Sir,
Remdesivir has been authorized for emergency use in patients with severe SARS-CoV-2 infection1 as it reduced the median time to recovery from COVID-19 in a randomized controlled trial.2 Due to a lack of data, remdesivir is not recommended for patients with an estimated glomerular filtration rate (‘eGFR’) ≤30 mL/min, including those with end-stage renal disease (ESRD), which represents a substantial limitation for COVID-19 treatment in this vulnerable population. Patients with ESRD have an increased risk for a severe course of COVID-19.3 Moreover, COVID-19 itself leads to acute kidney injury (AKI) and AKI-associated mortality in a significant proportion of critically ill patients.4 Thus, it is of high importance to assess the safety and pharmacokinetics of remdesivir in patients with renal impairment and those on renal replacement therapy.5
Intracellular remdesivir prodrug (GS-5734) is rapidly converted into its alanine metabolite GS-704277 and subsequently into the monophosphate that is finally converted into the active triphosphate GS-443902.6 Alternatively, dephosphorylation of the monophosphate yields the remdesivir nucleoside core (GS-441524), which becomes the predominant circulating metabolite providing a potential source of active drug by a slow re-phosphorylation process.7 In healthy volunteers, renal excretion of a remdesivir dose is about 10% as unchanged drug8 and about 50% as GS-441524.9
Here we report on the pharmacokinetics of remdesivir and its metabolites and the treatment outcome in a patient on renal replacement therapy without residual renal function suffering from severe COVID-19.
The study was approved by the local Ethics Committee of the University of Cologne. Informed consent was given prior to the study.
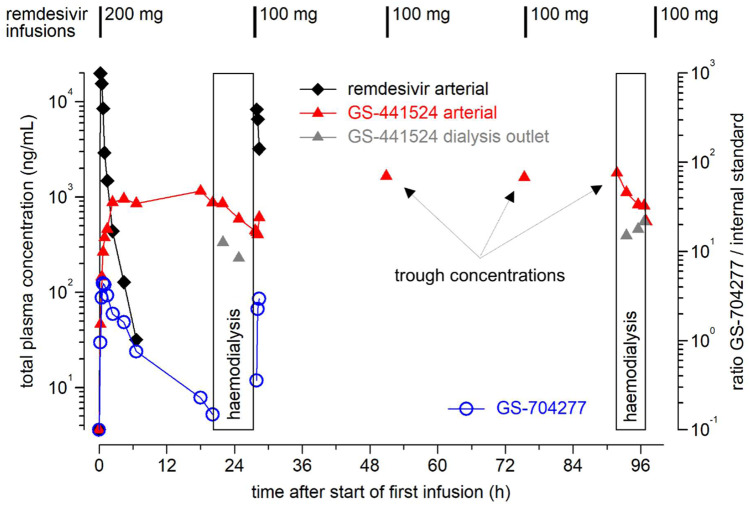
In Autumn 2020, a male patient in his mid seventies was admitted to the University Hospital Cologne and diagnosed with COVID-19 (Table S1 and Table S2, available as Supplementary data at JAC Online). Initially, he presented with typical pulmonary infiltrates and normal arterial blood gas results. However, the patient’s clinical status deteriorated rapidly and dexamethasone treatment was initiated. At this timepoint, remdesivir was not considered due to chronic renal impairment requiring renal replacement therapy. Sequential CT scans revealed progression of pulmonary infiltrates (Figure S1). Ten days after admission, the patient was transferred to the ICU with hypoxaemic lung failure requiring high-flow oxygen therapy. In this critical situation, we decided to apply remdesivir, while performing extensive therapeutic drug monitoring of the prodrug and two metabolites for safety reasons (see the Supplementary Methods for technical details). Remdesivir concentrations decreased rapidly after the first infusion with an apparent half-life of 1.1 h and were below the lower limit of quantification prior to dialysis (Figure 1, Table S3 and Table S4). GS-441524 concentrations increased up to 1 μg/mL and remained essentially constant. Exposure for remdesivir (AUC0–∞ = 13.0 μg/mL*h, Cmax = 19.8 μg/mL) and GS-441524 (AUC0–20.1h = 18.4 μg/mL*h, Cmax = 1.15 μg/mL) after the first dose was about 3-fold and 6-fold higher in our patient as compared with healthy volunteers (Table S3 and Table S4),8 which is in agreement with a preliminary observation in a critically ill patient with reduced renal function.10 Both remdesivir clearance and volume of distribution were clearly lower in our patient, which may in part be attributable to his low body weight (53.2 kg). Haemodialysis performed prior to the second and the fifth doses of remdesivir reduced GS-441524 plasma concentrations by about 50% (Figure 1). The initial extraction rate of dialysis was 72% and decreased to 42% at the end of dialysis (Table S5). Trough concentrations of GS-441524 were high but stable between dialysis sessions (range = 1.66–1.79 μg/mL), while remdesivir trough concentrations were always below the lower limit of quantification (Figure 1, Table S3 and Table S4). Thus, we did not observe significant accumulation of remdesivir and accumulation of GS-441524 was prevented by intermittent haemodialysis.
Figure 1.
Pharmacokinetics of the prodrug remdesivir (GS-5734), its intermediate alanine metabolite (GS-704277) and the predominant circulating metabolite GS-441524 during a standard 5 day regimen in a patient with intermittent haemodialysis due to long-lasting ESRD. Arterial blood samples were drawn in conjunction with initial administration of remdesivir and haemodialysis (day 1 and day 4). In addition, GS-441524 concentrations in samples from the dialysis outlet (grey triangles) are shown; the samples were taken in order to assess elimination by haemodialysis. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Accumulation of the vehicle sulfobutylether-β-cyclodextrin (SBECD) may occur in renal failure and high doses were associated with significant hepatic and renal toxicity in animals.11 However, reported toxic doses were 50–100 times higher than exposure during a 5–10 day course of remdesivir. In addition, it was shown that SBECD is eliminated by renal replacement therapy.12 Although we did not measure SBECD concentrations in this study, clinically significant accumulation of SBECD seems unlikely given the limited treatment duration and intermittent haemodialysis.
Overall, there were no signs of drug-related toxicity. Markers for hepatic injury remained within the reference ranges during treatment and up to 8 days of follow-up (Figure S2). An isolated increase in international normalized ratio (‘INR’) was observed, which improved after vitamin K supplementation and therefore was not considered as treatment related (Figure S2). By day 5 of remdesivir treatment, oxygenation parameters had improved allowing for patient transfer to a hospital ward.
Our observations support the safety of remdesivir in patients with ESRD on haemodialysis, which will assist clinical decision making and guide future clinical trial design.
Supplementary Material
Funding
This study was supported by internal funding.
Transparency declarations
None to declare.
Supplementary data
Tables S1 to S5, Figures S1 and S2 and Supplementary Methods are available as Supplementary data at JAC Online.
References
- 1.EMA. Veklury (Remdesivir). An Overview of Veklury and Why it is Authorised in the EU. https://www.ema.europa.eu/documents/overview/veklury-epar-medicine-overview_en.pdf.
- 2. Beigel JH, Tomashek KM, Dodd LE. et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med 2020; doi:10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ng JH, Hirsch JS, Wanchoo R. et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int 2020; doi:10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan L, Chaudhary K, Saha A. et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 2020; doi:10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adamsick ML, Gandhi RG, Bidell MR. et al. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol 2020; 31: 1384–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malin JJ, Suárez I, Priesner V. et al. Remdesivir against COVID-19 and other viral diseases. Clin Microbiol Rev 2020; 34: e00162-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eastman RT, Roth JS, Brimacombe KR. et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci 2020; 6: 672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Humeniuk R, Mathias A, Cao H. et al. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci 2020; 13: 896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. FDA. Fact Sheet for Healthcare Providers Emergency Use Authorization (EUA) of Veklury® (Remdesivir) 2020.
- 10. Tempestilli M, Caputi P, Avataneo V. et al. Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J Antimicrob Chemother 2020; 75: 2977–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luke DR, Tomaszewski K, Damle B. et al. Review of the basic and clinical pharmacology of sulfobutylether-β-cyclodextrin (SBECD). J Pharm Sci 2010; 99: 3291–301. [DOI] [PubMed] [Google Scholar]
- 12. Kiser TH, Fish DN, Aquilante CL. et al. Evaluation of sulfobutylether-β-cyclodextrin (SBECD) accumulation and voriconazole pharmacokinetics in critically ill patients undergoing continuous renal replacement therapy. Crit Care 2015; 19: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.