepandemic negative sera (RBD IgG end point titer ≥400). Individuals with undetectable antibodies were assigned an end point titer of 50 for plotting and calculation purposes. End point titers were log-transformed and compared between time points in mixed-effects models with adjustment for subject variation, age, sex, and other relevant demographic factors. **P < .01, ***P < .001. Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; HCW, health care workers; IgG, immunoglobulin G; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 3.
SARS-CoV-2 spike-specific IgG antibodies in HCW before and after COVID-19 patient admissions. A, The RBD-specific IgG levels were measured for HCW with positive or intermediate RBD screening results (n = 76), which were confirmed in a confirmatory spike IgG ELISA. B, Spike-specific IgG end point titers in high-risk and low-risk HCW groups. C, HCW were divided into seroconverters (blue circle) who were seropositive and had ≥4-fold increase in IgG titers at follow-up and nonseroconverters (gray circle) who were either seronegative or had <4-fold increase in IgG titers at follow-up. The fold changes are plotted on the right y-axis with horizontal lines representing the mean with standard error of the mean. Dotted lines represent cutoffs for positive results, calculated as 3 standard deviations above the mean of the prepandemic negative sera (spike IgG end point titer ≥485). Individuals with undetectable antibodies were assigned an end point titer of 50 for plotting and calculation purposes. End point titers were log-transformed and compared between time points in mixed-effects models with adjustment for subject variation, age, sex, and other relevant demographic factors. *P < .05, **P < .01. Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; HCW, health care workers; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
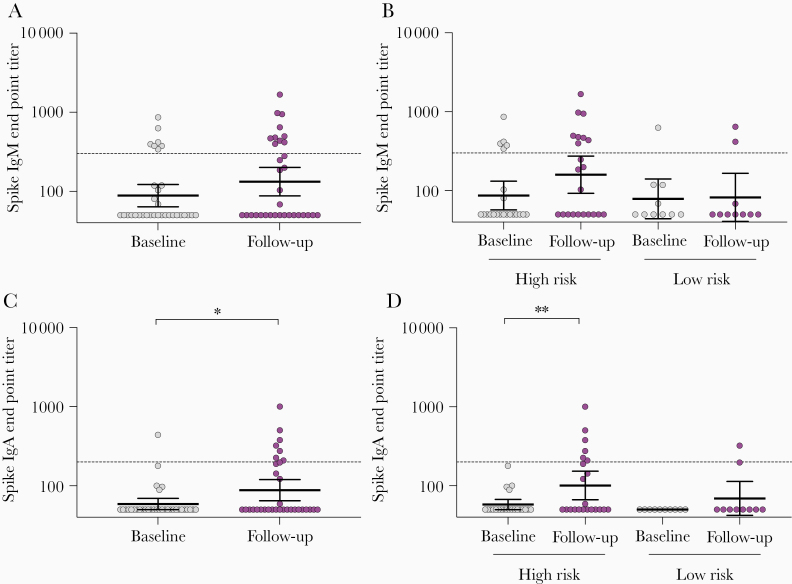
The spike-specific IgM and IgA antibody levels were measured in the 32 IgG-seropositive HCW (Figure 4A–4D and Supplementary Figure 4A–4D). An increase in both spike-specific IgM and IgA GMT was observed in HCW after the pandemic period, although this was only significant for IgA and high-risk HCW (Figure 4C and 4D). The IgM GMT increased from 87 to 159 (P = .068) and the IgA GMT increased from 58 to 101 (P = .005) from baseline to follow-up in the high-risk group (Figure 4B and 4D). Nine HCW had IgM antibodies above the positive cutoff of 300; 7 seroconverted (≥4-fold increase) at follow-up (6/7 were high-risk HCW) and 2 were seropositive at baseline (Supplementary Figure 4C). Six HCW had IgA antibodies above the positive cutoff of 200; 4 seroconverted (3/4 were high-risk HCW) and 2 had increases in titer but did not seroconvert (<4-fold increase) at follow-up from a negative titer at baseline (Supplementary Figure 4D). Four HCW in the high-risk group who IgG seroconverted had >2-fold increase in either IgM or IgA titers at follow-up but remained below the positive cutoff.
Figure 4.
SARS-CoV-2 spike-specific IgM and IgA antibodies in HCW before and after COVID-19 patient admissions. HCW with positive spike IgG results (n = 32) were further analyzed in spike IgM and IgA ELISA. A and C, Spike-specific IgM and IgA end point titers. B, and D, Spike-specific IgM and IgA end point titers in HCW in high-risk and low-risk groups. Each circle represents 1 HCW (gray baseline and purple follow-up). Horizontal lines represent geometric mean with 95% confidence interval. Dotted lines represent cutoffs for positive results, calculated as 3 standard deviations above the mean of the prepandemic negative sera (IgM end point titer ≥300, IgA end point tire ≥200). Individuals with undetectable antibodies were assigned an end point titer of 50 for plotting and calculation purposes. End point titers were log-transformed and compared between time points in mixed-effects models with adjustment for subject variation, age, sex, and other relevant demographic factors. *P < .05, **P < .01. Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; HCW, health care workers; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Neutralizing Antibodies
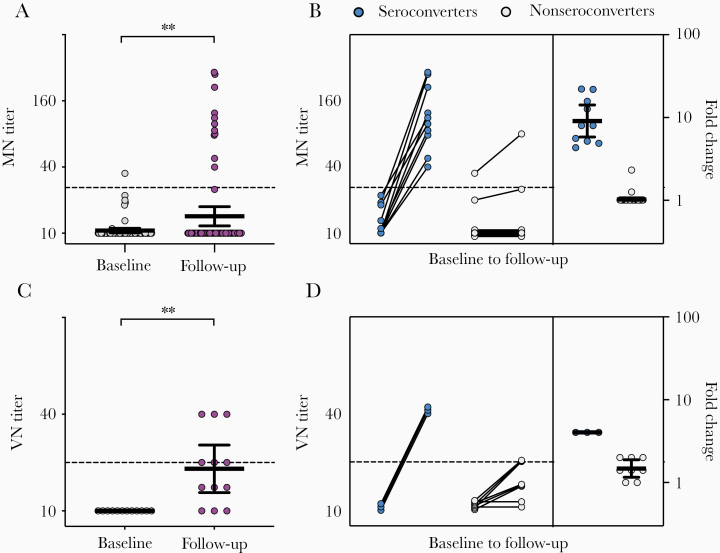
Virus neutralizing antibodies can potentially prevent reinfection with SARS-CoV-2 [17–19]. Therefore, we further assessed in vitro protective immunity against SARS-CoV-2 in HCW with positive or intermediate results by screening RBD ELISA (n = 76) using the MN assay to confirm infection. The MN titers increased significantly from baseline to follow-up (P = .002; Figure 5A). MN antibodies were found in the 11 HCW who IgG-spike seroconverted (titer range 35–291), of whom 10 seroconverted and 1 had 2.3-fold increase in MN titers (Figure 5B).
Figure 5.
SARS-CoV-2 neutralizing antibodies in HCW. A, MN titers of HCW with positive or intermediate SARS-CoV-2 RBD screening results (n = 76). C, Live VN titers of HCW positive with MN antibodies (n = 11). Each circle represents 1 HCW (gray baseline and purple follow-up). Horizontal lines represent geometric mean with 95% confidence interval. HCW were divided into seroconverters (blue circle) who had ≥4-fold increase in (B) MN and (D) VN titers and nonseroconverters (gray circle) who had <4-fold increase in titers. Their respective fold changes in MN and VN titers are plotted on the right y-axis with horizontal lines representing the mean with standard error of the mean. Dotted lines represent positive neutralizing antibody titers of 20. Individuals with undetectable antibodies were assigned a titer of 10 for plotting and calculation purposes. MN and VN titers were log-transformed and compared between time points in mixed-effects models with adjustment for subject variation, age, sex, and other relevant demographic factors. **P < .01. Abbreviations: HCW, health care workers; MN, microneutralization; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VN, virus neutralization.
We further extended our work to investigate the presence of sterilizing immunity that provides complete protection against SARS-CoV-2 infection in vitro in the 11 MN-positive HCW. The VN titers increased significantly from baseline to follow-up (P = .003), of which 6 HCW had VN antibodies (titer range 20–40) and 3 seroconverted with ≥4-fold increase in titer at follow-up (Figure 5C and 5D).
Infection Rates and Risk Factors
The overall SARS-CoV-2 seropositive rate was 5.3% (32/607) by using our 2-step ELISAs. Twenty-one HCW were seropositive at baseline, suggesting previous exposure to SARS-CoV-2 before the study, of which 6 had travelled internationally or treated confirmed/suspected COVID-19 patients before recruitment and were not tested or RT-PCR negative. The remaining 15 HCW did not recall any potential source of infection. Eleven HCW seroconverted during follow-up, indicating recent SARS-CoV-2 infection. Of these, 7 HCW with COVID-19–like symptoms were confirmed by RT-PCR, while 1 was RT-PCR negative and 3 HCW were not tested as they were asymptomatic (Supplementary Table 1). Interestingly, 1 RT-PCR–positive asymptomatic HCW did not develop anti-RBD antibodies. The total infection rate identified by either SARS-CoV-2 RT-PCR or serology testing was 2.0% (12/586, excluding 21 IgG-seropositive HCW at baseline). The infection rates by occupational exposure were 2.4% (9/370) in high-risk and 1.4% (3/216) in low-risk departments (P = .4). The majority of infected HCW in high-risk departments (7/9, 77.8%) were young nurses, aged 23–31 years. Three infected HCW (3/7, 42.9%) reported partial uses of PPE when treating COVID-19 patients. Among 3 infected HCW in low-risk departments, 2 had travelled internationally and 1 had community exposure.
Risk factors for SARS-CoV-2 seropositivity at baseline were recent travel history (OR, 1.8; 95% CI, .5–7.1), contact with confirmed or suspected patients (OR, 1.7; 95% CI, .7–4.3), having COVID-19–like symptoms, that is sore throat (OR, 1.9; 95% CI, .8–4.3) and myalgia (OR, 1.6; 95% CI, .6–4.3), young age (OR, 1.5; 95% CI, .7–3.4), and nursing occupation (OR, 1.3; 95% CI, .7–2.7) (Supplementary Table 2). At follow-up, HCW using partial PPE when treating COVID-19 patients had 2.5-fold higher odds (95% CI, .5–12.2) of being seropositive than HCW with no COVID-19 patient exposure.
DISCUSSION
During the COVID-19 pandemic, many countries experienced unprecedented increases in hospitalizations, overwhelming their health care systems, and rapidly depleting supplies of PPE, resulting in thousands of HCW infections and deaths [4, 20, 21]. In the UK, Spain, and the Netherlands, 6%–38% of HCW tested positive by RT-PCR [20, 22, 23], some requiring hospitalization and intensive care unit treatment [22], although most infections were community acquired. Here, we show a considerably lower SARS-CoV-2 infection rate among frontline HCW during the first wave of the pandemic in Bergen, Norway by RT-PCR and serological testing (5.3% overall and 2.0% during follow-up), probably due to low levels of community infection and strict occupational use of PPE. None of the HCW in our study died or were hospitalized. There was little overcrowding of emergency rooms and good compliance with infection prevention and control (IPC) measures. The early lockdown in Norway, implemented on 12 March 2020 before the first national deaths on 16 March 2020, contributed to the low number of community cases and hospitalizations, thus protecting the health care system and its most vital asset, the HCW. Recent modeling studies have shown that the unprecedented lockdowns have dramatically reduced mortality in many countries, preventing 12 000 deaths in Norway [24, 25]. No excess deaths were reported during the first pandemic wave in Norway, highlighting the success of the rapid deployment of effective public health responses. The Norwegian experience with early lockdown is especially important when compared to countries without or with delayed lockdown, which report higher infection and mortality rates in the community and HCW [4, 20, 21].
Several countries reported higher rates of SARS-CoV-2 infection among HCW treating COVID-19 patients, up to 11.6-fold increased risk, compared to other HCW or the community [5, 8, 26, 27]. In agreement, we found that the infection rate was 1.7-fold higher in HCW with COVID-19 patient exposure (2.4%) than in HCW with no exposure (1.4%), although not statistically significant. Unlike many other countries, Norway did not experience shortages of PPE, although the stockpile was alarmingly low. Only 1.7% (10/605) of our HCW reported occupational exposure without PPE to 1–2 COVID-19 patients and/or contact with an infected colleague; none of whom were positive by SARS-CoV-2 RT-PCR or serological assays. However, 42.9% (3/7) of seroconverted HCW reported having used partial PPE when treating ≥1 COVID-19 patients. HCW using partial PPE had 2.5-fold higher odds of being SARS-CoV-2 seropositive than HCW without patient contact. Nurses have a greater occupational exposure than other medical staff. Although we cannot exclude community infection, Norway had low levels of community spread and one of the highest levels of RT-PCR testing in Europe. In the UK, 48% of RT-PCR–positive HCW were nurses [23], while we found 77.8% (7/9) of seroconverted HCW treating COVID-19 patients were young nurses (23–31 years old). Indeed, working as a young nurse had higher odds of being SARS-CoV-2 seropositive, perhaps due to less experience in IPC measures. Better training in IPC for junior staff and ensuring adequate availability of PPE in the future will help to prevent the spread of SARS-CoV-2 in health care settings.
Serological assays can determine SARS-CoV-2 infection over a longer time period than RT-PCR, which can only detect acute infection. In our study, only 8 HCW tested positive by RT-PCR, one of whom had no symptoms but was tested due to recent international travel. In contrast, serological assays identified infection in 32 HCW, of whom 11 HCW seroconverted after the first COVID-19 pandemic wave, indicating recent infection. Seven seroconverted HCW had COVID-19–like symptoms and infection confirmed by RT-PCR; however, 4 seroconverted HCW (36.4%) were asymptomatic and either were RT-PCR negative or not tested. Similarly, other seroprevalence studies also reported asymptomatic and/or not RT-PCR–tested individuals who were positive by SARS-CoV-2 serology testing [6, 13, 28, 29]. In asymptomatic cases, T-cell immunity may control SARS-CoV-2 infection in the absence of antibodies. The role of asymptomatic HCW in SARS-CoV-2 transmission is not clear, particularly in nosocomial transmission to vulnerable patients and other HCW, with some studies reporting similar viral loads [30] and others reporting less infectious virus [31] found in asymptomatic than in symptomatic cases. Combined RT-PCR and serology testing is crucial to determine infection spread in the health care settings and improve IPC.
Importantly, virus neutralizing antibodies can potentially prevent reinfection with SARS-CoV-2 [17–19]. We developed neutralization assays to investigate protective antibodies against the live SARS-CoV-2 virus in HCW, which most studies have not reported [3–11, 13, 20–22, 26, 32–41]. We showed that 11 recently infected HCW had neutralizing antibodies, 6 of them had sterilizing immunity in vitro, which could provide protection from reinfection by SARS-CoV-2, perhaps during the second wave of the pandemic. Neutralizing antibodies were not detected in 21 HCW who were infected before the study with unknown time of infection. One explanation could be the change in the spike protein of SARS-CoV-2 viruses. In March 2020, a variant strain has replaced the previous SARS-CoV-2 originally isolated in Wuhan, China [42]. The 21 HCW infected before the study recruitment in March probably were exposed to the strain closer to the Wuhan isolate, which may not be recognized by our neutralization assays using the more recently isolated Norwegian strain. Recent reinfection of a previously confirmed SARS-CoV-2 infection highlights the importance of measuring neutralizing antibodies [43]. Future studies should investigate the correlates of protection of SARS-CoV-2 neutralizing antibodies against reinfection and its longevity to aid future COVID-19 vaccine development.
Home quarantine was introduced in Norway in late February for people with mild COVID-19 infection and their family members, HCW with close contact to COVID-19 patients without proper PPE, and travelers returning from countries with SARS-CoV-2 infection. Norway had good adherence with household quarantine, which was covered by statutory sick pay. Limitations in initial testing capacity led to 18% of our HCW being quarantined during the study, which reduced the available workforce and increased the workload for the remaining HCW. As increased RT-PCR testing became available, there was a lower threshold for testing of HCW with COVID-19–like symptoms and consequently reduced staff absenteeism.
The advantages of our prospective cohort study are the early recruitment of HCW before the first pandemic wave and prior to COVID-19 patient exposure, the use of stringent serological assays to define infection, and the investigation of neutralizing antibodies to assess protection from reinfection. Our moderate patient burden may have resulted in limited exposure but ensured correct characterization of HCW exposure as patients were only hospitalized on COVID-19–designated wards. The low infection rates in Norwegian HCW limited the statistical power of our study, thus the OR estimates should be interpreted with caution. Although the relevance of neutralizing antibodies using Vero cell-based in vitro neutralization assays for correlates of protection remains to be determined, neutralizing antibody titers correlated with in vivo protection after SARS-CoV-2 challenge of DNA-vaccinated rhesus macaque [44]. Our findings emphasize the importance of good IPC systems in health care facilities to isolate patients with suspected infection to reduce risk of exposure, and ensure HCW have suitable training and access to PPE.
Ensuring the safety of HCW and protecting them from infection, reinfection, and further transmission is one of the most important measures to sustain health care services during a pandemic. RT-PCR and serological testing of HCW are crucial to prevent infection within the hospital, as the tests complement each other. Our data document low infection rates in Norwegian frontline HCW, where the moderate burden of patients enabled rational patient management and compliance with IPC measures. Neutralizing antibodies were found in all infected HCW who seroconverted, which may provide protection against reinfection, perhaps during the future waves of the pandemic. HCW are vital for managing the ongoing outbreak and can be protected through timely national or local control of the outbreak, access to PPE, and through training in IPC. There is an urgent need to understand the most effective measures to protect HCW during this pandemic and our study highlights the importance of early control measures to protect society and the HCW.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Bergen COVID-19 research group members: Bård Kittang, Dagrunn Waag Linchausen, Håkon Amdam, Therese Bredholt Onyango, Geir Bredholt, Nina Ertesvåg, Sarah Lartey, Helene Heitmann Sandnes, Fredrik Grøvan, Hauke Bartsch, Heidi Syre, Francisco Real, and Åse Garløv Berg.
Acknowledgment. We thank all of the health care workers at Bergen Municipality, Haukeland University Hospital and Haraldsplass Deaconess Hospital for their altruistic participation in the study.
Financial support. This work was supported by the Helse Vest (grant number F-11628); the Trond Mohn Foundation (TMS2020TMT05); the Ministry of Health and Care Services, Norway; The Research Council of Norway Globvac (grant number 284930); the European Union (grant numbers EU IMI115672 FLUCOP and H2020 874866 INCENTIVE); the Faculty of Medicine, University of Bergen, Norway; and Nanomedicines Flunanoair ERA-NETet EuroNanoMed2 (grant number JTC2016).
Potential conflicts of interest. F. K. reports an ELISA assay used to screen for seroconversion was developed in his laboratory. Mount Sinai has filed patent applications to protect that assay and has licensed its use to several companies, and is also commercializing the assay. All other authors report no potential conflict of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Bergen COVID-19 Research Group:
Bård Kittang, Dagrunn Waag Linchausen, Håkon Amdam, Therese Bredholt Onyango, Geir Bredholt, Nina Ertesvåg, Sarah Lartey, Helene Heitmann Sandnes, Fredrik Grøvan, Hauke Bartsch, Heidi Syre, Francisco Real, and Åse Garløv Berg
References
- 1. World Health Organization. Timeline of WHO’s response to COVID-19 https://www.who.int/news-room/detail/29-06-2020-covidtimeline. Accessed 30 July 2020.
- 2. Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA 2020; 323:1335. [DOI] [PubMed] [Google Scholar]
- 3. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet 2020; 395:1225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lai X, Wang M, Qin C, et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open 2020; 3:e209666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen LH, Drew DA, Graham MS, et al. ; Coronavirus Pandemic Epidemiology Consortium Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 2020; 5:e475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol 2020; 128:104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia-Basteiro AL, Moncunill G, Tortajada M, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun 2020; 11:3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mansour M, Leven E, Muellers K, Stone K, Mendu DR, Wajnberg A. Prevalence of SARS-CoV-2 antibodies among healthcare workers at a tertiary academic hospital in New York City. J Gen Intern Med 2020; 35:2485–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA 2020; 324:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook T, Kursumovic E, Lennane S. Exclusive: deaths of NHS staff from COVID-19 analysed. Health Service Journal 22. April 2020. [Google Scholar]
- 11. Gao W, Sanna M, Tsai MK, Wen CP. Geo-temporal distribution of 1,688 Chinese healthcare workers infected with COVID-19 in severe conditions-a secondary data analysis. PLoS One 2020; 15:e0233255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Guardian. Lost on the frontline https://www.theguardian.com/us-news/series/lost-on-the-frontline. Accessed 11 August 2020.
- 13. Chen Y, Tong X, Wang J, et al. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect 2020; 81:420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 2020; 26:1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Folkehelseinstituttet. Statistikk om koronavirus og covid-19 https://www.fhi.no/sv/smittsomme-sykdommer/corona/dags--og-ukerapporter/dags--og-ukerapporter-om-koronavirus/. Accessed 11 August 2020.
- 16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Addetia A, Crawford KH, Dingens A, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with high attack rate. Clin Microbiol 2020; 58:e02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deng W, Bao L, Liu J, et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 2020; 369:818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med 2020; 383:1544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kluytmans-van den Bergh MFQ, Buiting AGM, Pas SD, et al. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open 2020; 3:e209673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Houlihan CF, Vora N, Byrne T, et al. ; Crick COVID-19 Consortium; SAFER Investigators Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet 2020; 396:e6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suárez-García I, Martínez de Aramayona López MJ, Sáez Vicente A, Lobo Abascal P. SARS-CoV-2 infection among healthcare workers in a hospital in Madrid, Spain. J Hosp Infect 2020; 106:357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keeley AJ, Evans C, Colton H, et al. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS foundation trust in the United Kingdom, March 2020. Euro Surveill 2020; 25:2000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flaxman S, Mishra S, Gandy A, et al. ; Imperial College COVID-19 Response Team Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 2020; 584:257–61. [DOI] [PubMed] [Google Scholar]
- 25. Hsiang S, Allen D, Annan-Phan S, et al. The effect of large-scale anti-contagion policies on the COVID-19 pandemic. Nature 2020; 584:262–7. [DOI] [PubMed] [Google Scholar]
- 26. Moscola J, Sembajwe G, Jarrett M, et al. ; Northwell Health COVID-19 Research Consortium Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA 2020; 324:893–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ran L, Chen X, Wang Y, Wu W, Zhang L, Tan X. Risk factors of healthcare workers with corona virus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis 2020; 71:2218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. ; Karolinska COVID-19 Study Group Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020; 183:158–68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea [published online ahead of print 6 August 2020]. JAMA Intern Med doi: 10.1001/jamainternmed.2020.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perera RAPM, Tso E, Tsang OTY, et al. SARS-CoV-2 virus culture and subgenomic RNA for respiratory specimens from patients with mild coronavirus disease. Emerg Infect Dis 2020; 26:2701–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. The Lancet. COVID-19: protecting health-care workers. Lancet 2020; 395:922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pallett SJC, Rayment M, Patel A, et al. Serological assays for delayed SARS-CoV-2 case identification—author’s reply. Lancet Respir Med 2020; 8:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hunter E, Price DA, Murphy E, et al. First experience of COVID-19 screening of health-care workers in England. Lancet 2020; 395:e77–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife 2020; 9:e58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin C, Montesinos I, Dauby N, et al. Dynamics of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk healthcare workers and hospital staff. J Hosp Infect 2020; 106:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Psichogiou M, Karabinis A, Pavlopoulou ID, et al. Antibodies against SARS-CoV-2 among health care workers in a country with low burden of COVID-19. PLOS ONE 2020;15:e0243025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chatterjee P, Anand T, Singh KJ, et al. Healthcare workers and SARS-CoV-2 infection in India: a case-control investigation in the time of COVID-19. Indian J Med Res 2020; 151:459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis 2020; 20:1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sikkema RS, Pas SD, Nieuwenhuijse DF, et al. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Infect Dis 2020; 20:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeng C, Evans JP, Pearson R, et al. Neutralizing antibody against SARS-CoV-2 spike in COVID-19 patients, health care workers, and convalescent plasma donors. JCI Insight 2020; 5:e143213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020; 182:812–27.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. To KK, Hung IF, Ip JD, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing [published online ahead of print 25 August 2020]. Clin Infect Dis doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020; 369:806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.