Abstract
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel pandemic virus. Mounting evidence supports the possibility of vertical transmission, which at the present time appears to be rare. We report a newborn with vertically acquired SARS-CoV-2 who developed acute respiratory failure and received remdesivir and coronavirus disease 2019 convalescent plasma.
Keywords: coronavirus, COVID-19, newborn, remdesivir, SARS-CoV-2, treatment, vertical transmission
There is growing evidence to support the possibility of vertical transmission of severe acute respiratory syndrome coronavirus 2 (SAR-CoV-2). For those neonates who have coronavirus disease 2019 (COVID-19), there is little evidence to guide treatment. We report a neonate with vertically acquired SARS-CoV-2 who developed acute respiratory failure and received remdesivir and COVID-19 convalescent plasma.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel pandemic virus. Mounting evidence supports the possibility of vertical transmission, which at the present time appears to be rare [1–3]. We report a newborn with vertically acquired SARS-CoV-2 who developed acute respiratory failure and received remdesivir and coronavirus disease 2019 (COVID-19) convalescent plasma.
CASE PRESENTATION
A 15-year-old primigravida woman presented at 40 weeks of gestation with contractions and amniotic fluid leakage. Maternal routine prenatal labs were negative, including human immunodeficiency virus and syphilis. At our institution, all pregnant women are tested for SARS-CoV-2 upon admission to labor and delivery. Her nasopharyngeal (NP) swab tested positive for SARS-CoV-2 by isothermal amplification (Abbott ID NOW, Abbott Park, IL). She was diagnosed with asymptomatic COVID-19; however, 5 days later, she developed anosmia. Rupture of membranes occurred 29 hours prior to delivery and fever 3 hours prior to delivery. She did not receive intrapartum antibiotics.
A female infant was delivered vaginally with a birth weight of 3936 g; the delivery was uncomplicated. Apgar scores were 8 and 9 at 1 and 5 minutes of life, respectively. The infant did not contact the parents in the delivery room and was immediately bathed. She was well appearing and the remainder of vital signs were normal. A blood culture was obtained and vital signs were closely monitored. The infant roomed in with the mother with the open bassinet situated 6 feet apart except when feeding. The mother attempted breastfeeding during the first day. When breastfeeding, mothers are instructed to wash their hands and breasts prior to each feed and wear a level 3 mask. The mother wore a level 3 American Society for Testing and Materials (ASTM) surgical mask at all times during the hospital stay. Both patients were under strict contact and enhanced droplet isolation per hospital protocol.
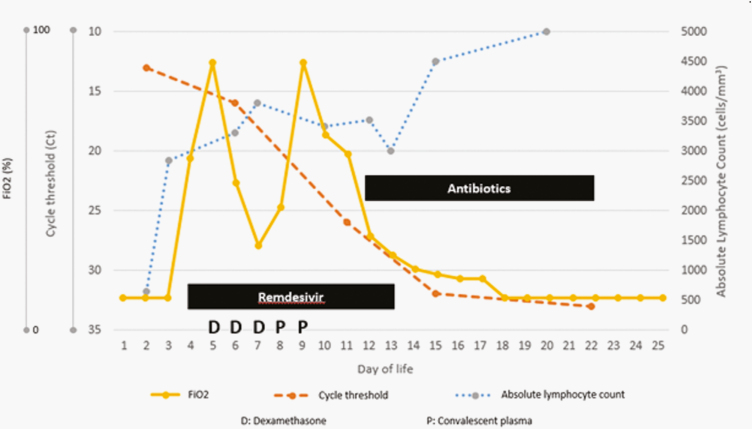
SARS-CoV-2 testing was obtained on the infant per hospital protocol at 24 hours of life. The infant’s NP swab tested positive for SARS-CoV-2 at 24 hours of life by reverse-transcriptase-polymerase chain reaction (RT-PCR) with a cycle threshold (Ct) of 13 (Cobas SARS-CoV-2, Roche Diagnostics Corporation, Indianapolis, IN) (Figure 1). At 25 hours of life, she was febrile (temperature 39.4°C), tachycardic, and tachypneic. Another blood culture was obtained and she was started on intravenous ampicillin and gentamicin. Chest radiograph was normal. Given her increased work of breathing, the infant received nasal continuous positive airway pressure (CPAP). Supplemental oxygen was added but quickly weaned to room air. Cerebrospinal fluid (CSF) profile was normal (Supplementary Material). A comprehensive metabolic panel, complete blood count with differential, and coagulation screen were obtained. Notably, she had hypoglycemia with serum blood glucose 36 mg/dL (2 mmol/L), corrected with total parenteral nutrition (TPN) adjustments. The white blood cell (WBC) count was 21.2 × 109/L, and the absolute lymphocyte count was 636 cells/uL. She had a mildly elevated partial thromboplastin time with normal prothrombin time, international normalized ratio, and fibrinogen. Blood cultures and CSF culture were negative after 48 hours, so antibiotics were discontinued.
Figure 1.
Clinical course of an infant with severe acute respiratory syndrome coronavirus-2 pneumonia treated with remdesivir and coronavirus disease 2019 convalescent plasma. Convalescent plasma was administered in 2 separate infusions to assess for tolerability and dose escalation. Nafcillin and vancomycin were given for 10 days to treat Staphylococcus lugdunensis pneumonia.
On day of life (DOL) 4, the infant had persistent desaturations to Oxygen saturation (SpO2) 88%, so she was placed on supplemental oxygen and increased CPAP support. A chest radiograph revealed prominent bilateral perihilar interstitial markings. Remdesivir was started under compassionate use with informed consent from both parents with a loading dose of 5 mg/kg and continued daily at 2.5 mg/kg for 10 days. Therapy was intended for 5 to 10 days but given the infant’s persistence of symptoms during therapy and elevated SARS-CoV-2 Ct values at day 5, we extended it for 10 days. On DOL 5, the infant had acute respiratory failure requiring intubation, so intravenous dexamethasone 0.15 mg/kg every 24 hours was added for 3 days. On DOL 6, she required increasing ventilator support and had episodes of bradycardia. Of note, she was receiving dexmedetomidine at the time of sedation. An electrocardiogram showed prolonged Corrected QT interval (QTc) 484 milliseconds, an echocardiogram revealed normal anatomy and no evidence of pulmonary hypertension, and serum troponin was 0.03 ng/mL. Repeat chest radiograph revealed prominent interstitial markings. An endotracheal tube aspirate was without WBCs and culture grew Staphylococcus lugdunensis. On DOL 7, serum SARS-CoV-2 Immunoglobulin G (IgG) N protein test was negative, and serum SARS-CoV-2 RT-PCR Ct value was 34.7, consistent with detection at low limits.
She deteriorated requiring increased Positive end-expiratory pressure (PEEP) and FiO2, so convalescent COVID-19 plasma was administered under compassionate use on DOL 8. After parental consent, she received 10 mL/kg and then 15 mL/kg 24 hours later. On DOL 19, she continued to decompensate, and a new tracheal aspirate was obtained, which grew methicillin-susceptible Staphylococcus aureus. We decided to treat both recovered staphylococci with vancomycin and nafcillin for a total of 10 days. Bradycardia resolved and QTc was normal on repeat Electrocardiogram (ECG). She was kept intubated and ventilated for a total of 13 days and was on positive pressure support including nasal CPAP for a total of 30 days. She was eventually weaned off respiratory support, where she remains in room air. Daily serum creatinine and transaminases remained normal while on remdesivir. She developed the late onset cholestatic jaundice with direct bilirubin 5.6 mg/dL and gamma glutamyltransferase 105 units/L thought due to TPN, which she received for 28 days. Repeated NP swabs for SARS-CoV-2 demonstrated increasing Ct values (Figure 1).
DISCUSSION
We report a case of presumed vertically acquired COVID-19 in an infant born to an asymptomatic SARS-CoV-2-positive mother. This infant’s respiratory distress leading to respiratory failure was most likely due to COVID-19, as other causes of neonatal sepsis were ruled out and an initial normal chest X-ray. With a reported incubation period of 2–14 days [4], postnatal SARS-CoV-2 transmission was considered unlikely.
There are now a few case reports of suspected vertical transmission of COVID-19 [1–3]. A study of mothers with COVID-19 reported 2 infants with SARS-CoV-2 Immunoglobulin M (IgM) after birth, suggesting in utero infection since IgM is not transferred across the placenta. Of note, 5 infants in the study had positive SARS-CoV-2 IgG, which may have been of maternal origin [1]. Our infant did not have IgM tested; SARS-CoV-2 IgG was negative on DOL 7. The absence of SARS-CoV-2 IgG may suggest the mother was in the early phase of COVID-19; unfortunately, maternal SARS-CoV-2 IgG was not tested. Viremia is usually highest in the first few days of SARS-CoV-2 infection [5]. We speculate that a high level of maternal viremia may have resulted in fetal infection.
Vivanti et al [2] demonstrated placental tissue positive by RT-PCR for SARS-CoV-2, immunostaining, and histology consistent with SARS-CoV-2 infection. They postulated that neonatal viremia occurred following placental infection given that infant blood and bronchoalveolar lavage fluid were positive for SARS-CoV-2 by RT-PCR [2]. Sisman et al [3] reported an infant with SARS-CoV-2 in NP samples as well as placental tissue by immunohistochemistry and electron microscopy, which strongly suggested transplacental transmission. We were unable to test the placenta or amniotic fluid for SARS-CoV-2. Infection prevention practices were consistent with Centers for Disease Control and Prevention (CDC) guidance and with those reported by Salvatore et al [6], where none of the 80 newborns from SARS-CoV-2-positive mothers acquired infection. In light of this evidence gathered by others and the clinical presentation of our infant, with limited contact with her mother and development of symptoms at 25 hours of life, we conclude that our patient most likely acquired COVID-19 by vertical transmission.
Treatment decisions were guided by the National Institutes of Health (NIH) treatment guidelines for COVID-19 [7] and the best available evidence [8–10]. The benefits and risks of each treatment were carefully weighed given the clinical deterioration of our patient and the paucity of evidence available in treating neonates with COVID-19. We decided to use remdesivir once the infant required supplemental oxygen, which is the strongest indication for remdesivir [7]. Han et al [9] recently described the viral kinetics in an infected newborn with viral shedding for 11 days. Our patient had prolonged detection with a progressive decline in viral load. Remdesivir therapy likely contributed to the decreased viral load, though we cannot speculate to what degree as the natural progression of viral load is to decrease over time. We opted to use remdesivir for 10 days to support the putative immature immunity with a direct antiviral agent [10]. We monitored serum creatinine and transaminases and they were within normal limits for age throughout treatment with remdesivir, which was tolerated well.
After intubation, the infant was started on dexamethasone, which at the time showed a marginal benefit in mechanically ventilated patients [7]. After 3 doses of dexamethasone, the infant was not showing clinical improvement, so it was stopped due to its high side-effect profile and the theoretical disadvantages of using corticosteroids in the setting of an acute viral infection in an infant with immature cellular immunity. We were especially concerned with the possibility of delayed viral clearance that has been described in patients with other causes of viral pneumonia that received corticosteroids [8].
With ongoing clinical deterioration and absent specific IgG, we decided to give her COVID-19 convalescent plasma with the goal of providing neutralizing antibodies, thereby suppressing viral replication and possibly modifying the host immune response. It has shown to have a modest beneficial effect in improving oxygen requirement and improving survival, while being overall safe [7]. Our patient tolerated the plasma transfusions well without evidence of adverse events. Notwithstanding, we did not observe an immediate improvement as it has been described in adult patients with severe respiratory COVID-19.
We must comment on the possible role of staphylococcal superimposed bacterial pneumonia. The initial sputum culture with S. lugdunensis was thought to be secondary to respiratory colonization given the lack of WBCs in the sample and chest X-ray with diffuse interstitial infiltrates. A repeat sputum culture after reintubation was positive for Methicillin-susceptible Staphylococcus aureus (MSSA). Due to her ongoing respiratory deterioration, we initiated antibiotics. It is difficult to discern the role of bacterial pneumonia and its treatment played in interpreting this infant’s clinical course and response to COVID-19 targeted therapy.
It is unclear whether the use of treatments for acute COVID-19 infection was of benefit; however, it is reassuring that we did not observe any adverse effects of these therapies. Studies to guide treatment in infants with congenital or neonatal COVID-19 are urgently needed.
Supplementary Material
Note
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 penumonia. J Am Med Assoc 2020; 323:1848–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vivanti A, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection [published online ahead of print July 14, 2020]. Nat Commun doi: 10.1038/s41467-020-17436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sisman J, Jaleel M, Moreno W, et al. Intrauterine transmission of SARS-CoV-2 infection in a preterm infant [published online ahead of print July 10, 2020]. Pediatr Infect Dis J doi: 10.1097/INF.0000000000002815 [DOI] [PubMed] [Google Scholar]
- 4. Rose D, Piersigilli F, Ronchetti M, et al. Novel coronavirus disease (COVID-19) in newborns and infants: what we know so far. Ital J Pediatr 2020; 46:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection – a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020; 9:727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salvatore CM, Han JY, Acker KP, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health 2020; 4:721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coronavirus disease 2019 (COVID-19) treatment guidelines Bethesda, MD: National Institutes of Health; Accessed July 16, 2020 https://www.covid19treatmentguidelines.nih.gov/ [PubMed] [Google Scholar]
- 8. Sanders J, Monogue M, Jodlowski T, et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19). J Am Med Assoc 2020; 323:1824–36. [DOI] [PubMed] [Google Scholar]
- 9. Han M, Seong M, Heo E, et al. Sequential analysis of viral load in a neonate and her mother infected with severe acute respiratory syndrome coronavirus 2 [published online ahead of print April 9, 2020]. Clin Infect Dis doi: 10.1093/cid/ciaa447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [published online ahead of print April 9, 2020]. N Engl J Med doi: 10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.