Abstract
Neutrophil extracellular traps (NETs) contribute to immunothrombosis and have been associated with mortality in coronavirus disease 2019 (COVID-19). We stimulated donor neutrophils with plasma from patients with COVID-19 and demonstrated that R406 can abrogate the release of NETs. These data provide evidence for how fostamatinib may mitigate neutrophil-associated mechanisms contributing to COVID-19 immunopathogenesis.
Keywords: COVID-19, neutrophil extracellular traps, immunothrombosis, fostamatinib
Novel therapies for the treatment of coronavirus disease 2019 (COVID-19) are urgently needed. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and resulting COVID-19 are characterized by an intense dysregulation of the host immune system accompanied by pathological coagulation. To date, only 2 therapies have demonstrated decreased morbidity or mortality in large clinical trials for severe COVID-19: remdesivir, an antiviral targeting the RNA-dependent RNA-polymerase, and dexamethasone, a broadly active immunosuppressant [1, 2]. While steroid therapy is an approach to dampen the immune response and other complications of COVID-19 such as acute respiratory distress syndrome (ARDS), concern remains about unintended consequences from steroid therapy, including prolonged viral shedding and risk of secondary infection [3]. Therefore, interest remains in identifying host-targeted immunomodulators to improve outcomes of patients with COVID-19 with limited or no adverse effects.
During systemic bacterial or viral infection, neutrophils release neutrophil extracellular traps (NETs) in a process called NETosis. NETs are web-like structures composed of DNA-decorated citrullinated histones and antimicrobial granules that limit dissemination of pathogens in the bloodstream [4]. However, elevated levels of serum NETs contribute to endothelial dysfunction and microvascular thrombosis that complicate severe COVID-19 and are associated with multiorgan pathology and mortality [5, 6]. Both host and microbial components in COVID-19 patient plasma contribute to NETosis [6]. One such pathway is the stimulation of FcγRIIA receptors on neutrophils by antigen-antibody complexes, which is mediated via spleen tyrosine kinase (SYK) phosphorylation and downstream signaling. R406, the metabolically active component of fostamatinib, a Food and Drug Administration (FDA)-approved drug, is a potent SYK inhibitor. R406 has been shown to limit inflammatory cytokine production of human macrophages stimulated by COVID-19 plasma by disrupting FcγRIIA receptor-mediated SYK signaling [7]. We sought to determine if SYK pathway inhibition limits NETosis in human neutrophils. We observed that R406 potently inhibits NETosis of healthy donor neutrophils stimulated with COVID-19 patient plasma, raising the potential for therapeutic benefit of fostamatinib for severe COVID-19.
METHODS
COVID-19 plasma was obtained from 6 patients treated at the National Institutes of Health Clinical Center (protocol number 10-I-0197) in accordance with National Institutes of Health institutional review board (IRB) and 1 patient treated under an IRB-approved protocol (HP-00086995) at the University of Maryland with extracorporeal membrane oxygenation. Healthy control plasma was obtained from 7 different donors. Healthy donor neutrophils were isolated from a single source (protocol number 17-CC-0148) using the EasySep Direct Human Neutrophil Isolation kit (Stemcell Technologies), following the manufacturer’s instructions. Stimulation assays were performed in duplicate by incubating healthy neutrophils with 10% plasma from healthy controls or plasma from patients with COVID-19 previously known to induce high levels of NETs. The formation of NETs was measured using the commercially available IncuCyte (Essen Bioscience), following manufacturer instructions. Briefly, IncuCyte was kept in an incubator at 37°C with 5% CO2. Cells were plated at 20 000 cells/mL in Gibco Opti-MEM I Reduced Serum Media (ThermoFisher) containing 250 nm of Cytotox Green Reagent (Essen Bioscience) to label extracellular DNA as a surrogate of NET formation. Phase and fluorescence images were captured using a 10× Plan Apo lens. For fluorescence, exposure time was 150 milliseconds and relative fluorescent units (RFU) were used to quantify NETs formation over 6 hours with data acquisition beginning approximately15 minutes after stimulation. For inhibition assays, R406 (Rigel Pharmaceuticals) was preincubated with healthy neutrophils at either 1 μM or 4 μM for 30 minutes prior to stimulation.
RESULTS
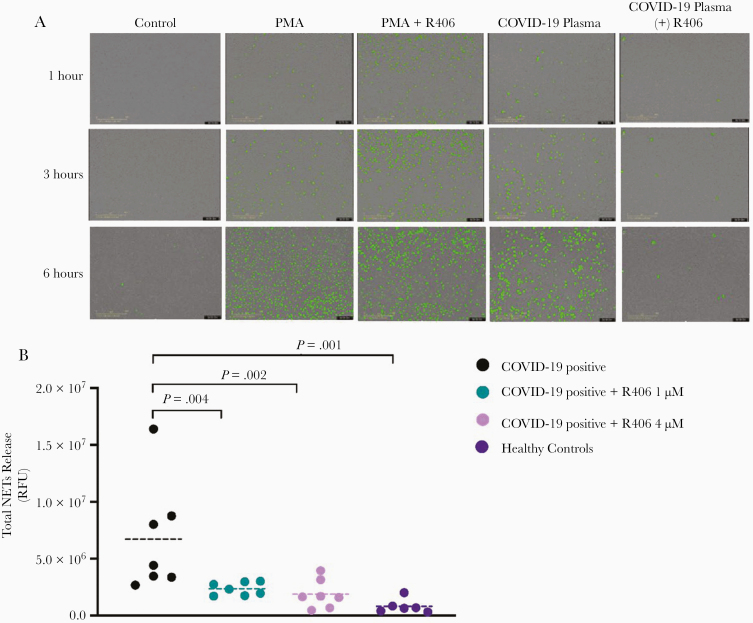
We stimulated healthy neutrophils with plasma from hospitalized patients with COVID-19 (n = 7) obtained between day 11 and 27 after symptoms onset, healthy controls (n = 6), or 1 μM phorbol myristate ester (PMA) as a positive control. The kinetics of NETs formation showed a gradual increase in NETs release over 6 hours when stimulated by plasma from patients with COVID-19 or PMA (Figure 1A). We analyzed the RFU values at 6 hours and demonstrated significant differences in total NETs release triggered by plasma from COVID-19–positive patients, when compared to healthy control plasma (7.7e6 vs 8.1e5; P = .01) (Figure 1B).
Figure 1.
R406 inhibits NETosis induced by plasma from COVID-19 patients. A, Representative images (10 ×) displaying the kinetics of NETs release visualized using the IncuCyte NETosis assay at 1, 3, and 6 hours comparing stimulation by healthy controls, plasma from a patient with COVID-19, PMA as a positive control, and R406 inhibition (4 μM) of plasma from a patient with COVID-19 and PMA. B, RFU at 6 hours after stimulation were used to compared groups using a Student t test. Horizontal dashed lines represent mean value of each group. Abbreviations: COVID-19, coronavirus disease 2019; NET, neutrophil extracellular trap; PMA, phorbol myristate ester; RFU, relative fluorescent unit.
Preincubation of neutrophils with R406 dramatically reduced NETs release triggered by COVID-19 plasma across all time points but did not alter NETosis induced by PMA (Figure 1A). The inhibitory effect of R406 was dose-dependent with a decrease of 72% (2.2e6 RFU, P = .04) at 1 μM and 81% (1.4e6 RFU, P = .02) at 4 μM compared to neutrophils stimulated with plasma from COVID-19 patients (Figure 1B).
DISCUSSION
This is the first report to identify that R406, a potent SYK inhibitor and the metabolically active component of fostamatinib, inhibits NETosis among healthy donor neutrophils stimulated with COVID-19 patient plasma. This finding was consistent across 7 different COVID-19 patients and R406 inhibition occurred in a concentration-dependent manner that is in the range of steady state concentration (800 nM to 1.6 μM) from an oral dose of 150 mg twice daily. Fostamatinib is currently FDA approved for adults with immune thrombocytopenia who have had insufficient response to previous treatment [8]. SYK is a cytoplasmic tyrosine kinase involved in intracellular signaling through Fc receptors or c-type lectin receptors on neutrophils, monocytes, macrophages, and platelets [9]. SYK signaling is therefore involved in a variety of cells types and pathways that have been implicated in the aberrant immune response and immunothrombosis that lead to worse outcomes in patients with severe COVID-19.
A major contributing factor to the development of immunothrombosis, a feature observed in COVID-19–induced ARDS that may associate with clinical outcomes and an area of much investigation, is the release of NETs [6]. A well-characterized mechanism for NETs production is stimulation of the FcγRIIA receptor and downstream SYK signaling, which contributes to heparin-induced thrombocytopenia [10]. Signaling through the FcγRIIA receptor is primarily driven by antigen-antibody complexes. In COVID-19 robust antibody responses have been associated with poor clinical outcomes, raising the possibility that antigen-antibody complexes contribute to pathogenic NETosis [11, 12]. Our results support the hypothesis that antigen-antibody complexes circulating in COVID-19 patient plasma might contribute to NETosis, which is inhibited by R406. By contrast R406 did not inhibit NETosis from neutrophils simulated with PMA in our experiments. This finding is likely attributable to the fact that PMA induces NETosis through redundant pathways, including pathways independent of SYK phosphorylation [13] (Supplementary Figure 1).
While immune complex-mediated NETosis may play a role in COVID-19 immunopathogenesis, a variety of microorganisms and endogenous stimuli, including cytokines and damage-associated molecular patterns, also have the ability to stimulate the release of NETs and definition of their role in COVID-19 disease remains to be elucidated [4]. We show that SYK inhibition using R406 prevents NETosis induced by COVID-19 patient plasma and this supports a potential therapeutic role for fostamatinib in the treatment of COVID-19, for which clinical trials are underway (clinical trials registration: NCT04579393) [7, 14].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the NIH-University of Maryland COVID-19 ECMO cohort for access to patient samples.
Disclaimer. The opinions expressed in this article are those of the authors and do not represent any position or policy of the National Institutes of Health, the US Department of Health and Human Services
Financial support. This work was supported in part by the Intramural Research Program of the National Institutes of Health Clinical Center, National Heart Lung and Blood Institute, National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Institute of Allergy and Infectious Diseases. M. J. R. was supported by a postdoctoral research associate fellowship from the National Institute of General Medical Sciences (grant number 1FI2GM137804-01). R406 was provided by Rigel Pharmaceuticals. This study is sponsored by the NHLBI which has a research collaboration with Rigel pharmaceutical through a Cooperative Research and Development Agreement (CRADA).
Potential conflicts of interest. J. R. S. is the principle investigator for a phase 2 study evaluating the safety of fostamatinib for COVID-19 and R. W. C., D. S. C., R. T. D., and A. F. S. are associate investigators on the same study (clinical trials registration: NCT04579393). All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Recovery Collaborative Group; Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print 17 July 2020]. N Engl J Med doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 3. Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA 2020; 324:1292–5. [DOI] [PubMed] [Google Scholar]
- 4. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018; 18:134–47. [DOI] [PubMed] [Google Scholar]
- 5. Middleton EA, He XY, Denorme F, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020; 136:1169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020; 5:e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoepel W, Chen H-J, Allahverdiyeva S, et al. Anti-SARS-CoV-2 IgG from severely ill COVID-19 patients promotes macrophage hyper-inflammation responses. bioRxiv, doi: 10.1101/2020.07.13.190140, 13. July 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
- 8. Rigel Pharmaceuticals. Tavalisse™ (fostamantinib disodium hexahydrate), 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209299lbl.pdf. Accessed 27 December 2020.
- 9. Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol 2010; 10:387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perdomo J, Leung HHL, Ahmadi Z, et al. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat Commun 2019; 10:1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Zhang L, Sang L, et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest 2020; 130:5235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu L, Wei Q, Lin Q, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 2019; 4:e123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gray RD, Lucas CD, MacKellar A, et al. Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J Inflamm (Lond) 2013; 10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alimova M, Sidhom EH, Satyam A, et al. A high-content screen for mucin-1-reducing compounds identifies fostamatinib as a candidate for rapid repurposing for acute lung injury. Cell Rep Med 2020; 1:100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.