Abstract
Background
Residential care homes for the elderly are important settings for transmission of the SARS-CoV-2 virus that causes COVID-19 disease.
Methods
We undertook secondary analysis of 248 care homes in Norfolk, UK. The dataset counted nurses, care workers and non-care workers, their status (available, absent due to leave or sickness and extra staff needed to address the coronavirus pandemic) and residents (if any) with suspected COVID-19 in the period 6 April to 6 May 2020. Concurrent descriptions of access by the home to personal protection equipment (PPE: gloves, masks, eye protection, aprons and sanitizer) were in the data. PPE access was categorized as (most to least) green, amber or red. We undertook two-stage modelling, first for suspected COVID-19 cases amongst residents and second relating any increases in case counts after introduction to staffing or PPE levels.
Results
Counts of non-care workers had strongest relationships (P < 0.05) to introduction of suspected SARS-CoV-2 to the homes. Higher staff levels and more severe PPE shortages were linked to higher case counts (P < 0.05) during the monitoring period.
Conclusion
Managing aspects of staff interaction with residents and some working practices might reduce ingression to and spread of COVID-19-like illness within care homes.
Keywords: care homes, COVID-19, Cox proportional hazards model, mixed effect models, personal protection equipment
Background
Residential care homes for the elderly have been important settings for transmission of the SARS-CoV-2 virus that causes COVID-19 disease.1 It became apparent early in the COVID-19 pandemic that infection control within care homes for the elderly would be especially challenging and yet important to reducing total mortality and wider disease spread. The viral disease has disproportionately more severe outcomes amongst the elderly2 while residential settings are well understood to be places where any disease outbreak can be especially difficult to control. That care homes for the elderly were key foci for transmission was quickly recognized in many countries and territories, including places that nominally were otherwise able to quite effectively contain and control spread of SARS-CoV-2.3 The high level of COVID-19 deaths in care home settings became highly politicized.4–7
Challenges in preventing or controlling infectious disease outbreaks in care settings are myriad. In many countries, the elderly care sector is acknowledged to be underfunded and staffed by relatively low-paid workers who may have insufficient training or experience in infection control.7,8 A detailed outbreak report on a COVID-19 outbreak in February–March 2020 focused on the Kirkland Care Facility in Washington State, USA.9 This nursing home provided care for >100 residents. Many factors were identified that contributed to late recognition and failure to prevent SARS-CoV-2 infection in many dozens of persons linked to this outbreak, who were mostly residents, some staff and at least one visitor. To recap, the most important vulnerability factors identified in the Kirkland outbreak were (in no particular order):
Delayed awareness of the disease (‘low index of suspicion’)
Staff who worked while symptomatic
Staff who worked in multiple care facilities (increasing their own personal risk of exposure and facilitating disease transfer between institutions)
Inadequate familiarity with and adherence to personal protection equipment (PPE) recommendations
Inadequate supplies of PPE and hand sanitizer
Limited availability of testing
Difficulty identifying symptomatic residents
Lack of both tests for COVID-19 and supplies of PPE (such as masks, gloves and protective gowns) were recognized as severe problems for infection control in the care home sector early in 2020.8 Shortage of diagnostic tests in early 2020 meant that many respiratory disease outbreaks in care homes could only be described as suspected rather than confirmed COVID-19 in surveillance monitoring.10,11 High numbers of excess deaths amongst care home residents and widely reported respiratory disease outbreaks in care homes during early 2020 are widely believed to have often been at least indirectly due to the pandemic, even if COVID-19 case status was not confirmed.12 Lack of PPE specifically was widely perceived to have contributed to greater disease ingression, greater transmission after introduction and higher mortality and morbidity within the UK care home sector. We used anonymized care home tracker data that reported on both staffing levels and PPE availability for individual care homes in the county of Norfolk, eastern England UK, in early 2020. We explored which identifiable care home-specific risk factors could be linked to either ingression or spread of suspected COVID-19 after ingression. It was hoped this analysis could help inform future infection control strategies specific to reducing ingression and spread of COVID-19 within elderly care homes.
Methods
Data
This is a secondary analysis of care home capacity tracker data (carehomes.necsu.nhs.uk) that is available to adult social care departments at English county councils. The data generator (North of England Commissioning Support Unit), data provider (Norfolk County Council) and Faculty of Medicine and Health Sciences Ethics Research Ethics Committee at the University of East Anglia (UEA, reference 2019/20-130) approved the research. Author TW extracted the information for Norfolk, removed true identifiers and added pseudonymized identifier codes. The pseudonymized data included all operational care homes within county boundaries during the monitoring period. Norfolk is a predominantly rural and coastal county in Eastern, UK that extends roughly 55 by 40 miles and has a population of ~906 000. Residents of Norfolk are relatively ‘old’ within the UK, with a median age ~45 years, which compares to a median age of 40.2 years for all UK residents in mid-2018.13 The county is neither especially affluent nor deprived but does have areas amongst the 10% most and least deprived areas in the UK.14 Norfolk residents are not ethnically diverse; 96.5% of residents in the 2011 census self-identified as White British or White Other.14 The percentage of ethnic minorities is even lower amongst persons age 60+. At this point of writing (end May 2020), death rates in Norfolk have been relatively lower than for rest of England.15,16
The data describe infection prevalence from COVID-19 in 307 care homes in Norfolk in the 30 days following 5 April 2020. The purpose of CapacityTracker was to monitor shortages of PPE, so PPE information was complete (not missing on any dates). PPE provision was reported in one of three categories (Red, Amber, Green) depending on the availability of each of five types of items: aprons, eye protection, gloves, masks and hand sanitizer on that date. The coding was converted to an ordinal numeric score with green coded as 1, amber 2 and red as 3. Hence, higher scores indicated progressive decrease in PPE supplies. This allowed us to investigate the impacts of variation in individual components of PPE as well as creating an overall score for ‘PPE problems’. There was a maximum score of 3 for severe deficiency in any single PPE category, and a maximum combined PPE score of 15, which meant severe (red) shortage of all types of PPE.
The bed capacity variable in the care home tracker data was mostly incomplete (not reported on any date for many of the homes). We therefore did not try to use bed capacity indicators in the models. Other data recorded (daily) were concurrent number of residents suspected or known to have COVID-19, counts of specific types of staff on payroll at the facility, bed capacity or occupancy. Care home managers were instructed to input ‘the number of residents you consider to be suffering from COVID-19 (including whether or not they have been tested)’.17 This surveillance dataset did not ask for only confirmed cases because of a national shortage of diagnostic tests in April 202012 when English care homes were provided with only enough diagnostic tests to confirm the first five cases of COVID-1918,19 and usually had to rely on clinical presentation to identify subsequent likely cases.
Staffing and case counts were reported completely for most homes on most dates. Staff were described in three categories: nurses, care workers and non-care staff. Nurses and care workers are both involved with personal and physical care but distinguished by different responsibilities, levels of training and pay grades. Non-care workers include cooks, maintenance, administrative and other employees who do not normally provide face-to-face care. Staff were further distinguished as actively available for work, absent (due to leave, sickness and vacancies) or additional (extra staff required to meet the needs of residents during the coronavirus pandemic). The counts of each type of staff were input to the model as one of four count categories: 0–10, 11–20, 21–30 or 31 and over (31+) staff in each of the nurse/care worker/non-care worker categories. Data on some dates were incomplete (staff counts not given on every date for every home), so we used the staff counts on the date with most staff (thus ignoring entries where staff counts were stated as all zeros or dates when only one staff category was reported). This was reasonable because, given the relatively short monitoring period (30 days), the total count of staff positions was unlikely to change. Subsequently, we tested whether case counts on any particular day could be linked to PPE status and/or counts in each staffing category. Any care homes that had no staff counts on any dates were excluded because this meant that the information had never been reported rather than the true counts of staff were zero in all staff categories on all dates.
Analysis
We considered but rejected a zero-inflated model form (such as zero-inflated negative binomial model) to predict case both incidence and total counts in a single-stage (unified) model because of risk of (too) perfect prediction, separation or partial separation that can bias coefficients. These problems can easily arise and distort interpretation of results when zero-inflated models are used to predict a (sparse data) low-incidence phenomenon.20,21 This was a legitimate concern because we had only 30 care homes with infection (9% of the total). Furthermore, the unified model could be less useful than two-part modelling for informing infection control policy. We hypothesized that factors predisposing disease ingress might be different to those dictating onward spread amongst residents. Therefore, we used a two-part modelling approach to assess the extent to which cases of suspected COVID-19 were associated with either the employed number of staff in the care home broken down by category (care, nurse and non-care worker) and/or the availability of PPE on presence and rate of spread of disease. The first model investigated correlates associated with the incidence of any suspected COVID-19 cases into care homes, whereas a separate (second) model investigated which factors were linked with onward spread after disease got into the home. We considered but rejected logistic regression for the (first part) ingression model because of strong underdispersion (high percentage of homes without recorded cases). Logistic regression in presence of such high underdispersion would lead to a strong underestimation of variable significance that we viewed as unhelpful for informing infection control policy.
Thus, to investigate correlates with disease introduction to homes we used a survival analysis to identify any factors that could be linked to timing of disease introduction. We effectively assumed that there was a baseline hazard of each care home becoming infected and that there would be features of care home management that would impact on this hazard. For the survival analyses, we used time to infection (measured in days after 5 April) as a measure of the survival time of care homes being free of disease, under the assumption that there was a baseline hazard of infection that was changed by covariates of risk. We analysed time to infection using Cox proportional hazards models with candidate predictors that were counts in categories of care home employee and PPE scores in either single PPE categories or total combined PPE score. We used a stepwise reduction procedure where the first model included all variables and followed by removal of non-significant variables from the model. Given that the timing of spread of disease in Norfolk and the outside community as a whole may have impacted on the baseline hazard of a care home becoming infected over the study period, we assessed the extent to which the hazard was proportional and not dependent on time by examining the Schoenfeld residuals for our final model.22 This test was useful because if community spread was changing over time during the monitoring period, then the hazard of infection would not have been proportional and our model result would be biased by consequential confounding.
Secondly, we used mixed effect modelling23 to analyse within care home spread following ingression. In the mixed effect models, care home was defined as a random effect to investigate the extent to which the care home employee counts and/or PPE parameters might affect spread of established disease (change in the number of cases in the home through time). We had considered using mixed effect log-linear models to investigate worker numbers and PPE status on each day after adjusting for time since the first case. However, the data were overdispersed and showed substantial aggregation, so it was preferable to use a generalized mixed effect model with a negative binomial error structure.24 This strategy accounted for aggregation in cases and let us extend the models to include a correlation structure that allowed for the repeated measures associated with repeated sampling. The best final models, at either ingression or spread stage, were chosen by keeping only predictors that were significant at P ≤ 0.05. Models were fit in the MASS, Survival and lme4 packages in R.25
Results
The supplied dataset comprised 307 care homes in Norfolk, UK. Of these, 30 had incursion by COVID-19 during in the period 5 April to 6 May 2020. In total, 59 homes were removed from the analyses as they had not supplied counts of employees in any category on any date; 5 of these 59 had reported any cases (total of 14 cases at peak). The useable dataset was therefore 248 Norfolk care homes of which 25 had any COVID-19 cases (total of 133 cases at end of monitoring period).
Predictors of disease incursion
Only ~10% of the care homes were subject to infection by COVID-19, indicating that the data were underdispersed. A generalized linear model with a quasi-binomial error structure to adjust for the underdispersion demonstrated that risk of any infection (dichotomous outcome) was significantly related to the number of non-care workers (t = 4.382, P < 0.001) employed in each establishment. Table 1 shows hazard ratios with 95% confidence intervals (CIs) and significance levels as P values for the survival analysis (Stage 1 model for any ingression of COVID-19 to care homes). In the final ingression model, the number of non-care workers at the care home was the only significant predictor.
Table 1.
Survival analysis, hazard ratios with 95% CIs and significance levels
| Variable | Hazard ratio | (95% CI) | P values |
|---|---|---|---|
| Non-care workers employed: ≤10 | 1.0 (ref) | — | — |
| 11–20 | 6.502 | 2.61–16.17 | <0.001 |
| 21–30 | 9.870 | 3.22–30.22 | <0.001 |
| ≥31 | 18.927 | 2.36–151.90 | 0.006 |
Model fit metrics: concordance = 0.742, standard error = 0.048, likelihood ratio test = 25.33 on 3 degrees of freedom (df; P < 0.001), Wald test = 24.38 on 3 df, P < 0.001.
For the survival analyses, timing to infection was significantly related to the number of non-care workers employed (Fig. 1). Risk of infection was 6.502 times higher (CI: 2.614–16.17) in care homes that employed 11–20 non-care workers; 9.870 times higher (CI: 3.224–30.22) in homes employing 21–30 care workers and 18.927 times higher (CI 2.358: 151.90) times higher in care homes employing >30 non-care workers. A test of the assumption of the proportionality of hazards remaining constant through time was not significant (χ2 = 1.81, P = 0.178) indicating that the baseline hazard and the hazard associated with number of care workers employed stayed constant over the sample period. This suggests that any changes in the prevalence of community infection did not impact on the risk of a care home becoming infected.
Fig. 1.
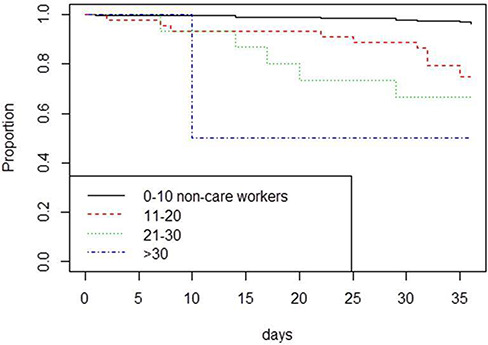
Survivorship curves investigating impacts of numbers of non-care workers employed in care homes on the risk of the care home suffering incursion by COVID-19. Incursion was more rapid in care homes employing more non-care workers.
Predictors of spread
The exponents of the regression parameters estimates for the best model are shown in Table 2 along with the 95% CIs. The data represent incremental risk (cases) in relation to the increment for each factor. The time increment is expressed in days since beginning of the study (5 April 2020) as the epidemic proceeded. The coefficients for PPE represent the increments in cases as the state of availability of PPE of different types moved from green through amber to red. It is clear that absence of masks and eye protection had the biggest impact on cases followed by the numbers of care workers. The employee increment is between categories, from 0 to 10, 11 to 20, 21 to 30 or 31+ employees in each group.
Table 2.
Regression diagnostics for the mixed effect model analysis in factors linked to spread of COVID-19 in care homes
| Variable | Incremental increase (95% CI) | P values |
|---|---|---|
| Increment after 5 April (days) | 1.0347 (1.02–1.05) | <0.001 |
| PPE eye protection score | 1.6571 (1.29–2.13) | <0.001 |
| PPE facemask score | 1.2602 (1.09–1.46) | 0.002 |
| Count of care workers employed | 1.0379 (1.02–1.05) | <0.001 |
| Count of nurses employed | 1.1814 (1.13–1.24) | <0.001 |
Data represent the incremental increase in cases per unit of the predictor variable.
The daily increment in suspected cases (i.e. spread) was 1.04. Reduced availability of PPE for eye protection and PPE for facemasks had the greatest impact on spread with coefficients increasing case load by, respectively, 1.66 and 1.26 per increment (both P < 0.001) on top of staff counts and daily increment effects. Spread (case count increments) also increased with higher staff levels.
The temporal patterns of spread in the 25 care homes and the predicted numbers from the final model are shown in Figure 2. Individual care homes are indicated by anonymized titles (e.g. N-00287). There is a reasonably close approximation (visually) between observed and predicted cases in the 25 care homes where disease was recorded and subsequently spread.
Fig. 2.
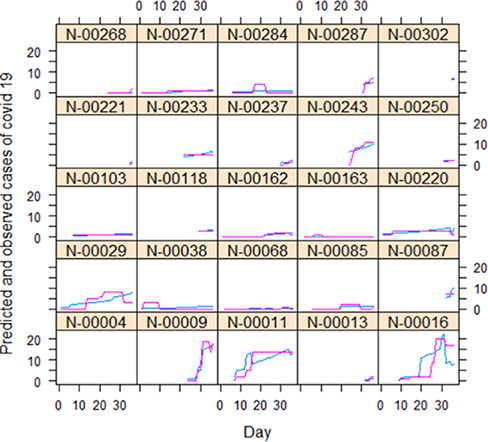
Predicted (blue line) and observed (pink line) numbers of COVID-19 cases in 25 care homes during April–May 2020. Horizontal axis = count of days after 5 April. Model form is mixed effect negative binomial. For predictors see text.
Discussion
We have shown that entry of suspected COVID 19 into a care home was primarily associated with the number of people not directly involved in the care of residents. We have also shown that once introduced into the home the subsequent spread of suspected COVID-19 was largely associated with inadequate access to PPE, most especially facemasks, and the number of resident-contact workers employed. These findings are not surprising. That the non-care worker category was important may indirectly correspond with low use of PPE amongst these employees, which meant they had more likelihood of passing the infection to other staff or during brief time spent near residents. Alternatively, non-care workers may be especially likely to work part time and possibly work across several locations. Our dataset did not indicate counts of agency workers (who move between multiple care homes), so we were unable to consider if working in multiple settings was directly relevant. The increased spread subsequent to introduction of suspected COVID19 into a home with inadequate access to PPE is equally unsurprising. Increased case counts linked to higher numbers of care workers may relate both to inadequate PPE and increased contact rates.
That care homes are particularly vulnerable to the rapid spread of infectious diseases and has been described for outbreaks of other infectious diseases, notably norovirus,26 influenza and other respiratory infections.27 Fell28 investigated the preparedness of care homes and concluded that at the time care homes were inadequately prepared for coping with an influenza pandemic. Fell observed that no planning had been made for where to obtain infection control guidance from, increased clinical needs of residents, increased staffing needs that staff might come to work while themselves infectious or likely impact on facility financial position. Similarly, four factors were identified that could exacerbate spread of norovirus in care homes29: ‘missing the diagnosis, care service under pressure, delay in outbreak control measures and patient/resident location and proximity’. All of these themes have clear resonance with issues around the management of COVID-19 outbreaks in care homes.
Although our research clearly indicated the importance of PPE to reduce disease spread, we argue here that infection prevention and reduction needs to be more multifaceted than simply supplying adequate PPE and training to use it. Investigation of the Kirkland’s long-term care facility9 outbreak highlighted many other problems that can exacerbate a COVID-19 outbreak in this type of setting. It is apparent that better data need to be collected to directly understand better how this sector is vulnerable to an emerging disease like COVID-19. Good understanding of the economic drivers that may compel staff to work in multiple facilities or while symptomatic could help to inform development of different employment policies and practices that might reduce the risks of disease introduction facilitated by common current work patterns. Understanding of how existing work patterns interact with COVID-19 spread is still developing and may be somewhat specific to individual countries.30 Low index of suspicion was also cited in the Kirkland’s outbreak, not least because it was difficult to clearly identify affected residents. Persistent coughing and fever are the most diagnostic symptoms for COVID-19 patients, but severity of symptoms is highly variable, even amongst the elderly.2 Moreover, care home residents often have respiratory problems,31 especially coughs due to chronic conditions such as chronic obstructive pulmonary disease32 and/or high susceptibility to minor self-limiting respiratory infections.33 Regular testing with fast results will be key to help distinguish other cough-inducing conditions from COVID-19.34
Limitations
It is interesting that the data for infection were underdispersed, whereas those for spread were overdispersed. The former outcome (whether or not a care home had any suspected COVID-19) provides a good justification for using the Cox proportional hazards model since infection was clearly not a straightforward binomial event; many more events should have occurred for this error structure to have been appropriate for the data. However, in reality, there is likely to have been a spatial component to the existence of disease in the wider community that would have meant that the binomial error model would have been inappropriate without consideration of spatial variation in community. Spatial and social network data interactions between homes were not available to us but would strengthen any future modelling efforts.
Lack of ethnic diversity in Norfolk meant we could not consider whether minority ethnic composition was a factor in disease spread or severity; ethnic diversity seems to be important to disease outcomes amongst affected care homes in other localities.5 Considerable efforts have been undertaken to increase supplies of PPE to UK care homes since these April 2020 data were collected.35 Improvements in procurement processes and supply chains may have changed the balance of future risk factors from what we see in these April 2020 data.
Conclusions
Ingression of COVID-19-like illness to Norfolk care homes in April 2020 was most strongly linked to the counts of employed staff in the non-care worker category. Specific detailed research should follow to examine the interaction patterns of all types of staff with each other and with patients. Understanding how often care home staff work in multiple institutions taking on which types of roles with what kinds of physical contact with patients and other staff may be key to improving infection control during a pandemic situation like COVID-19 has created. After disease was introduced, our models implicated lack of eye protection and face masks as the most important risk factors in spread of suspected COVID-19. This information may be helpful for prioritizing PPE procurement in future, at least with regard to this respiratory disease. It is worthwhile reiterating that residential care for the elderly is a generally underfunded sector with low-pay conditions where staff training has historically been undervalued; ameliorating this situation will not be quick.
Funding
National Institute for Health Research Health Protection Research Unit (NIHR200890, HPRU) in Emergency Preparedness and Response at King’s College London in partnership with Public Health England (PHE), in collaboration with the University of East Anglia to PRH and JB.
Acknowledgements
We appreciate Neil Stevenson of the CapacityTracker service (managed by NHS North of England Commissioning Support Unit) giving consent for the data to be used for research purposes and Pete Best of the Norfolk and Norwich University Hospitals Trust for facilitating local collaboration between NHS groups and UEA academics.
Julii Brainard, Senior Research Associate
Steven Rushton, Professor of Biological Modelling
Tim Winters, Head of Public Health Information
Paul R. Hunter, Professor of Health Protection
Contributor Information
Julii Brainard, Norwich Medical School, University of East Anglia, Norwich, NR4 7TJ, UK.
Steven Rushton, School of Natural and Environmental Sciences, Newcastle University, Newcastle Upon Tyne NE1 7RU, UK.
Tim Winters, Insight and Analytics, Norfolk County Council, Norwich NR1 2DH, UK.
Paul R Hunter, Norwich Medical School, University of East Anglia, Norwich, NR4 7TJ, UK.
Conflict of interest
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, UEA, the Department of Health or Public Health England. The authors declare no conflict of interest.
Approval to use the data to undertake the research
The data generator (North of England Commissioning Support Unit) and data provider (Norfolk County Council) approved use of the dataset for our research, which was confirmed by the Faculty of Medicine and Health Sciences Ethics Research Ethics Committee at UEA (UEA reference 2019/20-130).
References
- 1. Burki T. England and Wales see 20 000 excess deaths in care homes. Lancet 2020;395:1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu JT, Leung K, Bushman M et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med 2020;26:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. C-K P. Coronavirus: nursing homes emerge as South Korea’s new battleground for infections In: South China Morning Post, 19 March 2020. https://www.scmp.com/week-asia/health-environment (17 November 2020, date last accessed).
- 4. Clifton H, Cave R. Coronavirus: data delay left care homes ‘fighting losing battle’ In: BBC News, 19 May 2020. https://www.scmp.com/week-asia/health-environment/article/3075937/coronavirus-nursing-homes-emerge-south-koreas-new (17 November 2020, date last accessed).
- 5. New York Times COVID-19 and nursing homes: a striking racial divide In: New York Times, 21 May 2020. https://www.nytimes.com/article/coronavirus-nursing-homes-racial-disparity.html (17 November 2020, date last accessed).
- 6. Weiss S. ‘I’m furious’: failing care homes are the real coronavirus scandal are the real coronavirus scandal In: WIRED, 7 May 2020. https://www.wired.co.uk/article/care-homes-coronavirus (17 November 2020, date last accessed).
- 7. Bachega H. Coronavirus: inside story of Spain's care home tragedy In: BBC News, 30 April 2020. https://www.bbc.co.uk/news/world-europe-52188820 (17 November 2020, date last accessed).
- 8. Edwards N, Curry N. Deaths in care homes: what do the numbers tell us? Nuffield Trust. https://www.nuffieldtrust.org.uk/news-item/deaths-in-care-homes-what-do-the-numbers-tell-us (22 May 2020, date last accessed).
- 9. McMichael TM, Clark S, Pogosjans S et al. COVID-19 in a long-term care facility—King County, Washington, February 27–March 9, 2020. Morb Mortal Wkly Rep 2020;69:39–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holt A. Coronavirus: care home staff struggling to get tests In: BBC News, 26 April 2020. https://www.bbc.co.uk/news/uk-england-52418630 (17 November 2020, date last accessed).
- 11. House of Commons Commons Debate B5B3028B-C411-4CC0-A6EE-B7ED9B7955FB. Hansard: https://hansard.parliament.uk/Commons/2020-04-29/debates/B5B3028B-C411-4CC0-A6EE-B7ED9B7955FB/Engagements (2 October 2020, date last accessed). [Google Scholar]
- 12. Calcea N. Covid-19 involved in more than quarter of all care home deaths in recent months In: New Statesman, 15 May 2020. https://www.newstatesman.com/2020/05/covid-19-involved-more-quarter-all-care-home-deaths-recent-months (17 November 2020, date last accessed).
- 13. McCurdy C. Ageing fast and slow: when place and demography collide, 2019. https://www.resolutionfoundation.org/publications/ageing-fast-and-slow/ (17 November 2020, date last accessed).
- 14. Norfolk Insight Overview Report: Norfolk. Norfolk Insight; https://www.norfolkinsight.org.uk/population/ (1 June 2020, date last accessed). [Google Scholar]
- 15. Drury C. How Norwich achieved lowest coronavirus death rates in England In: The Independent, 6 May 2020. https://www.independent.co.uk/news/uk/home-news/coronavirus-norwich-death-rates-ons-lowest-highest-a9500106.html (17 November 2020, date last accessed).
- 16. Place C. Revealed: coronavirus death rates, with Norwich the lowest in England and Wales In: Norwich Evening News, 2 May 2020. https://www.eveningnews24.co.uk/news/health/ons-shows-norwich-lowest-death-rates-during-coronavirus-1-6634468 (17 November 2020, date last accessed).
- 17. NECS Capacity Tracker: User Guide for Care Homes, 2020.
- 18. Triggle N. Coronavirus: more tests promised for care homes In: BBC News, 15 April 2020. https://www.bbc.co.uk/news/uk-52289607 (17 November 2020, date last accessed).
- 19. Mason R. Sick care home residents not tested despite UK government pledge In: The Guardian, 24 April 2020. https://www.theguardian.com/world/2020/apr/24/sick-care-home-residents-not-tested-despite-uk-government-pledge (17 November 2020, date last accessed).
- 20. Marín RA, Christensen A, Atkins DC. Infidelity and behavioral couple therapy: relationship outcomes over 5 years following therapy. Couple Fam Psychol Res Pract 2014;3:1. [Google Scholar]
- 21. UCLA Institute for Digital Research & Education Zero-Inflated Poisson Regression | R Data Analysis Examples. https://stats.idre.ucla.edu/r/dae/zip/ (12 October 2020, date last accessed).
- 22. Therneau TM, Grambsch PM. The Cox model In: Modeling Survival Data: Extending the Cox Model. New York: Springer, 2000, 39–77. [Google Scholar]
- 23. Harrison XA, Donaldson L, Correa-Cano ME et al. A brief introduction to mixed effects modelling and multi-model inference in ecology. Peer J 2018;6:e4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrison XA. Using observation-level random effects to model overdispersion in count data in ecology and evolution. Peer J 2014;2:e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. RDC Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, https://www.r-project.org/ (17 November 2020, date last accessed).. [Google Scholar]
- 26. Inns T, Clough HE, Harris JP et al. Estimating the burden of care home gastroenteritis outbreaks in England, 2014–2016. BMC Infect Dis 2019;19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gallagher N, Johnston J, Crookshanks H et al. Characteristics of respiratory outbreaks in care homes during four influenza seasons, 2011–2015. J Hosp Infect 2018;99:175–80. [DOI] [PubMed] [Google Scholar]
- 28. Fell G. Preparedness of residential and nursing homes for pandemic flu. J Public Health 2008;30:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curran ET, Wilson J, Haig CE et al. The where is norovirus control lost (WINCL) study: an enhanced surveillance project to identify norovirus index cases in care settings in the UK and Ireland. J Infect Prev 2016;17:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Houtven C, Boucher N, Dawson WD. Impact of the COVID-19 Outbreak on Long-Term Care in the United States 1st LTCcovid webinar on: International responses to COVID-19 in care homes (18 May); May 18; (webinar). International Long-Term Care Policy Network, 2020. [Google Scholar]
- 31. Bentayeb M, Norback D, Bednarek M et al. Indoor air quality, ventilation and respiratory health in elderly residents living in nursing homes in Europe. Eur Respir J 2015;45:1228–38. [DOI] [PubMed] [Google Scholar]
- 32. Patel M, Steinberg K, Suarez-Barcelo M et al. Chronic obstructive pulmonary disease in post-acute/long-term care settings: seizing opportunities to individualize treatment and device selection. J Am Med Dir Assoc 2017;18:553. e17–22. [DOI] [PubMed] [Google Scholar]
- 33. Widdicombe J, Kamath S. Acute cough in the elderly. Drugs Aging 2004;21:243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roxby AC, Greninger AL, Hatfield KM et al. Outbreak investigation of COVID-19 among residents and staff of an independent and assisted living community for older adults in Seattle, Washington. JAMA Intern Med 2020; 180:1101–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. NHS Accessing supplies of Personal Protective Equipment (PPE). NHS England and NHS Improvement; https://www.england.nhs.uk/coronavirus/secondary-care/infection-control/accessing-supplies-of-personal-protective-equipment-ppe/ (9 June 2020, date last accessed). [Google Scholar]


