Abstract
Emergence of a new spike protein variant (D614G) with increased infectivity has prompted many to analyze its role in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. There is concern regarding whether an individual exposed to one variant of a virus will have cross-reactive memory to the second variant. Accordingly, we analyzed the serologic reactivity of both variants, and we found that antibodies from 88 donors from a high-incidence population reacted toward both the original spike and the D614 spike variant. These data suggest that patients who are exposed to either variant have cross-responsive humoral immunity. This represents an important finding both for SARS-CoV-2 disease biology and for therapeutics.
Keywords: SARS-CoV-2, coronavirus, spike protein, D614G, pandemic, COVID-19
As the D614G spike variant emerged as the main severe acute respiratory syndrome coronavirus 2 variant in the United States, concerns arose regarding possible differences in antibody binding and serologic assay performance. We show that antibodies cross-react with both variants.
The need to understand the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has led to the need to understand the rate of infection and acquisition of immunity in the community. The human immune system responds to SARS-CoV-2 infection through a variety of cellular and humoral effectors, including antibodies produced by B cells. Immune antibodies raised against SARS-CoV-2 have been detected that recognize multiple SARS-CoV-2 proteins, including nucleocapsid (N), envelope (E), and spike (S) proteins. Antibodies are highly specific for presented epitopes, and mutations in viruses can lead to reductions in immune responses based on acquired immunity to prior viral exposure [1], or even during the course of a single infection (so-called viral escape).
It was recently demonstrated by Korber et al [2] through variant tracking that the original SARS-CoV-2 genome spike protein sequence has been supplanted by a changed amino acid at position 614, from D614 to G614. Furthermore, evidence suggests that the emergent (and now dominant) G614 virus is more infectious, but a great deal remains to be elucidated, including a patient’s potential to be infected by both variants at once [3].
The SARS-CoV-2 spike protein is an outward-facing homotrimer presented on the surface of the nucleocapsid that mediates binding to the host cell’s angiotensin-converting enzyme 2. To test for the presence of antibodies against SARS-CoV-2, we and others have developed enzyme-linked immunosorbent assay (ELISA)–based seroassays. Our protocol uses 2 types of recombinant primary antigens—full-spike ectodomain protein (SARS-CoV-2 S2P) and receptor-binding domain protein—as the primary antigens in separate assays for immunoglobulin (Ig) G, IgA, and IgM levels [4]. The spike 614 position is not within the receptor-binding domain (although it is represented in full-spike ectodomain constructs), and reported seroassays (including ours) are based on the originally observed aspartic acid at position 614 (D614) [5].
The consequence of using D614 spike domain in seroassays is that it could affect the specificity of these assays, given the likelihood that most infections in the United States are now occurring with the G614 variant of SARS-CoV-2. Knowledge of cross-reactivity is essential to interpreting serosurveys and clinical antibody tests [6]. As serosurveys are well underway, we sought urgently to clarify whether serum from recovering/convalescent donors was cross-reactive to both forms of spike protein. In the current study, we generated a G614 full-spike ectodomain construct and incorporated this protein as antigen in an ELISA. This spike G614-based assay was compared with the original D614-based assay, and a set of 88 positive samples from a hard-hit (high-incidence) community were applied to both assays.
MATERIALS AND METHODS
ELISA Methods
ELISAs were performed as described elsewhere [4]. Briefly, plates are coated with 1-µg/mL full-spike ectodomain trimer (D614G) in 1× phosphate-buffered saline (PBS) overnight at 4ºC. Plates are washed 3 times with PBST (1× PBS + 0.05% Tween 20), then blocked in 5% nonfat dry milk in PBST for 2 hours at room temperature. Plates are washed again 3 times with PBST, and samples are then added at a 1:400 dilution of serum into blocking buffer and incubated for 1 hour at room temperature. Plates are again washed 3 times and then incubated with anti-IgG, IgM, or IgA cross-adsorbed horseradish peroxidase–linked secondary antibody (Thermo Fisher Scientific; 1:4000 in blocking buffer) for 1 hour at room temperature. Plates are washed again, and then 100 µL of tetramethylbenzidine (TMB) substrate (1 Step Ultra TMB Substrate; Thermo Fisher Scientific) is added for 10 minutes before stopping the reaction with 100 µL of stop solution (Thermo Fisher Scientific).
Plates are read on a BioTek Epoch2 plate reader at 450 and 650 nm. Resulting 650-nm reading is subtracted from the 450-nm reading before data analysis using GraphPad Prism software v9. D614G data were compared with wild-type spike data published elsewhere [4]. Assay limits of detection were described using monoclonal recombinant antibodies against the D614 spike protein, as described elsewhere, and were evaluated in comparison with 4 different alphacoronaviruses [4, 6].
Human Serum Samples
All archival pre-2019 clinical samples were used under a clinical protocol (NCT01386424; first posted 1 July 2011 and last updated 8 July 2020) approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board and conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. All participants signed written informed consent before enrollment. All other samples were collected under an institutional review board exemption since these were fully deidentified samples. Eighty-eight convalescent donors were fully deidentified and collected from a high-incidence community in New York and New Jersey between April and May 2020. Sequencing of virus was not completed to evaluate which variant these donors were infected with owing to sample availability and the scope of the study.
RESULTS
To evaluate the ability of antibodies developed during SARS-CoV-2 infection to react against both D614 and G614 variants of the spike protein, we measured serologic reactivity via an ELISA detecting IgG, IgM, and IgA binding to full-spike ectodomain trimers. Soluble spike trimers were produced that contained a protein sequence identical to the original S-2P spike variant [7] except for the addition of the D614G mutation. DNA constructs were generated by synthesis (ATUM) with gene optimization for expression in human cells and were subcloned into a high-yielding mammalian expression vector driven by a strong CMV51 promoter. Proteins were expressed in Expi293 cells and purified as described elsewhere [5].
In these vectors, the yields of both D614 and G614 spike proteins were similar (approximately 8–10 mg/L). Both proteins purified similarly, and no difference in protein behavior was observed with analytical size exclusion chromatography, demonstrating that both proteins equivalently formed the expected trimeric structures. Using the same ELISA conditions described previously, we tested 88 samples from a high-incidence community and 100 prepandemic negative controls (archived before 2019 and sourced from preexisting National Institutes of Health study NCT01386424) and compared them with our previously published data [4] for the original D614 spike.
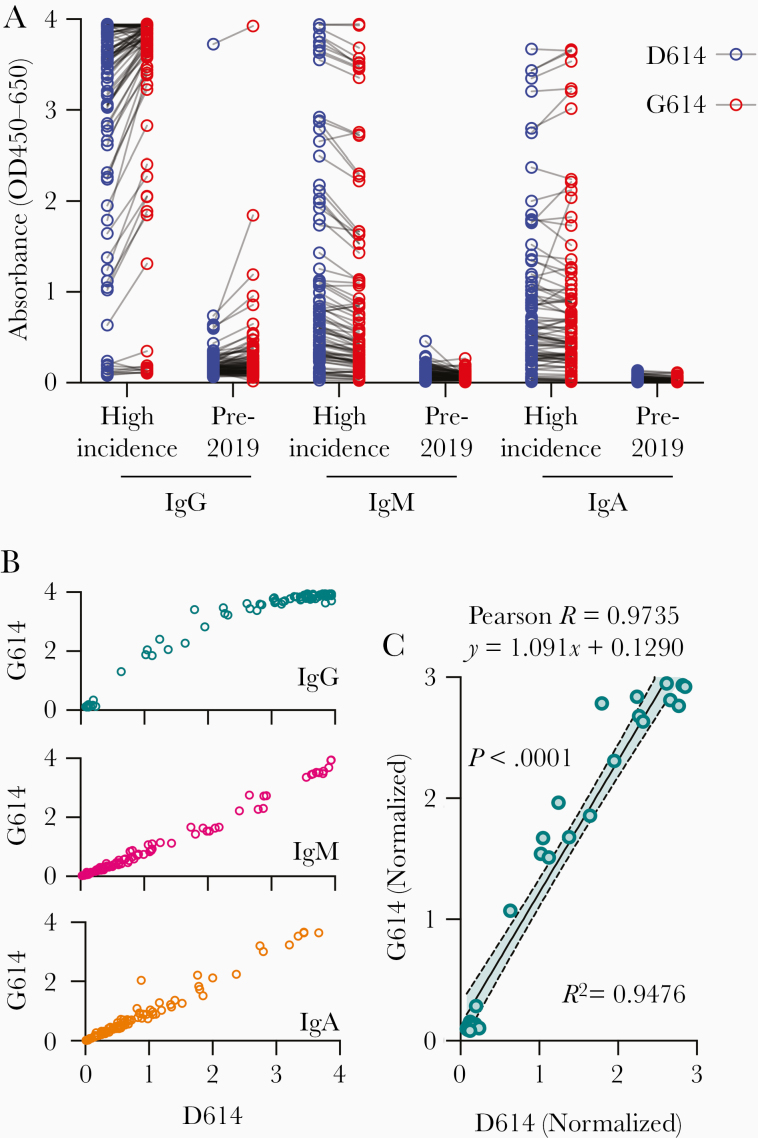
We found that serum samples from donors who tested positive for anti-spike antibodies using D614 spike also tested positive using G614 spike for IgG, IgM, and IgA antibodies (Figure 1A). These data were positively correlated for all antibody subclasses, and all donors who tested positive for D614 antibodies also displayed strong reactivity to G614 spike (Figure 1B). To further evaluate this correlation, we normalized the IgG values within the linear range of the detector (optical density <3) with the mean of the archival negative controls (Figure 1C). After running a linear regression and correlation analysis, we found a strong correlation (Pearson R = 0.9735; P < .001) and good fit (R2 = 0.9476) between both variants. Furthermore, the slope of the regression line was 1.091, suggesting a 1:1 signal intensity ratio.
Figure 1.
D614G mutation does not alter antibody binding to either spike variant. A, Raw absorbance (optical density [OD] at 450–650 nm) values for 88 donors from a high-incidence community and 100 archival pre-2019 donors. B, Absorbance signals of D614 (original) and G614 (new dominant variant) spike proteins for immunoglobulin (Ig) G, IgM, and IgA antibody classes. C, Correlation of signal intensity of IgG after normalization of high-incidence samples to mean of archival negative controls (linear range, OD <3).
DISCUSSION
A number of seroassays have been published that use various spike constructs, and ELISAs do not provide detection coverage for all possible SARS-CoV-2 antigens but rather use a single protein construct to ascertain seropositivity. Our data show that use of the full-spike protein construct should not affect seroassay performance or “miss” seropositive samples. The fact that D614 and G614 both elicited seropositivity is perhaps expected, given that the human immune response is polyclonal [8, 9]. Although there may be antibodies produced that recognize spike protein epitopes specific for D614 or G614, these would be among the many antibodies recognizing the SARS-CoV-2 spike protein used in our seroassays [10]. We conclude that human antibody response to SARS-CoV-2 can be detected using D614 or G614 spike protein in ELISAs. Further in-depth research on this topic should evaluate individual B-cell clones and responses correlated with viral genome sequencing.
Notes
Acknowledgments. The authors thank members of the FNLCR Protein Expression Laboratory (William Gillette, Simon Messing, and Vanessa Wall) for support in DNA production and protein purification.
Disclaimer. The National Institutes of Health, its officers, and its employees do not recommend or endorse any company, product, or service.
Financial support. This work was supported by the Intramural Research Program of the National Institutes of Health, including the National Institute of Biomedical Imaging and Bioengineering, the National Institute of Allergy and Infectious Disease, and the National Center for Advancing Translational Sciences, and also by the National Cancer Institute (contract HHSN261200800001E).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lucas M, Karrer U, Lucas A, Klenerman P. Viral escape mechanisms–escapology taught by viruses. Int J Exp Pathol 2001; 82:269–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Korber B, Fischer WM, Gnanakaran S, et al. ; Sheffield COVID-19 Genomics Group . Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 Virus. Cell 2020; 182:812–827.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grubaugh ND, Hanage WP, Rasmussen AL. Making sense of mutation: what D614G means for the COVID-19 pandemic remains unclear. Cell 2020; 182:794–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klumpp-Thomas C, Kalish H, et al. Standardization of ELISA protocols for serosurveys of the SARS-CoV-2 pandemic using clinical and at-home blood sampling. Nat Commun January 21, 2020; doi: 10.1038/s41467-020-20383-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Esposito D, Mehalko J, Drew M, et al. Optimizing high-yield production of SARS-CoV-2 soluble spike trimers for serology assays. Protein Expr Purif 2020; 174:105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hicks J, Klumpp-Thomas C, Kalish H, et al. Serologic cross-reactivity of SARS-CoV-2 with endemic and seasonal betacoronaviruses. medRxiv [Preprint: not peer reviewed]. 23 June 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.06.22.20137695v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020; 367:1260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neurath AR. Immune response to viruses: antibody-mediated immunity. Encyclopedia of Virology 2008; 56–70. [Google Scholar]
- 9. Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020; 584:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020; 26:845–8. [DOI] [PubMed] [Google Scholar]