Placental severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with a specific histiocytic intervillositis. Placental fibrin depositions can decrease the maternal-fetal interface causing fetal distress. Coronavirus disease 2019-related placental inflammation can lead to SARS-CoV-2-associated pediatric inflammatory multisystem syndrome.
Keywords: fetal distress, inflammation, Kawasaki-like syndrome, placenta, SARS-CoV-2
Abstract
Background
In general, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy is not considered to be an increased risk for severe maternal outcomes but has been associated with an increased risk for fetal distress. Maternal-fetal transmission of SARS-CoV-2 was initially deemed uncertain; however, recently a few cases of vertical transmission have been reported. The intrauterine mechanisms, besides direct vertical transmission, leading to the perinatal adverse outcomes are not well understood.
Methods
Multiple maternal, placental, and neonatal swabs were collected for the detection of SARS-CoV-2 using real-time quantitative polymerase chain reaction (RT-qPCR). Serology of immunoglobulins against SARS-CoV-2 was tested in maternal, umbilical cord, and neonatal blood. Placental examination included immunohistochemical investigation against SARS-CoV-2 antigen expression, with SARS-CoV-2 ribonucleic acid (RNA) in situ hybridization and transmission electron microscopy.
Results
RT-qPCRs of the oropharynx, maternal blood, vagina, placenta, and urine were all positive over a period of 6 days, while breast milk, feces, and all neonatal samples tested negative. Placental findings showed the presence of SARS-CoV-2 particles with generalized inflammation characterized by histiocytic intervillositis with diffuse perivillous fibrin depositions with damage to the syncytiotrophoblasts.
Conclusions
Placental infection by SARS-CoV-2 leads to fibrin depositions hampering fetal-maternal gas exchange with resulting fetal distress necessitating a premature emergency cesarean section. Postpartum, the neonate showed a fetal or pediatric inflammatory multisystem-like syndrome with coronary artery ectasia temporarily associated with SARS-CoV-2 for which admittance and care on the neonatal intensive care unit (NICU) were required, despite being negative for SARS-CoV-2. This highlights the need for awareness of adverse fetal and neonatal outcomes during the current coronavirus disease 2019 pandemic, especially considering that the majority of pregnant women appear asymptomatic.
In general, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy is not considered to be an increased risk for severe maternal outcomes but has been associated with an increased risk for fetal distress [1]. Localization of SARS-CoV-2 particles in placental tissue has been visualized [2, 3], and recently, a few cases of vertical transmission of SARS-CoV-2 have been reported [4–8]. Besides related to direct in utero infection with SARS-CoV-2, the mechanisms leading to the adverse perinatal outcomes are not well understood. We report an intra-placental SARS-CoV-2 infection at 31 + 4 weeks’ gestational age diagnosed by multiple methods, including immunohistochemistry, in situ hybridization, and transmission electron microscopy. Inflammation was characterized by histiocytic intervillositis with specific diffuse perivillous fibrin depositions and intervillous inflammatory infiltrates. Placental infection most likely resulted in fetal distress and related fetal cardiotocography abnormalities necessitating a premature emergency cesarean section. The neonate tested negative for SARS-CoV-2 but displayed severe multi-organ inflammatory symptoms including coronary artery ectasia for which admittance and care on the neonatal intensive care unit (NICU) were required.
RESULTS
Maternal
A 30-year-old obese primigravid woman with gestational diabetes was referred to our tertiary center at 31 + 4 weeks’ gestation due to lack of fetal movements during the last 2 days. She reported general malaise, myalgia, and fever 5 days earlier, which resolved within 3 days. At presentation to our perinatal center, she had no coronavirus disease 2019 (COVID-19)-related symptoms but mentioned that she shared a household with a COVID-19-positive person. Fetal cardiotocography showed signs of severe fetal distress, including loss of beat-to-beat variability and repetitive decelerations, for which an emergency cesarean section was performed. Because of her medical history resembling COVID-19-related symptoms, samples for SARS-CoV-2 diagnostics (polymerase chain reaction [PCR] and pathological analysis) were collected (see Table 1, Figure 1). Real-time quantitative PCR (RT-qPCR) was performed for the detection of SARS-CoV-2 using our in-house assay [9] or the Cobas SARS-CoV-2 test on the Cobas 6800 system (Roche Diagnostics) depending on the availability of platforms. Cycle threshold values were converted to log10 ribonucleic acid (RNA) copies/mL by using calibration curves based on quantified E-gene in vitro transcripts as previously described [9]. All collected PCR samples during delivery, including placental tissue slices, tested positive for SARS-CoV-2, except for the umbilical cord blood, feces, and breastmilk. Over a period of 11 days, maternal PCR sampling was repeated (Table 1), which all remained positive for SARS-CoV-2, except for breastmilk and feces. Results for repeated neonatal PCR sampling are described in the “Neonatal Outcome” section later. SARS-CoV-2 serology was performed using the commercially available Beijing Wantai Biological Pharmacy assay. At 1 day after delivery, maternal serology for SARS-CoV-2 was positive. Additional maternal blood tests showed a slightly elevated C-reactive protein (CRP) (41 mg/L) and IL-6 (11 pg/mL) levels, a positive interferon type 1 (IFN-1) signature, and normal levels of ferritin (90 ug/L), leukocytes (7.9 x 109/L), and D-dimers (0.40 mg/L). To exclude the presence of other viral infections, serology (Immunoglobulin G [IgG] and Immunoglobulin M [IgM]) against ToRCH (toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus) and parvovirus B19 pathogens were determined. IgG for cytomegalovirus (CMV) and parvoB19 tested positive, whereas IgG and IgM tested negative for the other pathogens. To exclude the presence of other viral infections, serology (IgG and IgM) for ToRCH and parvoB19 was determined. Mother was positive for IgG against CMV and parvoB1, but IgM tested negative for all. Routine RT-PCR was performed for influenza A and B virus, parainfluenza virus type 1–4, human respiratory syncytial virus, rhinovirus, human metapneumovirus, adenovirus, coronavirus, bocavirus, and enterovirus. All PCRs were negative. During admission, maternal vital parameters (temperature, heart frequency, saturation, and blood pressure) remained within normal ranges. After 3 days, the patient was discharged home without complaints.
Table 1.
SARS-CoV-2 PCR Results (Log10 RNA Copies/mL)
| April 28 | April 29 | April 30 | May 1 | May 4 | May 5 | May 8 | |
|---|---|---|---|---|---|---|---|
| Maternal | |||||||
| Blood | POS (4.26) | ||||||
| Urine | POS (6.70) | ||||||
| Nasopharynx | POS (5.63) | POS (4.80) | POS (4.96) | POS (5.44) | |||
| Vagina | POS (4.58) | POS (3.91) | |||||
| Feces | NEG | ||||||
| Breastmilk (2×) | NEG | ||||||
| Placental | |||||||
| Maternal side | POS (4.42) | ||||||
| Fetal side | POS (7.15) | ||||||
| Neonate | |||||||
| Umbilical cord blood | NEG | ||||||
| Blood | NEG | NEG | |||||
| Sputum | NEG | NEG | |||||
| Nasopharynx | NEG | NEG | NEG | ||||
| Urine | NEG | NEG | |||||
| Feces | NEG | NEG |
Abbreviations: PCR, polymerase chain reaction; POS, positive PCR; NEG, negative PCR; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 1.
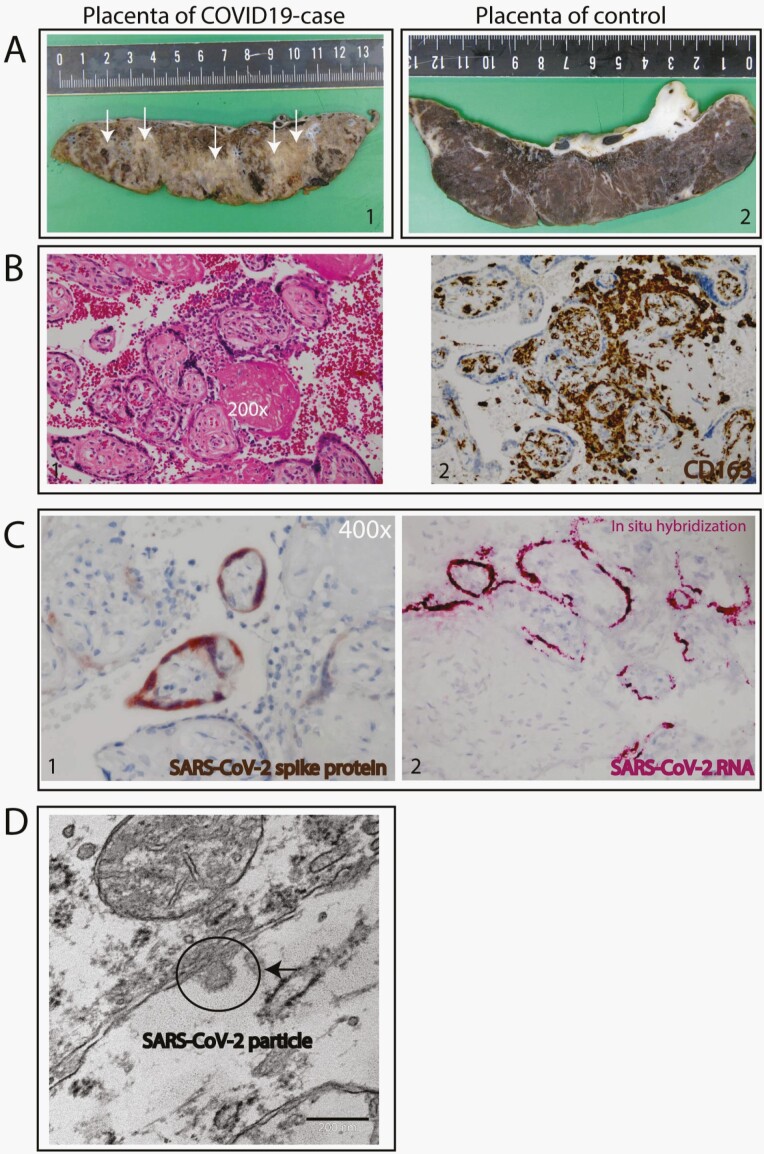
Placental syncytiotrophoblast severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection detected by histochemical staining, specific SARS-CoV-2 RNA probe, and electron microscopy. (A) Gross pathology of the placenta. Case placenta slice is abnormal and shows pale trabeculae in a lattice-like network (1) compared with control, age-matched placenta slice with normal appearance (2). (B) Histopathology of the placenta: diffuse perivillous fibrin and an intervillous inflammatory infiltrate. (1) The intervillous inflammatory cells have a monomorphonuclear, mostly histiocytic appearance by hematoxylin and eosin staining (200×). (2) The macrophages are of the M2 phenotype as shown by CD163+ staining. (C) SARS-CoV-2 infection of the syncytiotrophoblasts; (1) immunohistochemical staining for SARS-CoV-2 spike protein-specific antibody localizing to the cytoplasm (400×) (2) In situ hybridization for SARS-CoV-2 RNA. (D) Electron microscopy of SARS-CoV-2 particle.
Placental Examination
Gross examination showed a dense and stiff placenta with pale trabeculae in a lattice-like network (arrows, Figure 1A), in line with the histological results of diffuse perivillous fibrin deposition (Figure 1B-1). There was diffuse damage to the syncytiotrophoblasts associated with an intervillous inflammatory infiltrate, characterized by immunohistochemistry as M2 macrophages (CD163+ [Figure 1B-2] and CD68+ [Supplementary Figure 1]), cytotoxic (CD8), and helper T-cells (CD4) as well as activated B-lymphocytes (PAX5 and CD38) (Supplementary Figure 1). No plasma cells were detected by immunostaining for CD138 (Supplementary Figure 1). There were no signs of villous parenchyma invasion, villitis, or decidual vasculopathy. Immunohistochemical investigation for SARS-CoV-2 antigen expression in combination with SARS-CoV-2 RNA in situ hybridization demonstrated predominant localization of SARS-CoV-2 in the syncytiotrophoblast cells of the placenta (Figure 1C). Electron microscopy confirmed the presence of SARS-CoV-2 particles in the syncytiotrophoblast (Figure 1D), whereas villous and fetal parenchyma showed no evidence of SARS-CoV-2 infection (immunohistochemistry, in situ hybridization, or electron microscopy).
Neonatal Outcome
A female preterm infant was delivered at 31 + 4 weeks of gestation with an Apgar score of 1, 4, and 6 at, respectively, 1, 5, and 10 minutes postpartum, with an umbilical cord blood pH of 6.90, a base excess of −19 mmol/L, and a birth weight of 1880 grams (75th percentile). Neonatal life support was initiated in the absence of spontaneous breathing and an undetectable heart rate. Because of persistent insufficient breathing and a high oxygen demand, the infant was intubated, mechanically ventilated, and admitted to the NICU. During the physical examination, no heart murmur, mucocutaneous signs, rash, or hepatosplenomegaly was noted. Chest radiography showed bilateral opacities consistent with respiratory distress syndrome for which she received repetitive dosages of pulmonary surfactant. Intravenous antibiotics were started on admission and stopped after 36 hours as blood cultures remained negative, and CRP levels were low. Shortly after admission, the infant showed signs of multiple organ failure (elevated creatinine, liver, and cardiac enzymes) and developed a bilateral intraventricular hemorrhage (on the left side a grade 3 and the right side a grade 1). The patient also developed a thrombopenia and leukopenia; however, differentiation showed no lymphopenia. All recovered spontaneously.
The high oxygen demand raised the suspicion of persistent pulmonary hypertension of the neonate (PPHN), which was confirmed by echocardiography. Besides the flattened interventricular septum, mild-to-moderate tricuspid regurgitation, a small patent ductus arteriosus with predominantly right-to-left shunt, and a significantly enlarged left main coronary artery (LMCA) were observed (Supplementary Table 1 and Supplementary Figure 2). To treat PPHN and systemic hypotension, inhaled nitric oxide (iNO), inotropic agents, and hydrocortisone were started. Repeated echocardiograms were performed and showed an improvement of the PPHN but increasing diameter (aneurysmatic lesions) of the LMCA (maximum 0.34 mm Z-score + 6.5). Because of the clinical presentation resembling a paediatric multisystem inflammatory syndrome—temporally associated with SARS-CoV-2 (PIMS-TS) [10], immunoglobulins (2 gr/kg) were administered on day 4 and aspirin was started on day 6 to prevent further coronary dilation and thromboembolic complications [11]. At day 14 of life, only mild dilatation of the LMCA was observed. At 4 months postpartum, no signs of coronary artery dilation were observed during echocardiography (Supplementary Table 1).
Severely elevated levels of ferritin (14272 ug/L at day 3) as a sign of activated macrophages and significantly elevated D-dimers as a sign of an endotheliitis were seen, both described in fetal inflammatory response syndrome (FIRS) [12]. An active COVID-19 infection was ruled out as all sampling of umbilical cord blood, urine, feces, blood, nasopharynx, and sputum from a deep tracheal aspirate over a period of 9 days tested negative for SARS-CoV-2. Furthermore, the neonate did not develop antibodies to SARS-CoV-2, which were tested in umbilical cord blood, at days 1–3 and week 3 postpartum. The IFN-1 signature was (repetitively) negative. In the course of the first week, inotropic support could be gradually weaned and eventually stopped at day 6 after delivery. In addition, the infant was weaned from iNO and oxygen supplementation followed by detubation at day 6 of life.
Discussion
Our case shows that a maternal SARS-CoV-2 infection during the third trimester of pregnancy is associated with an adverse neonatal outcome based on a placental inflammatory reaction with subsequent dysfunction of the placenta. Remarkable is that the affected mother was in the postinfection period and had no symptoms during the event. The maternal positive IFN-1 signature indicates a strong maternal antiviral response despite the absence of clinical signs of SARS-CoV-2 infection during presentation. We hypothesize that the SARS-CoV-2-associated damage to the placenta early in pregnancy potentially can lead to fetal growth restriction and distress as in our case, while fetal demise may occur when not recognized in time. Although the effects of SARS-CoV-2 infection on pregnancy and neonatal outcomes in the majority of cases seem relatively mild, complications such as miscarriage due to placental infection by SARS-CoV-2 [13], placental abruption [14], (iatrogenic) preterm birth (21.5%), fetal distress (10.1%), and perinatal death [1] have been reported.
We diagnosed placental inflammation caused by SARS-CoV-2 infection, based on the detection of virus infection in syncytiotrophoblasts, which was colocalized with syncytiotrophoblast necrosis and a specific B-lymphocyte presentation of histiocytic intervillositis with subsequent placental failure, fetal distress, and perinatal asphyxia. The observed prominent infiltrate with B-lymphocytes has not previously been described in histiocytic intervillositis [15, 16], indicating that it might be one of the histopathological hallmarks that differentiates the histiocytic intervillositis of SARS-CoV-2 infection from chronic histiocytic intervillositis of unknown origin [15, 16]. Our findings are in line with more recent reports of histopathological placental findings in SARS-CoV-2-infected women [3, 17, 18]. Further studies in pregnant women with an active or a COVID-19 infection during pregnancy are warranted to investigate if morphological and histopathological placental characteristics are specific for SARS-CoV-2 infection and if these findings are associated with clinical perinatal outcome. Until now, only a few confirmed cases of vertical transmission of SARS-CoV-2 have been reported [4–8]. In our case, despite the massive placental infection, all neonatal samples were negative for SARS-CoV-2, and we found no evidence for vertical transmission. This is puzzling since angiotensin-converting enzyme 2 receptors seem essential in the transmission and infection by SARS-CoV-2 and are highly expressed on the placental maternal-fetal interface cells [19]. We cannot rule out that the detected SARS-CoV-2 RNA by PCR on the fetal side of the placenta is caused by contamination at the time of sampling, although the number of RNA copies was substantially higher than in maternal blood. During pregnancy, the placenta forms a natural barrier against maternal viral infections although the local immune-tolerant environment might permit viral replication. The specific mechanisms allowing some viruses, such as the rubella virus and the Zika virus, to cause transplacental fetal infection are not well understood [20, 21]. Placental examination of pregnant women infected with the related SARS-CoV of 2002–2003 revealed increased subchorionic and intervillous fibrin with extensive fetal thrombosis [22]. However, in contrast to our case, intervillositis, a histologic characteristic of maternal hematogenous infections that can lead to congenital infection, was not observed in those placentas in 2002–2003 [22]. Unraveling the mechanism by which the placenta prevents the passage of coronaviruses onto the fetus would be of great general interest for better understanding the placental barrier function.
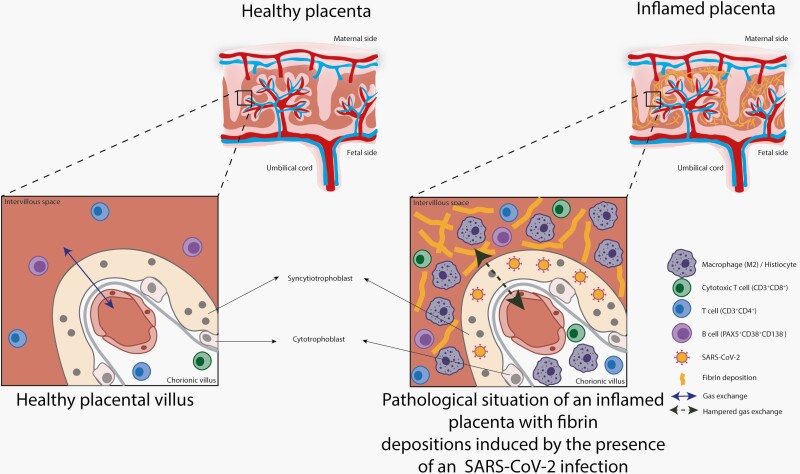
Placental SARS-CoV-2 infection can lead to massive local inflammation with the formation of fibrin depositions, thereby decreasing the available maternal-fetal interface necessary for effective gas exchange (Figure 2), which is essential for fetal growth and development. More recent reports have confirmed our immunological findings in the placenta after a COVID-19 infection [3, 17, 18]. We speculate that fetal and subsequent neonatal distress due to placental dysfunction caused by inflammation explain the clinical course resembling a fetal presentation (FIRS) of the pediatric inflammatory multisystem syndrome temporarily associated with SARS-CoV-2 (PIMS-TS) [23]. However, some clinical hallmarks (such as a dilated coronary arteries, extremely high ferritin, and high D-dimer levels) were not consistent with asphyxia alone and suggest a hyperinflammatory response as seen in macrophage activation syndrome [11, 24–26]. In perinatal asphyxia, increased coronary blood flow (myocardial sparing) has been described, but enlargement of coronary arteries has not [27]. Enlargement of coronary arteries is associated with neonatal viral myocarditis, although in combination with severe myocardial dysfunction and left ventricle dilatation [28], and the presence of a coronary arteriovenous fistula, which in our case was ruled out by echocardiography. Vasculitis of the coronary arteries in neonates, and specifically directly postpartum, is extremely rare [29–32]. The observed dilation of the LMCA and left anterior descending artery might be a sign of endotheliitis, which has been described in older children with COVID-19-related disease (PIMS-TS) recently [10]. In these older pediatric patients, high levels of ferritin and D-dimers were described, as seen in our neonate [10]. Also, by the use of pattern recognition receptors [12], the fetal immune system can detect signals produced in the context of structural damage, which can activate a severe immunological response leading to FIRS. This is normally associated with severe bacterial chorioamnionitis [12]. In our case, it might be that placental infection by SARS-CoV-2 resulted in a FIRS-like phenomenon.
Figure 2.
Graphical representation of a healthy placenta compared with perivillous fibrosing and placental inflammation caused by infection of severe acute respiratory syndrome coronavirus 2.
In conclusion, we here report a SARS-CoV-2-associated inflammation of the placenta in a mother who was asymptomatic at presentation with severe fetal and neonatal consequences, including coronary artery dilation. This highlights the need for awareness of adverse fetal and neonatal outcomes during the current COVID-19 pandemic, especially considering that the majority of pregnant women appear asymptomatic [33] who are still at risk for developing histopathological placental abnormalities [18].
Supplementary Material
Notes
Financial support. This work was supported by the European Union Commission COVID 19 grant (RECoVER 101003589) to P.F.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Yang Z, Wang M, Zhu Z, Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med 2020; 33:1–4. [DOI] [PubMed] [Google Scholar]
- 2. Algarroba GN, Rekawek P, Vahanian SA, et al. . Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol 2020; 223:275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hosier H, Farhadian SF, Morotti RA, et al. . SARS-CoV-2 infection of the placenta. J Clin Invest 2020; 130:4947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fenizia C, Biasin M, Cetin I, et al. . Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun 2020; 11:5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kotlyar AM, Grechukhina O, Chen A, et al. . Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol 2020:S0002–9378(20)30823-1. doi: 10.1016/j.ajog.2020.07.049. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz DA. Vertical transmission of severe acute respiratory syndrome coronavirus 2 from the mother to the infant. JAMA Pediatr 2020; 174:1004–5. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz DA, Morotti D, Beigi B, Moshfegh F, Zafaranloo N, Patane L. Confirming vertical fetal infection with COVID-19: neonatal and pathology criteria for early onset and transplacental transmission of SARS-CoV-2 from infected pregnant mothers. Arch Pathol Lab Med 2020; 144:451–1456. [DOI] [PubMed] [Google Scholar]
- 8. Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. . Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020; 11:3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corman VM, Landt O, Kaiser M, et al. . Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020; 25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045.25(3):2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verdoni L, Mazza A, Gervasoni A, et al. . An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395:1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agarwal S, Agrawal DK. Kawasaki disease: etiopathogenesis and novel treatment strategies. Expert Rev Clin Immunol 2017; 13:247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gotsch F, Romero R, Kusanovic JP, et al. . The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007; 50:652–83. [DOI] [PubMed] [Google Scholar]
- 13. Baud D, Greub G, Favre G, et al. . Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 2020; 323:2198–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuhrt K, McMicking J, Nanda S, et al. . Placental abruption in a twin pregnancy at 32 weeks’ gestation complicated by coronavirus disease 2019 without vertical transmission to the babies. Am J Obstet Gynecol MFM 2020; 2:100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parant O, Capdet J, Kessler S, et al. . Chronic intervillositis of unknown etiology (CIUE): relation between placental lesions and perinatal outcome. Eur J Obstet Gynecol Reprod Biol 2009; 143:9–13. [DOI] [PubMed] [Google Scholar]
- 16. Contro E, deSouza R, Bhide A. Chronic intervillositis of the placenta: a systematic review. Placenta 2010; 31:1106–10. [DOI] [PubMed] [Google Scholar]
- 17. Sharps MC, Hayes DJL, Lee S, et al. . A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta 2020; 101:13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patberg ET, Adams T, Rekawek P, et al. . COVID-19 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol 2020:S0002-9378(20)31194-7. doi: 10.1016/j.ajog.2020.10.020. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li M, Chen L, Zhang J, et al. . The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One 2020; 15:e0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pereira L. Congenital viral infection: traversing the uterine-placental interface. Annu Rev Virol 2018; 5:273–99. [DOI] [PubMed] [Google Scholar]
- 21. van der Eijk AA, van Genderen PJ, Verdijk RM, et al. . Miscarriage associated with Zika virus infection. N Engl J Med 2016; 375:1002–4. [DOI] [PubMed] [Google Scholar]
- 22. Ng WF, Wong SF, Lam A, et al. . The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology 2006; 38:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia . Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. . Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehta P, McAuley DF, Brown M, et al. ; HLH Across Speciality Collaboration, UK . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jinkawa A, Shimizu M, Nishida K, et al. . Cytokine profile of macrophage activation syndrome associated with Kawasaki disease. Cytokine 2019; 119:52–6. [DOI] [PubMed] [Google Scholar]
- 27. Baschat AA, Gembruch U, Reiss I, et al. . Demonstration of fetal coronary blood flow by Doppler ultrasound in relation to arterial and venous flow velocity waveforms and perinatal outcome–the ‘heart-sparing effect’. Ultrasound Obstet Gynecol 1997; 9:162–72. [DOI] [PubMed] [Google Scholar]
- 28. Rached-D’Astous S, Boukas I, Fournier A, et al. . Coronary artery dilatation in viral myocarditis mimics coronary artery findings in Kawasaki disease. Pediatr Cardiol 2016; 37:1148–52. [DOI] [PubMed] [Google Scholar]
- 29. Altammar F, Lang B. Kawasaki disease in the neonate: case report and literature review. Pediatr Rheumatol Online J 2018; 16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grasa CD, Fernandez-Cooke E, Sánchez-Manubens J, et al. ; Spanish Network for the Study of Kawasaki disease, KAWA-RACE . Kawasaki disease in infants 3 months of age and younger: a multicentre Spanish study. Ann Rheum Dis 2019; 78:289–90. [DOI] [PubMed] [Google Scholar]
- 31. Krapf R, Zimmermann A, Stocker F. Lethal vasculitis of coronary arteries in a neonate and two infants: possible neonatal variant of the MLNS/IPN complex? Helv Paediatr Acta 1981; 36:589–98. [PubMed] [Google Scholar]
- 32. Parashar R, Lysecki PJ, Mondal T. Diffuse coronary artery dilatation in a neonate: a case report. J Neonatal Perinatal Med 2013; 6:263–6. [DOI] [PubMed] [Google Scholar]
- 33. Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020; 382:2163–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.